Introduction
Even though the production of persistent organic pollutants (POPs) has been banned for over 35 years, their levels still remain in the environment, and because they can be transported by wind and water, their effects can be evidenced far from where they were released (Geisz and others Reference Geisz, Dickhut and Cochran2008). Polychlorinated biphenyls (PCBs) and chlorinated pesticides such as DDTs are toxic chemicals that adversely affect people, wildlife and the environment (Bright and others Reference Bright, Grundy and Reimer1995; Roosens and others Reference Roosens, van Den Brink and Riddle2007; Llansola and others Reference Llansola, Montoliu, Boix and Felipo2010). Antarctica is the most remote continent of the planet, but despite of this it is not exempted from anthropogenic impacts (Bargagli Reference Bargagli2008; Corsolini Reference Corsolini2009). Since 1970s some POPs have been found in biota (Corsolini and others Reference Corsolini, Ademollo and Romeo2003), air (Bidleman Reference Bidleman, Walla and Roura1993), water (Bicego and others Reference Bícego, Weber and Ito1996), sediments (Montone and others Reference Montone, Taniguchi and Weber2001) and soil (Roosens and others Reference Roosens, van Den Brink and Riddle2007).
Only 0.34 % (about 50,000 km2) of the Antarctic continent is ice-free exposed rock mostly in the Peninsula area and remote mountain regions (BAS 2004). The ice-free areas constitute the main habitat for much of the wildlife and plants of Antarctica, in addition to fauna that inhabit Antarctica seasonally. There also concentrate most of the research stations and most human activities on the continent (Tin and others Reference Tin, Fleming and Hughes2009). This thin coastal strip represents only 0.05% of the total land area of Antarctica or about 6,000 km2 (Poland and others Reference Poland, Riddle and Zeeb2003). This habitat is globally important as the home for most of the terrestrial biota of Antarctica, including iconic Antarctic species such as Pygoscelis penguins, which are found nowhere else in the world (Woehler and Poncet Reference Woehler and Poncet1993). These ice-free areas are the sites of most environmental contamination (Tin and others Reference Tin, Fleming and Hughes2009).
Some evidence has shown that Antarctic fauna are susceptible to contaminants at lower concentrations than similar temperate species (Poland and others Reference Poland, Riddle and Zeeb2003). On the other hand, polar animals have a high content of lipids and a slow metabolism to save energy, and therefore they have a very slow process of pollutants detoxification (Chapman and Riddle Reference Chapman and Riddle2005). This implies that Antarctic organisms may be more vulnerable to the adverse effects of persistent pollutants than animals from other regions (Bargagli Reference Bargagli2008). Moreover, many contaminants in the polar environments may remain for decades (Poland and others Reference Poland, Riddle and Zeeb2003). Therefore, more research is necessary to investigate now baseline levels of POPs to monitor possible future changes in Antarctica and to understand the effects of contaminants on the environment in the cold regions.
Biomonitoring can provide valuable data of chemical levels in seabirds, like penguins, because they are considered sensitive species of environmental perturbations (Boersma Reference Boersma2008). Moreover, seabirds have long life cycles and provide subtle responses to contaminant exposure (Joshi and others Reference Joshi and Bakre2013). Penguins represent about 90% of the bird biomass in the Southern Ocean, where they breed on the Antarctic continent and sub-Antarctic islands (Williams and Rothery Reference Williams and Rothery1990; Williams Reference Williams1990). Antarctic penguins usually nest in colonies on sub-Antarctic islands and the Antarctic Peninsula area (Woehler and Poncet Reference Woehler and Poncet1993). Since 2000, this seabird species has been listed as near threatened (IUCN 2012). These animals can be excellent indicators of POPs exposure because they rely on their fat reserves to mitigate extreme cold during the winter, an energy burden that is one of the main targets for bioaccumulation of the highly lipophilic pollutants (Roosens and others Reference Roosens, van Den Brink and Riddle2007; Taniguchi and others Reference Taniguchi, Montone and Bícego2009). Although some studies have documented the presence of POPs in Antarctic organisms (Corsolini and others Reference Corsolini, Romeo and Ademollo2002; Corsolini and others Reference Corsolini, Ademollo and Romeo2003; Cipro and others Reference Cipro, Colabuono and Taniguchi2013), little is still known about their potential adverse effects on penguins.
