Introduction
The extraction of fish and other biotic resources from marine systems has had dramatic effects on ocean ecosystems (Pauly and others Reference Pauly, Christiansen, Dalsgaard, Froese and Torres1998; Halpern and others Reference Halpern, Walbridge, Selkoe, Kappel, Micheli, D'Agrosa, Bruno, Casey, Ebert, Fox, Fujita, Heinemann, Lenihan, Madin, Perry, Selig, Spalding, Steneck and Watson2008; Baum and Worm Reference Baum and Worm2009; Estes and others Reference Estes, Terborgh, Brashares, Power, Berger, Bond, Carpenter, Essington, Holt, Jackson, Marquis, Oksanen, Oksanen, Paine, Pikitch, Ripple, Sandin, Scheffer, Schoener, Shurin, Sinclair, Soulé, Virtanen and Wardle2011). The process by which this has been accomplished, described by Pauly and others (Reference Pauly, Christiansen, Dalsgaard, Froese and Torres1998) as ‘fishing down the food web’, generally has been to initially target larger, high-trophic level species and then, as these become depleted, to transition to short-lived pelagic fish and low-trophic level invertebrates. These changes result in a reduction of the mean trophic level of the catch, now called the marine trophic index (MTI; Pauly and Watson Reference Pauly, Watson and Alder2005). An alternative explanation for the change in the average trophic level of species exploited, argued by Essington and others (Reference Essington, Beaudreau and Wiedenmann2006) and called ‘fishing through the food web’, is that exploitation of upper-trophic level species continues but lower-trophic level ones are added. One problem in disentangling these processes and subsequent interpretation of ecosystem effects is that the most studied ocean areas, where a large portion of fishery extraction has occurred, are coastal, with the ocean there subject to numerous other natural and unnatural, confounding impacts (see, for example, Halpern and others Reference Halpern, Walbridge, Selkoe, Kappel, Micheli, D'Agrosa, Bruno, Casey, Ebert, Fox, Fujita, Heinemann, Lenihan, Madin, Perry, Selig, Spalding, Steneck and Watson2008). Most recently, Branch and others (Reference Branch, Watson, Fulton, Jennings, McGilliard, Pablico, Ricard and Tracey2010) proposed that the catch statistics used in ‘fishing down’ and ‘fishing through’ studies are not informative as to trophic changes in fisheries and food web health, and that direct abundance-survey data are required to gauge current food-web structure and fishing impacts.
Herein an attempt is made to review how the most remote ocean area in the world, the Antarctic continental shelf and slope, has fared in the face of human exploitation. This location, with its daunting environmental conditions, has no resident human population, and only a relatively short history of human exploitation, contrary to the situation in the Arctic (Zeller and others Reference Zeller, Booth, Pakhomov, Swartz and Pauly2011). Thus, the Antarctic continental shelf and slope are destinations that can serve few other purposes than exploitation. Indeed, other than the taking of biotic resources there has been little other anthropogenic influence on the food webs of these waters, except for recent effects of global climate change (Atkinson and others Reference Atkinson, Siegel, Pakhomov and Rothery2004; Halpern and others Reference Halpern, Walbridge, Selkoe, Kappel, Micheli, D'Agrosa, Bruno, Casey, Ebert, Fox, Fujita, Heinemann, Lenihan, Madin, Perry, Selig, Spalding, Steneck and Watson2008).
The waters covered in this review are those included in the continental margin portion of the Antarctic large marine ecosystem (LME; Aquarone and Adams Reference Aquarone, Adams, Sherman and Hempel2008; see also Sea Around Us Project 2012). These waters are also contained within Food and Agriculture Organization (FAO) fishery statistical areas, subareas and divisions 48.1; 48.5; 48.6 (in part); and the continental margin portions of 58.4.1, 58.4.2 and 88.1–88.3. They are, obviously, also part of the area covered by the Convention for the Conservation of Antarctic Marine Living Resources (CCAMLR; Fig. 1). Overall, the Antarctic continental shelf and slope in many sectors have received relatively little attention by commercial sealers, whalers and fishers, in large part owing to persistent sea ice that deters vessel operations, especially vessels that are not ice capable. Very few fishing vessels that ply the Southern Ocean are ice capable.

