Introduction
Pumpkins (Cucurbita spp.) are cultivated throughout the world for their food, seed oil and medicinal value. The term ‘pumpkin’ is commonly applied to various species within the genus Cucurbita (Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008). These include C. pepo L., C. moschata Duchesne, C. maxima Duchesne, C. argyrosperma C. Huber and C. ficifolia Bouche, although it has been applied to other species of Cucurbita and to members of other genera (i.e. Caili et al., Reference Caili, Huan and Quanhong2006; Stevenson et al., Reference Stevenson, Eller, Wang, Jane, Wang and Inglett2007). The seed oil of these Cucurbita spp. is of local and regional importance. Pumpkin oil is highly digestible (Jacks et al., Reference Jacks, Henserling and Yatsu1972) and grown on a semi-industrial scale in Yugoslavia (Earle et al., Reference Earle, Glass, Geisinger, Wolff and Jones1960; Markovic and Bastic, Reference Markovic and Bastic1976), Austria, Hungary, the former USSR and India (Schormuller, Reference Schormuller1969; Lal et al., Reference Lal, Datta and Madaan1983). Pumpkin seed oil is credited with having anti-hypertension, anti-tumour, immune modulation, antibacterial, anti-hypercholesterolaemia, anti-inflammatory and analgesic properties (Caili et al., Reference Caili, Huan and Quanhong2006).
C. moschata is a tropical herbaceous annual (Saade and Hernandez, Reference Saade, Hernandez, Bermejo and Leon1995) with fruits that vary considerably in size and shape. Current data suggest that C. moschata originated in the lowlands of northern South America from an ancestral species closely related to C. argyrosperma (Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008). The history of this species in cultivation can be traced to 3000 BC in coastal Peru and 2000 BC in Guatemala (Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008). C. moschata is the most frequently consumed vegetable in Korea (Kim et al., Reference Kim, Kim, Kim, Choi and Lee2012). Seeds of C. moschata contain high levels of various minerals and oil (Madaan and Lal, Reference Madaan and Lal1984; Fokou et al., Reference Fokou, Achu and Tchouanguep2004, Reference Fokou, Achu, Kansci, Ponka, Fotso, Tchiegang and Tchouanguep2009) in addition to all nine essential amino acids (Kim et al., Reference Kim, Kim, Kim, Choi and Lee2012). Seeds of C. moschata cv. Butternut have been reported to have an oil content of approximately 33.5% (Bemis et al., Reference Bemis, Berry, Kennedy, Woods, Moran and Deutschman1967). Linoleic acid is typically the predominant fatty acid (Fokou et al., Reference Fokou, Achu, Kansci, Ponka, Fotso, Tchiegang and Tchouanguep2009). Numerous commercial varieties have been developed (Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008).
C. argyrosperma is a complex of domesticated, feral and wild forms closely related to C. moschata (Merrick, Reference Merrick, Bates, Robinson and Jeffrey1990). C. argyrosperma and C. moschata were originally considered a single species. However, two subspecies and four varieties of C. argyrosperma are now recognized (Merrick, Reference Merrick, Bates, Robinson and Jeffrey1990). Each of the subspecies and varieties has a distinctive seed morphology (Merrick, Reference Merrick, Bates, Robinson and Jeffrey1990). Seeds of both subspecies are consumed (Merrick, Reference Merrick, Bates, Robinson and Jeffrey1990). Oil content in the seed of three varieties of C. argyrosperma ranged from 30.6 to 43.0% (Applequist et al, Reference Applequist, Avula, Schaneberg, Wang and Khan2006). The principal fatty acid is typically linoleic (Stevenson, 2007). Despite its long history of cultivation, relatively few varieties of C. argyrosperma have been developed (Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008).
Although there is a substantial amount of data available on pumpkin seed oil, there are considerably less data available on seed oil content within and among individual species of Cucurbita. This study was undertaken in order to examine inter- and intraspecific variability for seed oil content and fatty acid composition within a genebanks’ collections of Cucurbita moschata and C. argyrosperma.
Materials and methods
Plant material
All seeds used in this study were obtained from the USDA/ARS Plant Germplasm Collection in Griffin, GA (Jarret et al., Reference Jarret, Spinks, Lovell and Gillaspie1990). Individual taxa were identified by R.L.J. and L.C.M. based on field observations, examination of seed or digital images of seed, and/or herbarium records. Prior to analysis, all seeds (stored at − 20°C in foil pouches) were brought to room temperature for a minimum of 24 h. All analyses were conducted on intact seed, unless noted otherwise. Materials analysed in this study included: C. moschata Duchesne; C. argyrosperma C. Huber subsp. argyrosperma var. argyrosperma; C. argyrosperma subsp. argyrosperma var. callicarpa L. Merrick & D. M. Bates; C. argyrosperma subsp. argyrosperma var. palmeri (L. H. Bailey) L. Merrick & D. M. Bates; C. argyrosperma subsp. argyrosperma var. stenosperma (Pangalo) L. Merrick & D. M. Bates; C. argyrosperma subsp. sororia (L. H. Bailey) L. Merrick & D. M. Bates.