Bird excreta have proven to be one of the best non-destructive matrices for measuring persistent pollutants (Sun and others Reference Sun, Yin and Liu2006; Espejo and others Reference Espejo, Celis and González-Acuña2014), due to minimal intervention on the animal during sampling, and provide a net result between consumed and intestinal absorbed fractions of food. The use of porphyrins in excreta as a biomarker of exposure in birds has showed a close link between the contaminant and induction of a specific porphyrin in target organs (Fossi and others Reference Fossi, Casini and Marsili1996; Casini and others Reference Casini, Fossi and Gavilan2001). Porphyrins are a group of biomolecules generated in the synthesis of hemoglobin and cytochroms, which are metabolised and accumulated in trace amounts in erythropoietic tissues such as liver or kidney, and then disposed with urine and guano (Lim Reference Lim, Smith and Witty2002). Likewise, the resulting oxide by-products such as copro-, uro- and protoporphyrins are not toxic at physiological levels, but accumulated in excess may affect liver function and bone marrow. Some contaminants can interfere with heme biosynthesis leading to altered levels of porphyrins that are accumulated or excreted (Fossi and others Reference Fossi, Casini and Marsili1996). We predicted that the levels of organic pollutants in excreta would correlate with those of porphyrins.
Determining organic pollutant levels in penguin excreta can provide a quantitative indication of environment impact caused by POPs in the marine Antarctic fauna as well as in the terrestrial ecosystem. The aim of this research was to determine the levels of some POPs and porphyrins in excreta of penguins that inhabit the Antarctic Peninsula area. A secondary objective was to validate the use of the QuEChERS method (Asensio-Ramos and others Reference Asensio-Ramos, Hernández-Borges, Ravelo-Pérez and Rodríguez-Delgado2010) to detect organochlorine pesticides and PCBs in penguin excreta. QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) is an analytical method originally developed for measuring a wide range of pesticides in fruits and vegetables (Anastassiades and others Reference Anastassiades and Lehotay2003). Recently, some studies have shown that this method can be used for environmental purposes, and can be adapted to other kinds of contaminants and other biological matrices, such as fish tissue (Norli and others Reference Norli, Christiansen and Deribe2011) and penguin blood samples (Jara-Carrasco and others Reference Jara-Carrasco, González and González-Acuña2015).
Material and methods
Field work
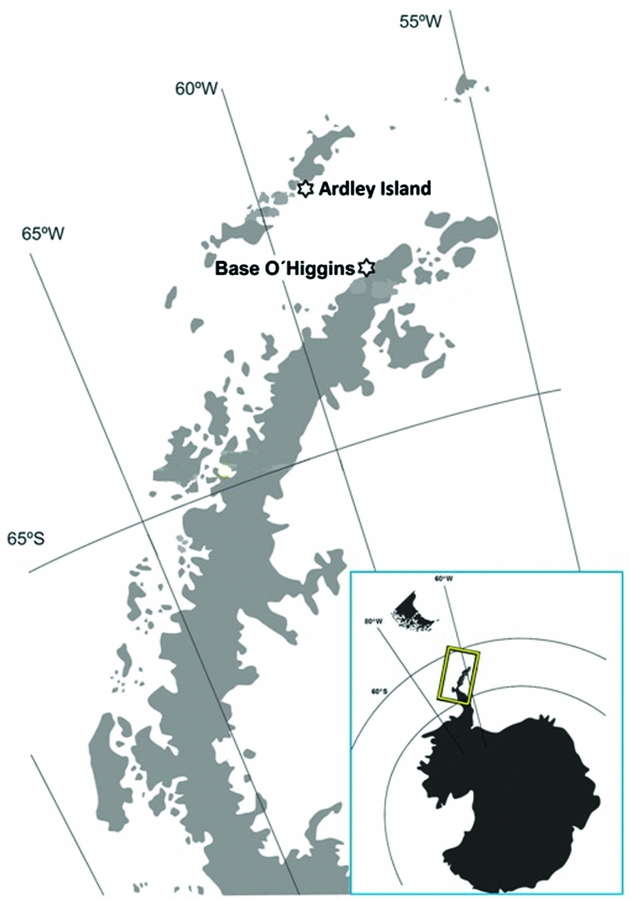
This study was carried out at two locations of the Antarctic Peninsula area (see Figure 1) during the southern summer (January-February 2011). Twenty excreta samples (5 to 10 g each) were collected from nesting sites of Adélie penguin and chinstrap penguin colonies at Base O'Higgins (63°19′01″S, 57°54′29″W) – situated in the Antarctic Peninsula, while 20 samples of gentoo penguin colonies were obtained on the Ardley Island (62°13′48″S, 58°54′34″W) – situated south of King George Island. Before collecting samples, a bioethic letter was provided by the Universidad de Concepción, which was strictly required to obtain authorization from the Chilean Antarctic Institute (INACH) to carry out the field campaign. Each sample collected was from many individuals of the breeding colony. Excreta were collected immediately after bird defecation. Careful sampling and storage procedures were observed to avoid contamination. Fresh penguin droppings were taken with stainless steel spoon from the top of the mass of excrements deposited on the ground, then placed in aluminum foil and stored at -20°C in a steel container until their analysis in the laboratory.
