Wheat suffers from a number of pathogens, and one of the most important of its foliar fungal diseases is leaf rust. More than 50 leaf rust resistance (Lr) genes have been documented (McIntosh et al., Reference McIntosh, Yamazaki, Devos, Dubcovsky, Rogers and Appels2003), but most have been overcome by the pathogen. Wild relatives and related species represent a valuable source of genes for crop improvement, and more than 20 Lr genes have been introduced into bread or durum wheat from a range of species (Schachermayr et al., Reference Schachermayr, Messmer, Feuillet, Winzeler, Winzeler and Keller1995; Friebe et al., Reference Friebe, Badaeva, Hammer and Gill1996; Cenci et al., Reference Cenci, D'Ovidio, Tanzarella, Ceoloni and Porceddu1999; Xie et al., Reference Xie, Sun, Ni, Yang, Nevo and Fahima2003; Leonova et al., Reference Leonova, Börner, Budashkina, Kalinina, Unger, Röder and Salina2004). The diploid Aegilops markgrafii (syn. Ae. caudata; genome CC) is a valuable source of genes encoding resistance to powdery mildew, leaf rust and stripe rust (Schubert et al., 1995), and is therefore a good candidate to be a donor of exotic alleles and genes of utility for wheat improvement.
Microsatellite (simple sequence repeat, SSR) loci are associated with significant levels of sequence polymorphism. A growing number of SSR loci have been incorporated into the wheat genetic map (Röder et al., Reference Röder, Korzun, Wendehake, Plaschke, Tixier, Leroy and Ganal1998; Somers et al., Reference Somers, Isaac and Edwards2004). The specificity of these markers can help to determine which chromosome(s) are involved in alien introgression materials (Peil et al., Reference Peil, Korzun, Schubert, Schumann, Weber and Röder1998; Iqbal et al., Reference Iqbal, Miller, Reader and Calligari2000). We therefore used SSRs to characterize a set of wheat/Ae. markgrafii introgression lines.
Experimental
Five leaf rust-resistant sister introgression lines, which all shared a wheat-like growth habit, were selected from a cross between the leaf rust-susceptible wheat cultivar ‘Alcedo’ and the resistant Ae. markgrafii accession ‘S740-69’ (Schubert, Reference Schubert2001; Weidner, Reference Weidner2004). One of these (N43) was crossed to the susceptible wheat cultivar ‘Borenos’, and the resulting segregating 140 member F2 population was used for genetic mapping. A pathogenicity test was performed on seedlings at the two-leaf stage in a growth chamber. The leaf rust inoculum carried virulences against Lr1, Lr2a, Lr2b, Lr2c, Lr3, Lr3bg, Lr3ka, Lr10, Lr11, Lr13, Lr14a, Lr14b, Lr15, Lr16, Lr17, Lr18, Lr20, Lr21, Lr23, Lr26, Lr28, Lr30, Lr32, Lr33, Lr37, Lr38 and Lr44. After a 24 h dark incubation at 14–15°C and 100% humidity, the temperature was reset to 20°C, and permanent light and normal humidity was maintained. After 10 days, the plants were scored for the intensity of leaf rust infection, using a measurement scale of 0 (leaves without any visible symptom) to 4 (clearly visible red-brown pustules) (McIntosh et al., Reference McIntosh, Wellings and Park1995). For SSR analysis, DNA was isolated from individual seedling leaves and PCR reaction conditions were as described by Röder et al. (Reference Röder, Korzun, Wendehake, Plaschke, Tixier, Leroy and Ganal1998). The profiles were generated by an Automated Laser Fluorescence (ALF) express semi-automatic DNA sequencing device.
A set of 226 SSR loci of known intrachromosomal location (Röder et al., Reference Röder, Korzun, Wendehake, Plaschke, Tixier, Leroy and Ganal1998; unpublished data) were tested. Of these, four (Xgwm614, Xgwm636, Xgwm497 and Xgwm1176), all mapping to chromosome arm 2AS, were absent from the introgression lines, as were Xgwm148, Xgwm374 and Xgwm972 (2BS), Xgwm160 (4AL) and Xgwm732 and Xgwm1103 (6DL). The GWM830 (2AL), GWM1053 (2AL) and GWM1005 (3BL) primer pairs also amplified Ae. markgrafii alleles, replacing the wheat alleles at these loci. These results are graphically illustrated in Fig. 1.