Fig. 1. Antarctica, its Large Marine Ecosystem (LME, in grey) and the FAO areas, subareas and divisions used in this report (which are also those used by CCAMLR). Note that subarea 48.6 actually extends further north, but was cut here (dotted line), as this contribution deals only with the shelf and slope of the Antarctic continent. The present report covers largely just the grey area: shelf and slope.
In the present review, details are given of the continued exploitation of Antarctic continental shelf and slope waters only. The fish catch data reported here have been taken from CCAMLR statistical bulletins (CCAMLR 1990–2012).
Early history: the marine mammals
The South Shetland and South Orkney islands (FAO subareas 48.1 and 48.2, respectively), continental islands located off the northern coast of the Antarctic Peninsula, were discovered by sealers in 1819–1820, and subsequently the Antarctic fur seal Arctocephalus gazella was extirpated (O'Gorman Reference O'Gorman1961; as were southern elephant seals Mirounga leonina in more northern Southern Ocean waters). Thus, human exploitation of Antarctic resources began with a high-trophic level species (Table 1). The last fur seal seen breeding in this area, on Nelson Island, South Shetlands, was in 1904. Not long after, in 1911, the hunt for baleen whales (Balaenoptera and Megaptera spp.) began among the South Shetlands, and by 1915 well over 6,000 whales had been taken, as well as thousands of tonnes of guano, presumably from penguin colonies (Tønnessen and Johnsen Reference Tønnessen and Johnsen1982: 190–200). By the late 1920s few whales could be found, but by then the industry had shifted to pelagic whaling in neighbouring waters and elsewhere in the Southern Ocean. In so doing, the whale catch was shifted to shelf and slope waters to the west, including the Ross Sea and the offshore Balleny Islands. By the early 1930s, blue whales B. musculus could no longer be found along the Ross Sea slope (Tønnessen and Johnsen Reference Tønnessen and Johnsen1982: 346–353; Ainley Reference Ainley2010). By the 1970s, few great whales remained in Southern Hemisphere waters, including those of the Antarctic continental shelf and slope (Hilborn and others Reference Hilborn, Branch, Ernst, Magnusson, Minte-Vera, Scheuerell and Valero2003: 363–365). Blue whales, for example, which concentrated along the Antarctic continental slope, were reduced to no more than 10% of their historical numbers by the 1950s (Kareiva and others Reference Kareiva, Yuan-Farrell, O'Conner, Estes, DeMaster, Doak, Williams and Brownell2006; IUCN 2011). A take of Antarctic minke whales B. bonaerensis followed in the late 1960s to early 1980s, mainly in Antarctic continental shelf and slope waters, but this ended when a moratorium was placed on commercial whaling by the International Whaling Commission (IWC) in 1982 (Ainley Reference Ainley2010). A ‘scientific catch’ of minke whales by Japan, 400–1,000 individuals per year, was instituted in 1987 and has continued in slope waters off East Antarctica to the present day (IWC areas III–VI, FAO areas 58 and 88; see Brown and Brownell Reference Brown and Brownell2001).
Table 1. Scientific name, common name, mean maximum length and trophic levels of Antarctic marine mammals, fish and invertebrates mentioned in this article

a From Trites and Pauly (Reference Trites and Pauly1998) for marine mammals, FishBase (2012) for fish and Rosenberg and others (Reference Rosenberg, Beddington and Basson1986) for krill.
b From Pauly and others (Reference Pauly, Christiansen, Dalsgaard, Froese and Torres1998) for marine mammals, Fishbase (2012) for fishes and SeaLifeBase (2012) for krill.
Fishery for groundfish
The early 1960s saw the emergence of fisheries for demersal species in the Southern Ocean, with the early years of this activity being well summarised by Kock (Reference Kock1992: 173–201). Fishing first around South Georgia (FAO subarea 48.3) and the Kerguelen Islands (58.5), exploitation began in waters of the South Orkney Islands (48.2) in 1977 and South Shetland Islands (48.1) in 1978 (a split season, such as 1978/79, is indicated here by the first year, in this case 1978). Target species in continental margin waters included Notothenia rossii, Champsocephalus gunnari, Lepidonotothen squamifrons and Gobionotothen gibberifrons (Table 1). Highest catches were made initially, with stocks becoming greatly depleted within 10 years (Fig. 2; Tables A1 and A2). These waters of the southern Scotia Arc and southward were closed to fin-fishing in 1990 by the CCAMLR, an international convention that came into force in 1982. At that time, stocks of almost all groundfish species were estimated to be <10% of pre-fished size (Kock Reference Kock1992). To this day, fin-fishing in subareas 48.1 and 48.2 is prohibited (CCAMLR 2011), and stocks have yet to recover (Marschoff and others Reference Marschoff, Barrera-Oro, Alescio and Ainley2012). When the fish stocks disappeared in these Antarctic continental waters, industry turned more intensively to Antarctic krill Euphausia superba (see below).