Fig. 1. Map of the Antarctic Peninsula area showing the sampling sites on Ardley Island (King George Island) and Base O'Higgins (Antarctic Peninsula).
Laboratory work
The levels (wet weight) of PCBs (congeners 138, 153 and 180), hexachlorobenzene (HCB), heptachlor, dichlorodiphenyltrichloroethanes (p,p' -DDT, -DDE, -DDD) and endrin aldehyde were determined at the Environmental Chemistry Laboratory (accredited ISO 17025) of the Universidad de Concepción. The extraction of the contaminants in excreta was performed by using the QuEChERS method (Asensio-Ramos and others Reference Asensio-Ramos, Hernández-Borges, Ravelo-Pérez and Rodríguez-Delgado2010) used for sediments, here modified. First, all the samples were thawed and then weighed. Five grams of wet sample was put in a 50 ml centrifuge tube with 10 ml of n-hexane and stirred for 1 min in a vortex mixer. Gram quantities of salts (4 g of Mg SO4 and 2 g of NaCl) were added to each tube (to drive analytes partitioning between the aqueous residue and the solvent) by stirring (vortex mixer) for 1 min, followed by sonication (15 min) in an ultrasonic bath, and centrifugation (4°C, 20 min at 5000 rpm). After vortex mixing and centrifugation, clean-up and removal of residual water was performed using a d-SPE procedure (PSA adsorbent and anhydrous MgSO4 were mixed with the sample extract). After centrifugation (5000 rpm, 5 min), the volume of the extracts was reduced in a rotary evaporator (40°C). The final extract (1 ml) was stored in an amber vial, brought to dryness under a nitrogen stream. An internal standard (PCNB, 5 ng ml−1) and the samples were injected into a gas chromatograph equipped with an electron capture detector (GC-ECD Perkin Elmer, series 9000). The separation of the analytes was performed in a PTE-5 capillary column (30 m × 0.25 mm inner diameter, 0.25 μm thick stationary phase) and helium as the carrier gas. The temperatures of the injector and detector were 240°C and 360°C, respectively. The temperature program of the oven was: 100°C for 10 min, then increased until 280ºC at 5°C per minute, and kept for 12 min. The analytes of interest were separated within 58 min. Analysis of POPs in penguin excreta was accompanied by a quality control program, which consisted of repetitive analysis of blank samples and excreta samples spiked with known concentrations of the analytes. The detection limits were 0.002 µg l−1. Surrogate recovery ranged from 68% up to 110% for PCB and 60% up to 108% for pesticides. The results were within the specified values with recovery rates between 67.9 and 108.2 % for all the analytes at the monitoring rates. Blank sample values were 0.5, 0.05, 1.0, 0.2 and 1.0 % for HCB, heptachlor, DDTs, endrin aldehyde and PCBs, respectively.
The levels (dry weight) of porphyrins (uro-, copro- and copro-) were determined at the Biomarkers Laboratory of the Universidad de Concepción. Porphyrins were determined as described by Lockwood and others (Reference Lockwood, Poulus and Rossi1985). Briefly, 1 ml of a 5 N HCl solution was placed into a centrifuge tube containing 100 mg of excreta and vortex-mixed for 1 min. Then, diethyl ether (3 ml) was added and mixed to obtain an emulsion. Afterwards, 3 ml of distilled water were added and the extract was again mixed and then centrifuged at 700 g for 10 min. The supernatant was removed, and the precipitant containing the porphyrins was analyzed in a spectrophotofluorimeter (Perkin Elmer model LS50). The porphyrins were detected using the following excitation/emission wavelengths: 405/595 nm for uroporphyrin, 400/595 nm for coproporphyrin, and 410/605 nm for protoporphyrin (Casini and others Reference Casini, Fossi and Gavilan2001). The quantitation of porphyrins was performed with external standards (uroporphyrin III octamethyl ester, coproporphyrin III dihydrochloride, protoporphyrin IX dimethyl ester) obtained from Porphyrin Products Inc. (Logan, UT).
The levels of POPs and porphyrins (X) were subjected to logarithmic transformation [ln (X+1)] for statistical analyses. Statistical elaboration of data was performed using Statistica software (Statsoft). Comparisons of mean levels were performed using one-way ANOVAs. Correlations for pollutants and porphyrins were calculated through Pearson's test. Differences were considered significant at p ≤ 0.05.