Fig. 1 The characterization of wheat/Aegilops markgrafii introgression lines. Alien segments indicated as dark zones, with corresponding and flanking markers shown. Underlined markers (on chromosome 2A) were those employed for linkage analysis. Genetic distances in cM. c, centromere.
Only 19 of the 140 F2 plants were leaf rust-susceptible, a highly significant deviation from the ratio expected for a monogenic trait. The segregation of SSR loci located on chromosome arm 2A was also highly distorted, although in contrast, the segregation of the other SSR loci detecting the presence of an alien introgression was consistent with the expected 3:1 ratio (Table 1). Simple linkage calculation between leaf rust resistance and SSR loci was hindered by the segregation distortion, and so a quantitative trait locus (QTL) approach was attempted. This resulted in the identification of a significant QTL (LOD score 5.14) on chromosome arm 2AS (Table 2Fig. 1), designated QLr.ipk-2A.
Table 1 Segregation patterns for leaf rust resistance and SSR loci in an F2 population derived from the cross introgression line×normal bread wheat
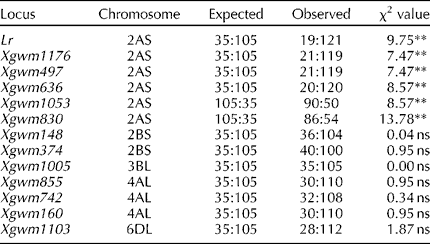
** P < 0.01. ns, not significant.
Table 2 QTL mapping of leaf rust resistance with respect to SSR loci

* P < 0.05.
Discussion
The homoeologous relationships between the A, B, D genomes of wheat and the C genome of Ae. markgrafii have been described by Peil et al. (Reference Peil, Korzun, Schubert, Schumann, Weber and Röder1998) and Schubert (Reference Schubert2001). Although the authors have shown that there are rearrangements between the wheat and Aegilops genomes, recombination may occur. Chromosome B of Ae. markgrafii carries gene(s) for resistance to Puccinia recondita and this chromosome shares homoeology with those in groups 2 and 4 of wheat (Peil et al., Reference Peil, Korzun, Schubert, Schumann, Weber and Röder1998; Schubert, Reference Schubert2001). The SSR analysis of the introgression lines was consistent with the presence of Ae. markgrafii segments on homoeologous group 2 and 4 chromosomes, as well as detecting additional transfers involving the long arms of chromosomes 3B and 6D.
Distorted segregation ratios are commonplace in mapping populations. Gametocidal effects of chromosomes derived from Aegilops species have been described earlier (Endo, Reference Endo1983, Reference Endo, Miller and Koebner1988, Reference Endo1996). The gametocidal action of Ae. markgrafii chromosomes was reported by Endo and Katayama (Reference Endo and Katayama1978). Therefore, it is possible that the segregation distortion observed in the present mapping population is caused by the activity of such a gametocidal gene. An alternative explanation for the distortion may be a poor level of compensation between the introduced segment and the substituted wheat segment.
Of the more than 65 Lr loci (both major genes and QTLs) described to date (McIntosh et al., Reference McIntosh, Yamazaki, Devos, Dubcovsky, Rogers and Appels2003), about 15 originate from Aegilops species. However, this is the first report of a resistance locus from Ae. markgrafii. The potential of this species as a donor for disease resistance (including leaf rust) was already well understood by the 1980s (Frauenstein and Hammer, Reference Frauenstein and Hammer1985; Valkoun et al., Reference Valkoun, Hammer, Kucerova and Bartoš1985) and so the species should be considered to be a strong candidate as a source of exotic genes to improve the disease resistance of advanced wheat germplasm.
Acknowledgements
Nayyer Iqbal thanks the Alexander von Humboldt Foundation (grant no. PAK/1117127 STP) for financial support.