Fig. 2. Catch by taxon (A) and by fishing country (B) in the shelf and slope waters of area 48 (48.1, the northern Antarctic Peninsula; 48.5; and shelf/slope waters of 48.6).
Following closure of subareas 48.1 and 48.2 to fin-fishing in 1990, waters of the Antarctic continental shelf and slope saw no fishing for fin-fish until 1996. In that year, with the fishery for Patagonian toothfish Dissostichus eleginoides and other fin-fish fully exploited (or off limits) in insular waters of the northern Southern Ocean (northern portions of areas 48 and 58), a New Zealand vessel conducted exploratory fishing for Antarctic toothfish D. mawsoni in the continental shelf and slope waters of the Ross Sea (subareas 88.1 and 88.2; Fig. 1). Until then, the long distance from home ports and persistent pack ice of these waters had discouraged fishing (Ainley and others Reference Ainley, Nur, Eastman, Ballard, Parkinson, Evans and DeVries2012). Less than one tonne was caught that first year, but by 1999 the fishers had figured out the technique, finding the most productive areas (slope, Antarctic ridge seamounts) and depths (800–1,400m: slope and seamounts to the north). In 2000, after several years of what CCAMLR terms ‘exploratory fishing’, vessels of other CCAMLR members entered the fishery, though its ‘exploratory fishing’ status remained (owing to insufficient data to conduct a proper assessment). Permits were issued as long as vessels agreed to take observers and abide by CCAMLR rules (such as no fishing in waters <550m deep to protect benthic communities, or location to be changed if the by-catch becomes too high; CCAMLR 2011). By 2003, vessels flagged in 12 countries had fished in area 88 and in that year the quota, based on stock models with the initial stock level unknown, was reached for the first time: the catch was around 3,000–3,500t (Fig. 3A). The catch has remained at about that level since (Table A3), but the number of countries and vessels fishing has decreased (Table A4). Presumably, the distances to port, conditions and competition encountered have proved too daunting for some. In the last three years about 15 vessels from only five or six countries have fished (for example, four vessels from New Zealand and up to six from the Republic of South Korea), in an Olympic-style scenario in which permitted vessels vie for the same total allowable catch (TAC) (Fig. 3B). Since inception of the fishery, and attracted by the high price that ‘Chilean sea bass’ commands, several vessels have foundered, caught fire or been holed by collision with ice thus requiring rescue. The Ross Sea (especially subarea 88.1; see Fig. 1) is regularly patrolled by aircraft from New Zealand and this activity is thought to discourage almost all IUU fishing in this area.

Fig. 3. Catch by taxon (A) and by fishing country (B) in the shelf and slope waters of area 88 (88.1, 88.2 (Ross Sea) and 88.3 (southern Antarctic Peninsula), as well as catches in 88 not allocated to one of these subareas).
In 1998, a cruise was undertaken by Chilean vessels to determine the level of toothfish catch that could be achieved in subarea 48.1, as well as at the base of the Antarctic Peninsula (88.3). Yields were very low, <20g/hook and were equalled or surpassed by by-catch fishes (Arana and Vega Reference Arana and Vega1999). This subarea, characterised by year-round sea-ice cover, subsequently has seen very limited fishery.
The by-catch in the toothfish fishery of non-target, unused species, principally macrourid fish Macrourus spp. (Macrouridae) and fish of the genus Muraenolepis (Muraenolepidae), and to a lesser extent skates Raja spp., has been significant. In the legal fishery, it has ranged to almost 170t, which in some years has been close to 10% of the toothfish catch in the respective subarea (Fig. 4). The ratio of the catches of macrourids to toothfish caught has decreased following the onset of fishing; in other words, the catch is initially high then gradually declines in subsequent years (Fig. 4).