Results
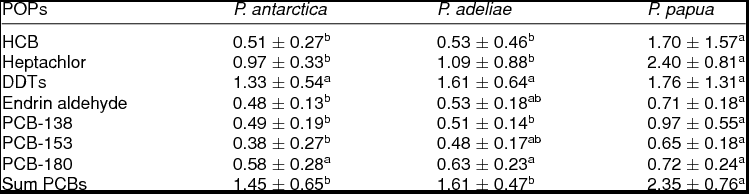
Table 1 shows the mean concentrations of POPs in penguin excreta. Across the three penguin species studied here, PCBs (1.45-2.35 ng g−1 ww) and DDTs (1.33-1.76 ng g−1 ww) presented the highest levels of all analyzed POPs. On the contrary, the lowest concentrations corresponded to endrin aldehyde with 0.48, 0.53 and 0.71 ng g−1 ww in excreta of P. antarctica, P. adeliae and P. papua, respectively. The concentrations of HCB, heptachlor and PCB-138 were significantly higher in gentoo penguin excreta than those levels found in chinstrap and Adélie penguin excreta. The levels of endrin aldehyde and PCB-153 were higher (ANOVA, p ≤ 0.05) in gentoo penguin droppings than in chinstrap excreta. The levels of DDTs and PCB-180 showed not significant differences in excreta of the three penguin species studied. Not significant differences in the concentration of HCB, heptachlor, endrin aldehyde, PCB-153 and PCB-138 were found in chinstrap penguin excreta as compared to Adélie penguin excreta. The contaminant concentration patterns in the three species of penguins studied was: gentoo penguin > Adélie penguin > chinstrap penguin. The levels of POPs found in excreta of the three penguin species, considering all the samples together, indicated the following relation: PCBs > DDTs > heptachlor > HCB > endrin aldehyde.
Table 1. Concentration (ng g−1 wet weight) of persistent organic pollutants in excreta samples of different Pygoscelis penguins (mean ± standard error) from the Antarctic Peninsula area.
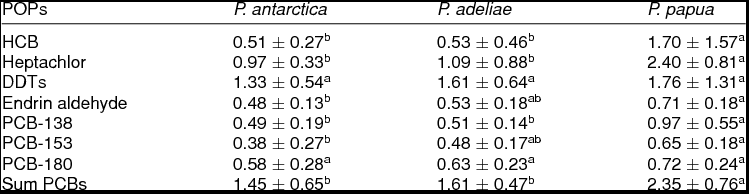
HCB = hexachlorobenzene; DDTs (dichlorodiphenyltrichloroethanes) = p,p' –DDT, p,p' –DDD and p,p' –DDE; PCB = polychlorinated biphenyl. Different letters in a row indicate statistical significant differences (ANOVA, p ≤ 0.05).
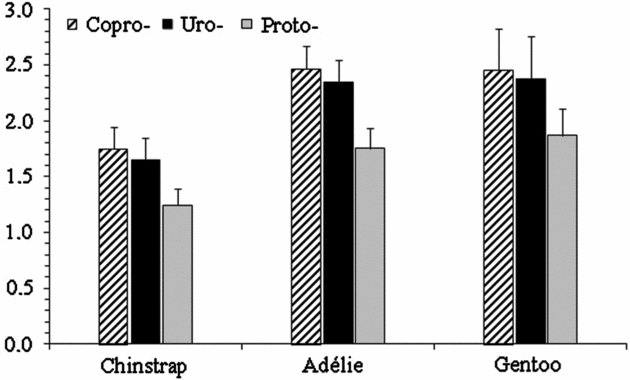
The porphyrin levels in the samples analysed are shown in Figure 2. The total porphyrins levels were 4.6, 6.6 and 6.7 nmol g−1 in chinstrap, Adélie and gentoo penguin excreta, respectively. The levels of copro- (p = 0.132), uro- (p = 0.121) and protoporphyrin (p = 0.068) did not show significant differences among the penguin species. Copro- (1.01-2.81 nmol g−1), uro- (0.99-2.76 nmol g−1) and protoporphyrin (0.77-2.11nmol g−1) found in the excrements of chinstrap penguins were similar to the levels of copro- (1.69-3.66 nmol g−1), uro- (1.72-3.26 nmol g−1) and proto- (1.26-2.46 nmol g−1) obtained in Adélie penguins excreta, and also were similar to the levels of copro- (1.09-3.51nmol g−1), uro- (1.05-3.53 nmol g−1) and proto- (0.87-3.12 nmol g−1) found in gentoo penguin excreta. Persistent PCB congeners 180 and 138 contributed more to the total PCB content in the excreta samples than congener 153.
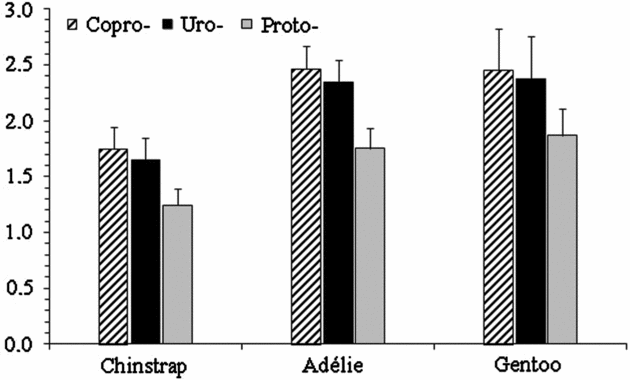
Fig. 2. Mean concentration (ng g−1 dry weight) and standard error of porphyrins in excreta samples of different Pygoscelis penguins from the Antarctic Peninsula area.
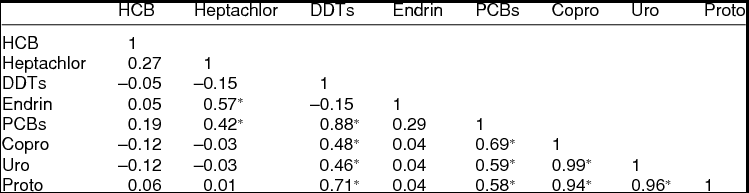
There was a significant positive correlation between the concentrations of PCBs and DDTs with the levels of porphyrins (Table 2). The levels of PCBs found in penguin excreta were significantly correlated (p ≤ 0.05, Pearson, r > 0.5) with those of porphyrins (copro-, uro-, proto-). Also, the levels of DDTs were highly correlated with the levels of protoporphyrins. There was a medium positive correlation (p ≤ 0.05, Pearson, r > 0.3) between the levels of uroporphyrins and coproporphyrins with those of DDTs. Moreover, a positive significant correlation was observed in the levels of endrin/heptachlor, PCBs/heptachlor, and PCBs/DDTs, which could indicate that these contaminants all together induce porphyrin synthesis.
Table 2. Pearson correlation among POPs and porphyrins (copro-, uro-, proto-) in excrements of the three species of penguins from the Antarctic Peninsula area.
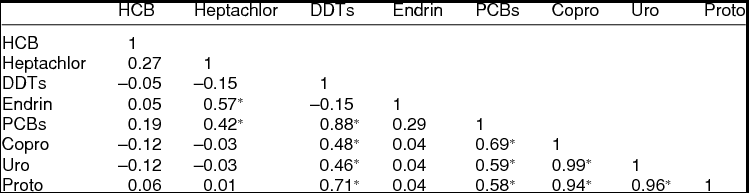
*Statistically significant (ANOVA, p ≤ 0.05).
Discussion
Our levels detected of PCBs, DDTs, HCB, endrin and heptachlor in penguin excreta reveal that these organic pollutants are still relevant within the Antarctic food web. These organic pollutants are been incorporated into penguins mainly through diet, where are metabolised and then eliminated via excretion (Falkowska and others Reference Falkowska, Reindl and Szumilo2013; Joshi and others Reference Joshi and Bakre2013). Because gentoo penguins do not travel outside the Antarctic Peninsula area (Williams Reference Williams1990), these seabirds are concentrating POPs that have already been transported into the area via water or air and further redistributing them through local bird activities.
The lack of published data on POPs and porphyrins levels in penguin excreta makes it difficult to discuss these results. In the present study, the levels of DDTs (1.33–1.76 ng g−1) found in excreta of penguins from the Antarctic Peninsula area were about 106–80 times lower than those reported in excreta of penguins from marine environments of lower latitudes (Falkowska and others Reference Falkowska, Reindl and Szumilo2013). Overall, our levels of POPs were generally lower than those reported in the same penguins species, but measured in other biological materials such as eggs (Cipro and others Reference Cipro, Taniguchi and Montone2010) and lipid tissue (Taniguchi and others Reference Taniguchi, Montone and Bícego2009). Our levels of HCB, DDTs, PCB-138, PCB-153 and PCB-180 found in chinstrap penguin excreta were 1.7, 5.5, 3.1, 5.8 and 3.4 times lower than those levels observed by Jara-Carrasco and others (Reference Jara-Carrasco, González and González-Acuña2015) in blood of the same species, respectively. Taniguchi and others (Reference Taniguchi, Montone and Bícego2009) found that the concentrations of HCB, DDTs and PCBs in the fat tissue of penguins (average levels of Pygoscelis adeliae, Pygoscelis antarctica and Pygoscelis papua, pooled together) found at King George Island were 373, 193 and 256 ng g−1, which is about 220, 110 and 109 times higher than our highest levels in excreta, respectively. The lower levels of POPs found in penguin excreta can be explained as these pollutants tend to bioaccumulate mainly in the organism. PCB-138, 153 and 180 have a low excretion rate in birds and tend to accumulate in the animal (Maervoet and others Reference Maervoet, Chu and Vos2004). A study found that 8.4% of PCBs ingested were excreted in the excrements of otters (Lontra canadensis), which revealed that PCBs of 16,000 ng/g lipid weight excreta is equivalent to 50,000 ng g−1 lipid weight tissue, a critical level for otters based on reproductive toxicity (Smit and others Reference Smit, Leonards and van Hattum1994). POPs in excreta as related to internal tissue threshold levels for effects on reproduction, neurobehavioral development and immune suppression of Antarctic species is an issue that need to be more investigated.