Fig. 4. The ratio of the by-catch of grenadiers (family Macrouridae) to the catch of toothfish (family Nototheniidae) in areas 58 and 88 (1977–2010). The by-catch is actually much higher in area 58 owing to the large IUU effort in the 58.4.1 and 58.4.2 divisions, for which the level of by-catch is not known.
The waters off East Antarctica (divisions 58.4.1, 58.4.2), the parts of the Antarctic continent facing the Indian Ocean, began to be fished for krill and some bottom fish as early as 1974. Fishing was short-lived and initially discontinued after the early 1990s. However, the success of fishing in area 88 inspired fishing companies to explore the continental margin elsewhere for Antarctic toothfish. Mainly this led to re-opening of the continental margin of subarea 58 to fishing less than 10 years ago (58.4.1, 58.4.2). This ‘exploratory’ fishery for Antarctic toothfish is conducted by many of the same countries fishing area 88 (Table A5). The TAC has been lower here than elsewhere (Fig. 5), but the IUU take has been huge, often surpassing the legal take. By-catch is sparsely reported (Table A6), the IUU by-catch not at all, and, therefore, the pattern relative to tonnes of toothfish caught in area 88 is difficult to ascertain (Fig. 4).
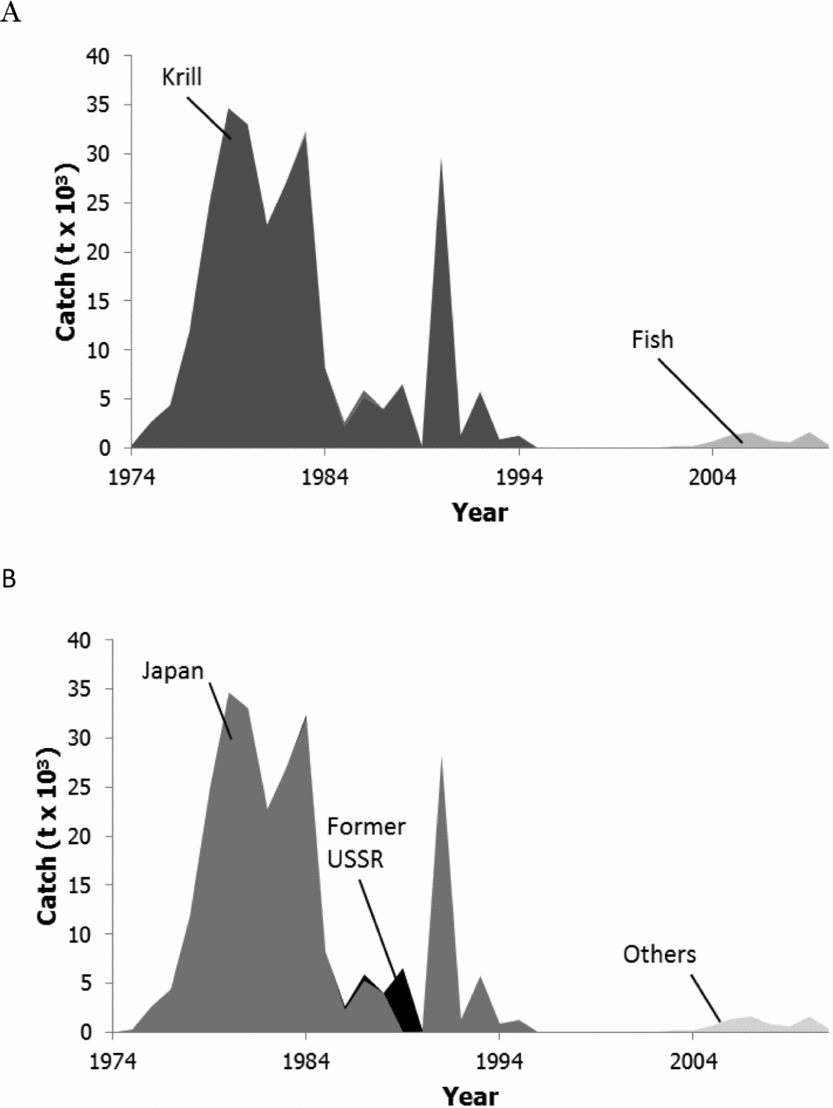
Fig. 5. Catches by taxon (A) and by fishing country (B) in the shelf and slope portions of area 58 (divisions 58.4.1 and 58.4.2).