The presence of PCBs in excreta is highly related to their molecular structure, as most birds are capable of eliminating PCBs with low molecular weight (Norstrom and others Reference Norstrom, Simon and Muir1988). The congeners 138 and 180 are hexa-and hepta-chlorinated PCBs, respectively, which constitute most of the residues of PCBs from food ingested by penguins (Focardi and others Reference Focardi, Corsolini and Bargagli1995). A study performed in the Lake Ontario ecosystem stated that twelve PCB congeners (including 153, 138 and 180) constituted over 50% of the PCBs in fish and are considered to be environmentally important (Oliver and Niimi Reference Oliver and Niimi1988). The tetra-, penta-, and hexa-chlorinated isomer groups exhibit greater toxicity than the other chlorinated forms, thus congener PCB-138 is classified in a group more potent than PCB-180 in inducing hepatic function alterations and immunotoxicity in humans (Llansola and others Reference Llansola, Montoliu, Boix and Felipo2010). In the present study, only gentoo penguin excreta showed PCB-138 levels higher than those of PCB-180.
When comparing the POPs levels measured in the current study with those reported recently in other marine species and biological materials from the Northern Hemisphere, our findings appear to be much lower. A study on eggs of white-tailed sea eagles (Haliaeetus albicilla) from Baltic Sea, showed a depressed nest productivity related to eggshell thickness caused by PCBs (500,000 ng g−1) and p,p' –DDE (120,000 ng g−1) (Helander and others Reference Helander, Olsson and Bignert2002). In blubber of seals from White Sea (Russia) the levels of DDTs and PCBs were about 4,000 and 5,000 ng g−1 lw, respectively, whereas the levels of HCB were 62 ng g−1 lw (Muir and others Reference Muir, Savinova and Savinov2003). In some species from contaminated environments, PCBs have usually been the dominant contaminant class, whereas levels of DDTs are lower (Evenset and others Reference Evenset, Christensen and Kallenborn2005). Recent studies have found that organic pollutant concentrations in tissue, body compartment or egg of several Arctic marine mammal species and populations exceed a general threshold level of concern of 1,000 ng g−1 ww (Letcher and others Reference Letcher, Bustnes and Dietz2010).
The presence of POPs can be a serious threat to Antarctic ecosystems and their endemic animals, because they bioaccumulate and biomagnify in aquatic food webs, resulting in high concentrations in top-predatory wildlife species (Guertin and others Reference Guertin, Harestad and Ben-David2010). In Antarctica, trophic chains are relatively simple and short, where seabirds at the top of the food webs, like penguins, depend mainly on the silverfish (Pleuragramma antarcticum) and krill (Euphausia superba) (Corsolini Reference Corsolini2009). Concentrations (ng g−1 ww) of DDTs (0.41), PCB-153 (0.1) and HCB (0.06) have been reported in krill (Cipro and others Reference Cipro, Taniguchi and Montone2010), the main prey of Antarctic penguins, which are about 28, 5 and 4 times lower than our mean levels in penguin excreta, respectively. Levels (ng g−1 ww) of HCB (0.66) and DDTs (3.04) have been found in Antarctic fish (Cipro and others Reference Cipro, Colabuono and Taniguchi2013), which are about twice lower than our mean levels in excreta. Biomagnification is the main route of contamination at the higher levels, where the feeding habits of penguins play a crucial role in POPs intake (Corsolini Reference Corsolini2009). Our data showed that P. papua excreta had the highest concentrations of HCB, heptachlor and PCB-153 as compared to P. antarctica and P. adeliae. Diet could explain the differences found in the concentration of POPs in penguin excreta (Falkowska and others Reference Falkowska, Reindl and Szumilo2013), because the three colonies studied are distant and this may affect the diet of penguins and consequently the contaminant and porphyrin levels. The differences found in penguin excreta could be a result of increased bioaccumulation of contaminants for these seabirds, probably associated to changes in diet through time and space. Gentoo penguin feed mainly on Antarctic fish, but its diet may be supplemented with an intake of krill and different crustaceans and cephalopods (Berón and others Reference Berón, Néstor and Coria2002).
Our results provide important details for interpretation of contaminant exposure. The fingerprint of the porphyrins in the excreta samples from the three localities studied showed the following pattern: coproporphyrins > uroporphyrins > protoporphyrins. This pattern observed is probably due to the same nature of chemical stress in the area studied (Casini and others Reference Casini, Fossi and Gavilan2001). Results from the present study showed that the highest total porphyrin levels (6.7 nmol g−1) detected in gentoo penguin excreta are 2.24 lower than those levels reported by Fossi and others (Reference Fossi, Casini and Marsili1996) in droppings of Japanese quail (Coturnix japonica), which were treated with 50 mg kg−1 of PCBs in the diet for 25 days. In a study performed in Talcahuano (Chile), a highly impacted area by anthropogenic activities, levels of total porphyrins (8.6 ng g−1 dw) measured in excreta of kelp gulls (Larus dominicanus) showed that this species is at high toxicological risk (Casini and others Reference Casini, Fossi and Gavilan2001). Japanese quail treated with 500 mg of HCB per kg body weight for 60 days was associated with 4.8 nmol g−1 of total porphyrins in excreta (Casini Reference Casini1997), which was similar to our levels in chinstrap penguins, and about 30% lower than our levels in Adélie and gentoo penguins. The levels of porphyrins in penguin excreta studied here are similar to those found by Celis and others (Reference Celis, Jara and González-Acuña2012) in gentoo penguin excreta at Base O'Higgins. Also, our total porphyrins levels were lower than those levels (9.1 nmol g−1 dw) found in Humboldt penguin (Spheniscus humboldti) excreta, a species of seabird that naturally inhabits the coast between Peru (5°S) and Chile (43°S), which were linked to heavy metal contamination (Celis and others Reference Celis, Espejo and González-Acuña2014).
The positive correlation between porphyrins (uro-, copro-, and proto-) and POPs indicates a high affinity between them, which is linked to physiological problems in the pancreas, liver and kidneys of birds (Casini and others Reference Casini, Fossi and Leonzio2003). This means that colonies of penguins living on Base O'Higgins and Ardley Island might be suffering some negative biological effects from POPs contamination. Some local pollution (fuel combustion, waste incineration, sewage disposal, paint or accidental oil spills) or the impact of the tourism increases (6,704–36,875 tourists from 1992–1993 to 2009–2010) and scientific bases with their associated activities as plane and ship trips have been noted in the northern area of the Antarctic Peninsula (Lynch and others Reference Lynch, Crosbie and Fagan2010; IAATO 2010; Barbosa and others Reference Barbosa, de Mas, Benzal and Diaz2013). Most Antarctic research stations are located on the King George Island within 5 km of the coast. Base O'Higgins is a permanently staffed base, where around a hundred pairs of gentoo penguins inhabit in the surrounding area, which is frequently visited by tourists. Research stations are the major land-based activity in the Antarctic, and, as a consequence, are the source of most locally derived pollutants (Poland and others Reference Poland, Riddle and Zeeb2003).
Porphyrins have a typical absorption spectrum, thus their detection levels are very sensitive and specific (Fossi and others Reference Fossi, Casini and Marsili1996). Porphyrins are produced and accumulated in small amounts in erythropoietic tissues, liver and kidney and excreted by urine or faeces, which can be identified as coproporphyrin, uroporphyrin and protoporphyrin (Casini and others Reference Casini, Fossi and Leonzio2003). It has been noted that polycyclic aromatic hydrocarbons (PAHs), metals, and organic contaminants might interfere with heme synthesis (Fossi and others Reference Fossi, Casini and Marsili1996). In our study, POPs and porphyrins levels indicate gentoo penguins from Base O'Higgins and Ardley Island are exposed to these pollutants at some level. Certain organic pollutants such as DDTs and PCBs can affect the immuno-haematological response of Pygoscelis antarctica that nest in some sites of the South Shetland Islands and Base O'Higgins (Jara-Carrasco and others Reference Jara-Carrasco, González and González-Acuña2015). As a consequence, the use of porphyrins in droppings of seabirds can be considered as a valid biomarker of exposure to chemicals, which is consistent with some other reports (Casini and others Reference Casini, Fossi and Gavilan2001; Celis and others Reference Celis, Jara and González-Acuña2012).