Fishery for forage fish
On occasion, attempts have been made to determine the feasibility of taking some of the mid-water fish in continental waters. Principally, this has involved species of Myctophidae (lanternfish, perhaps mostly Electrona spp.) along the slope and the nototheniid Pleuragramma antarcticum over the shelf. In both cases these species could be characterised as loosely shoaling, a level of dispersal that is problematic for fuel-efficient trawling (Gjøsaeter and Kawaguchi Reference Gjøsaeter and Kawaguchi1980). Fished during less than 10 seasons in total and not in every area, peak catches for Pleuragramma were reported as 653t and 1,517t in subareas 58.4.2 and 88 (subarea not specified), and for myctophids 129t in subarea 88, in all cases by USSR vessels (Table A3).
Fishery for Antarctic krill
Antarctic krill (Table 1) is the small, shrimp-like crustacean supporting, directly or indirectly, most larger animals in the Antarctic LME, or at least those of the outer shelf and slope (Pikitch and others Reference Pikitch, Boersma, Boyd, Conover, Cury, Essington, Heppell, Houde, Mangel, Pauly, Plagányi, Sainsbury and Steneck2012). Fisheries, since the 1970s, have exploited krill for making fish meal and related products. Japan reported taking 283t in the Indian Ocean sector (58.4.2) in 1974, Chile reported taking 276t and 92t in waters of the South Shetland Islands (48.1) in 1976 and 1977, and the USSR reported taking 3,355t from area 88 (subarea unspecified) in 1977. Subsequently, the fishery for krill in area 88 was largely confined to the western portion, including waters off Wilkes Land and the Balleny Islands (Dolzhenkov and others Reference Dolzhenkov, Zhigalov, Kovalev, Timonin and Spiridonov1990). The fisheries in areas 58 and 88 were discontinued by the 1990s but never reached more than 6,000t or 35,000t, respectively (Fig. 6A). On the other hand, the krill fishery in Antarctic Peninsula waters has been much greater, ranging up to 150,000t, taken by vessels flagged in 14 different countries (Fig. 6B). During the 1980s and early 1990s, the catch in subarea 48.1 was consistently 20–25% of the total Southern Ocean catch. The total has been halved in recent years. However, the subarea 48.1 catch contributed 72% of the total in 2009 (26% in 2008, 5% in 2010). In recent years, vessels from South Korea, Japan (less so than initially) and Norway have replaced those from Eastern European countries as those taking the largest proportion of the annual catch in continental margin waters (Fig. 6B).

Fig. 6. Catches by taxon (A) and by fishing country (B) on the shelf and slope of the Antarctic continent (= portion of the Antarctica large marine ecosystem (LME) corresponding to the grey area of Figure 1).
Krill trawlers seek to fish on swarms that are as pure as possible, to avoid contamination by other organisms, especially gelatinous ones (Kelleher Reference Kelleher2005). At times, however, the by-catch can be significant, but it is not routinely reported. Observers take samples for analysis, but not every trawl is observed. The low level of reporting is related to difficulties in separating out by-catch owing to the method of catch and initial processing of krill. In most operations, krill are smashed together in fine-mesh trawls and the catch then has to be processed (oil removed, meat freeze- or air-dried) within a few hours to avoid the rapid enzymatic breakdown releasing toxic fluorides in the stored product (FAO 2005). Specific studies of by-catch have shown that lanternfish (Myctophidae) and the larvae of other fin-fish can be caught in significant amounts (Pakhomov and Pankratov Reference Pakhomov and Pankratov1994; Watters Reference Watters1996) to the point that it is a concern for CCAMLR (CCAMLR 2006). Management thus far seeks to avoid secondary effects of an elevated by-catch of fin-fish juveniles, and competition with land-based predators, by spreading the krill fishing as broadly as possible among spatial management units (CCAMLR 2011). The degree to which the catch of fin-fish larvae and juveniles affects the stocks of by-catch species is open to question (Marschoff and others Reference Marschoff, Barrera-Oro, Alescio and Ainley2012). Some researchers posit that krill exploitation by fishing where predators concentrate is limiting the recovery of some top trophic-level species, such as cetaceans (Leaper and Miller Reference Leaper and Miller2011).