The correlations between POPs and porphyrins indicate despite of the low chemical concentrations at which Antarctic penguins can be exposed, compared to seabirds of the Arctic and other latitudes (Bustnes and others Reference Bustnes, Moe and Herzke2010; Letcher and others Reference Letcher, Bustnes and Dietz2010; Falkowska and others Reference Falkowska, Reindl and Szumilo2013), the levels of POPs found in our study suggest that these contaminants could potentially be altering the synthesis of porphyrins in the species studied. There is evidence showing that even low concentrations of some toxic pollutants may have hazardous effects on penguins (Subramanian and others Reference Subramanian, Tanabe and Fujise1986). However, Antarctic penguins are exposed to a wide range of other contaminants, and therefore the influence of some other pollutants in Antarctica cannot be neglected (Roosens and others Reference Roosens, van Den Brink and Riddle2007; Corsolini Reference Corsolini2009; Taniguchi and others Reference Taniguchi, Montone and Bícego2009; Espejo and others Reference Espejo, Celis and González-Acuña2014). It is possible that POPs in combination may act additively, antagonistically or synergistically. A study showed that while PCB-153 alone did not result in porphyrin accumulation in rats, co-administration of PCB-153 with dioxin revealed a strong synergistic effect (Van Birgelen and others Reference Van Birgelen, Fase and van Der Kolk1996).
Long-range atmospheric transport appears to be the main mechanism for exposing cold regions to POPs (Bargagli Reference Bargagli2008). However penguin excreta can also increase contaminant levels in some environments (Sun and others Reference Sun, Yin and Liu2006). Penguins form large breeding colonies on locations where they can number in the hundreds or tens. Because they feed exclusively off shore but nest on land, penguins can act as biovectors of contaminants from marine ecosystems to terrestrial ecosystems (Roosens and others Reference Roosens, van Den Brink and Riddle2007). Through this transport pathway, penguins can also concentrate POPs that are biomagnified and bioaccumulated through the marine food webs. This may have serious implications for any biota and possible incorporation into the terrestrial food web. Polar invertebrates have low detoxifying activity to metabolise PCB 138, 153 and 180 (Bright and others Reference Bright, Grundy and Reimer1995). Evidence on sediment quality shows that the incidence of benthic macroinvertebrate effects increase when concentrations of PCBs > 61.2, DDTs > 12.6, endrin > 4.7 and heptachlor > 0.11 ng g−1 ww (Burton Reference Burton2002). Our heptachlor levels (0.97-2.40 ng g−1) detected in penguin excreta are above threshold values (defined as the concentration above which adverse effects are expected to occur) for this organic pollutant, indicating that penguins are transporting this chemical from the sea to the nesting areas to toxic levels for the surrounding biota that live on Base O'Higgins and Ardley Island, such as the Antarctic whelk (Neobuccinum eatoni) or the Antarctic scallop (Adamussium colbecki). The scallop is a filter feeder, therefore it can accumulates more POPs than be expected in relation to its tropic position in the Antarctic food web (Corsolini Reference Corsolini2009). Some evidence from the Antarctic Peninsula indicated high heavy metal levels in coastal soils near penguin rockeries at Base O'Higgins (Celis and others Reference Celis, Barra and Espejo2015). The effects of POPs in freezing ground are of concern because of their potential for detrimental effects on biota (Poland and others Reference Poland, Riddle and Zeeb2003). As practically no data on POPs are available for this area, our findings suggest the need for further studies.
Conclusions
The levels of PCBs, DDTs, endrin, heptachlor and HCB found in this study indicate these contaminants are still relevant in the Antarctic environment and can affect the food chain through bioaccumulation and biomagnification processes. Our study showed that QuEChERS is an useful tool in the detection of POPs in excreta. The detection of these POPs in excreta of the three species of penguins that inhabit the Antarctic Peninsula area, showed a clear capacity of these population of seabirds to accumulate and eliminate these pollutants, where the feeding habits of penguins play a crucial role in chemical uptake.
Porphyrins can be used as nondestructive indicators of exposure to organic pollutants of penguin species potentially at risks, especially when sequential sampling must be carried out for long-term studies. The levels of porphyrins detected in excreta indicate that these POPs may be producing some biological effects in the colonies of penguins studied. Gentoo penguin is the species that showed the highest concentrations of these contaminants in excreta, which can be attributed to their diet and trophic level.
The organic pollutants found in excreta of Antarctic penguins can be a source of contamination of terrestrial ecosystems from marine environments. The levels of heptachlor found in penguin guano may be affecting some benthonic organisms that live around nesting sites.
Acknowledgements
We are grateful to the personnel of the Base O'Higgins for their valuable assistance in the field. Also, special thanks to Diane Haughney for her kind help in improving the language of the manuscript.
Financial support
This study was supported by the Chilean Antarctic Institute (through projects INACH T-12-13 and INACH RG 09–14) and by the Universidad de Concepcion (through project VRID 216.153.025-1.0).