Discussion
To date, owing to severe conditions and destinations a long way from well-equipped ports, there has been no reason for humans to be active along the Antarctic continental margin except for scientific research or biotic exploitation. Coordination of research was instituted in 1959 by the signing of the Antarctic Treaty (Fogg Reference Fogg1992). Subsequently, treaty nations established a number of logistics hubs along the coast of FAO areas 48, 58 and 88 (Tin and others Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009). Treaty parties excluded future minerals development by an agreement that came into force in 1998. On the other hand, biotic exploitation along the continental margin (and elsewhere in the Southern Ocean), which had been underway for almost 200 years, was allowed to continue. Populations of certain seals and whales were decimated. To prevent a repetition of the fur (and elephant) seal decimation, a treaty banning the take of fur seals and controlling commercial take of other species (pack ice ‘true’ seals) entered into force in 1978 (Convention for the Conservation of Antarctic Seals). A few very small areas (two in subarea 88.1) were closed entirely to sealing to protect scientifically important populations. The International Whaling Commission, recognising that the stocks of large whales had been decimated, banned commercial whaling of all cetacean species in 1986. Fin-fishing then increased but quickly decimated the targeted demersal fishes. One of the first conservation measures adopted by CCAMLR, which the members brought into force in 1982, was to close subareas 48.1 and 48.2 to fin-fishing (by 1990). Fishing for Antarctic krill became regulated during the 1980s, and the management of this has been continually refined since then (CCAMLR 2011). To this day, krill fishing occurs in areas closed to fin-fishing as well as other areas.
Fur seals have recovered in the South Orkneys and South Shetlands (Boyd Reference Boyd2002), but baleen whales have not (IUCN 2011), although one species, humpback whale M. novaeangliae, is beginning to do so (Branch Reference Branch2011). Some fin-fish species in area 48.1 are showing signs of recovery (for example, N. rossii), while others are not (Marschoff and others Reference Marschoff, Barrera-Oro, Alescio and Ainley2012). Fishing for Antarctic krill continues in this region, with the continental margin fishery being important, its contribution (5–72% in recent years) depending on conditions and conservation measures in force (CCAMLR 2011; see Figures 2, 3, 5 and 6).
The history of fishing in Antarctic continental waters is a good example of ‘fishing down the food web’. Figure 7 summarises ‘fishing down’ as it occurred in the vicinity of the Antarctic Peninsula (subarea 48.1) and, by extension, around the Antarctica margin as a whole. Clearly, what occurred was not ‘fishing through’, as described by Essington and others (Reference Essington, Beaudreau and Wiedenmann2006): the mean trophic level of the fishery (or previously the hunt for marine mammals) declined not (only) because new, low trophic-level species came under exploitation, but because the high trophic-level animals that were targeted had been decimated and their exploitation therefore had to cease for economic reasons. Thus, the MTI, in this case, does its job as an indicator of high-trophic level biodiversity (Pauly and Watson Reference Pauly and Watson2005) and points to a vastly altered ecosystem (see also Ballance and others Reference Ballance, Pitman, Hewitt, Siniff, Trivelpiece, Clapham, Brownell, Estes, Demaster, Doak, Williams and Brownell2006; Ainley and Blight Reference Ainley and Blight2009; Trivelpiece and others Reference Trivelpiece, Hinke, Miller, Reiss, Trivelpiece and Watters2011), a victim of the ‘tragedy of the commons’ (Hardin Reference Hardin1968). Ironically, the MTI in recent decades may be increasing, not so much owing to the recovery of fur seals and baleen whales (which is still in its infancy), but because krill abundance is decreasing due to climate change (Atkinson and others Reference Atkinson, Siegel, Pakhomov and Rothery2004), perhaps exacerbated by decimation of the top trophic levels (Nicol and others Reference Nicol, Bowie, Jarman, Lannuzel, Meiners and van der Merwe2010).

Fig. 7. The extraction of biotic resources from the Antarctic Peninsula (subarea 48.1) best illustrates the ‘fishing down’ process that occurred in Antarctic waters as a whole, starting with Antarctic fur seals, then on to baleen whales, fishes and, finally, krill (based on trophic levels in Table 1 and catches in Table A1). The open dots for 1976 and 1977 refer to miniscule catches (276t and 92t, respectively) taken by Chile, which were probably exploratory trawls.
In the southern portions of areas 58 and 88, fur seals were never present but large baleen whales, once plentiful, were severely reduced. The take of smaller minke whales was greatly curtailed before that stock was depleted, and since appears to have recovered; stocks of humpback whales are beginning to recover in spectacular fashion in slope waters of western area 88 (Balleny Islands) and eastern area 58, but stocks of blue whales remain greatly depressed (Ainley Reference Ainley2010; Branch Reference Branch2011). Nowadays, fishing in these areas, which once included krill, is confined to bottom fishes (many demersal fishes in the northern parts of area 58 were depleted by the late 1970s; Kock Reference Kock1992: 191–192). The target species in waters of the Antarctic margin is Antarctic toothfish, but it appears that grenadiers (Macrouridae), caught as by-catch, are significantly taken as well. Ironically, these macrourids are an important prey for the target species in the main fishing grounds along the continental slope (Fenaughty and others Reference Fenaughty, Stevens and Hanchet2003). Impacts to the toothfish have not been studied, but the principles of ecosystem-based fishery management are probably being challenged.
The total catch of Antarctic toothfish in areas 58 and 88 accounts for almost all the catch for this species in the Southern Ocean or 26% (in 2010) of the total toothfish catch (~15,000t). The remainder of the total is contributed by D. eleginoides taken from northern areas of the Southern Ocean. Although krill were taken in relatively small amounts from areas 58 and 88 early, in recent years the fishery continues only in the SW Atlantic sector (area 48), probably owing to the greater densities of krill there as well as easier access by fishing vessels (as it is closer to ports and population centres).
In summary, then, exploitation of biotic resources along the continental margin of Antarctica has played a role in humans’ progressive exploitation of global marine resources: ever farther away, deeper and more species (see, for example, Pauly and others Reference Pauly, Watson and Alder2005; Swartz and others Reference Swartz, Sala, Tracey, Watson and Pauly2010), and, with successive depletions, has not been something of which human society can be proud. During the last few decades, efforts have been made to try to recover this reputation in these Antarctic continental waters, though whether continued severe reduction in toothfish stocks can be offered as an example of enlightened management, that is, fishing to 50% of grossly estimated pre-fished spawning biomass (Constable and others Reference Constable, de la Mare, Agnew, Everson and Miller2000), is debatable (Ainley and Brooks Reference Ainley, Brooks, Liggett and Hemmings2013). To what degree the affected food webs will respond or can recover from this form of exploitation remains to be seen, despite the fact that the CCAMLR articles require that exploited populations are not so badly affected that they cannot recover in 20–30 years. With rapid climate change now one of fishery management's challenges (Cheung and others Reference Cheung, Lam, Sarmiento, Kearney, Watson, Zeller and Pauly2010, Reference Cheung, Sarmiento, Dunne, Frölicher, Lam, Palomares, Watson and Pauly2012), the latter requirement may have become especially problematic.
Acknowledgements
This summary was prepared under NSF grant ANT-0944411; views expressed here do not necessarily represent those of NSF. The authors acknowledge support from The Sea Around Us, a research collaboration between the University of British Columbia and the Pew Environment Group. They thank A. Ulman for her assistance in preparing figures and tables, and P. Penhale, P. Trathan, T. Branch and an anonymous reviewer for very helpful comments that improved the article greatly.
Appendices
Table A1. Catches (t) by taxon in the parts of FAO area 48 covered in this article (that is, subareas 48.1, 48.5 and the southern portion of 48.6; note that until 1990, all of these catches were from 48.1). The ‘Other icefish’ category includes blackfin icefish, ocellated icefish, spiny icefish, South Georgia icefish and other crocodile icefish categories

Table A2. Total catches (t) by country in the parts of FAO area 48 covered in this article (that is, subareas 48.1, 48.5 and 48.6, catches from the shelf area only)

Table A3. Catches (t) by taxon in the parts of FAO area 88 covered in this article (that is, catches in subareas 88.1–88.3 and catches in area 88 not allocated to any subarea)

Table A4. Total catches (t) by country in the parts of FAO area 88 covered in this article (that is, catches in subareas 88.1–88.3 and catches in which the subarea was not recorded)

Table A5. Catches (t) by taxon in the parts of FAO area 58 covered in this article (that is, subareas/divisions 58.4.1 and 58.4.2)

Table A6. Catches (t) by country in the parts of FAO area 58 covered in this article (that is, subareas/divisions 58.4.1 and 58.4.2)

