Preparation of oil standards
Oil standards were prepared from C. moschata cv. Waltham Butternut [Stokes Seed Company (Lot No. 200854), Buffalo, New York, USA] and C. argyrosperma cv. green-striped cushaw (Eden Brothers, Dahlonega, GA, USA) essentially as described by Jarret et al. (Reference Jarret, Wang and Levy2011). For each of these, 200 g of dried seed were ground to a fine powder in a coffee mill (Black & Decker Model CBM205 – medium setting) and the powder transferred to a 1-litre round bottom flask. To the flask was added sufficient heptane (Acros Organics) to bring the volume of the mixture to ca. 500 ml. The flask was sealed, transferred to a rotary shaker (Thermolyne AROS 160) and the contents mixed for 24 h. The mixture was then allowed to settle for several hours and then twice vacuum filtered through Fisher Scientific P5 (Atlanta, GA, USA) filter paper. The filtrate was concentrated by rotary evaporation, yielding a yellow oil. Yields were typically 18–20% oil by seed weight.
TD-NMR analysis
Seed oil and moisture measurements were carried out by Time Domain Nuclear Magnetic Resonance (TD-NMR) essentially as described by Krygsman and Barrett (Reference Krygsman, Barrett and Luthria2004) and Jarret et al. (Reference Jarret, Wang and Levy2011) on a Bruker (Madison, WI, USA) mq10 Minispec NMR operating at a resonance frequency of 9.95 MHz and maintained at 40°C. For each signal acquisition, spin-echo parameters consisted of a 90° pulse of 10.44 μs and reading at 50 μs followed by a 180° pulse of 21.38 μs (pulse spacing = variable) and reading at 7 ms. A 2 s recycle delay between scans was used, and a total of 16 scans were collected for each sample. Bulk seed measurements were made in a 40 mm glass sample tube, and NMR signals were compared with oil and moisture calibration curves, generated by sample weight. All samples were measured in triplicate and the results were averaged. Moisture standards were prepared using seeds of known moisture content and calculating the mass of water present in different seed lots. Moisture content was predetermined by measuring the differences in masses of seeds before and after baking at 130°C for 3 h. All NMR oil analyses were conducted using separate seed samples drawn from the available inventory. Seeds were drawn from the 01 (first regeneration) inventory of each accession, unless noted otherwise. Seed oil in the samples of C. moschata was determined utilizing a standard curve prepared from oil extracted from cv. Waltham Butternut. Seed oil in the samples of C. argyrosperma was determined utilizing a standard curve prepared from oil extracted from cv. green-striped cushaw.
Isolation and analysis of fatty acids
For isolation of fatty acids, replicates of 50 seed samples were ground to a fine powder in a coffee bean mill. Approximately 50 mg of ground powder were transferred into a 16 × 100 mm test tube, and 5.0 ml of n-heptane (Fisher Scientific) were added to extract the oil. For conversion of fatty acids to methyl esters (FAME), 500 μl of 0.5 M sodium methoxide (NaOCH3) in methanol (Fisher) were added to the test tube and mixed with the sample. The reaction was allowed to proceed for 2 h. Then, 7 ml of distilled water were added to separate the organic layer from the aqueous layer and residue (45 min). An aliquot of the organic layer (1.5 ml) containing the methyl esters was transferred to a 2.0 ml autosampler vial for GC analysis.
FAME extracts were diluted 100-fold in hexane containing 25 μg/ml of methyl nonadecanoate (C19:0) and analysed with a ThermoQuest Finnigan DSQII GC-MS system (ThermoFisher, San Jose, CA, USA). C19:0 was used as an internal standard. The mass spectrometer was operated in the electron impact mode and scanned at m/z= 50–400 during data acquisitions. Chromatographic separations were performed on a 30 m DB5® column (0.25 mm inner diameter, 0.25 μm film thickness; Agilent, San Jose, CA, USA). Helium carrier gas flow was held constant at 1.5 ml/min and injection port temperature was 220°C. Injection was in the splitless mode. An oven initial temperature of 60°C was held for 1 min after injection and increased to 250°C at 8°C/min and held for 5 min. Peak assignments and quantification were based on the analysis of serial dilutions of a commercially available FAME mixture. The FAME mixture (GLC-10) and internal standard were purchased from Matreya LLC (Pleasant Gap, PA, USA). Data were collected on C16:0 (palmitic), C18:0 (stearic), C18:1 (oleic) and C18:2 (linoleic) acids, which in total accounted for more that 97% of the FAME present.
Determination of physical properties
Various physical properties of the oils used as standards (C. moschata cv. Waltham Butternut and C. argyrosperma cv. green-striped cushaw) were evaluated.
Pour point
Pour points were measured in accordance with Method D 97-96a (American Society for Testing Materials, 1996) to an accuracy of ± 3°C. The pour points were determined by placing a test jar with 50 ml of the sample into a cylinder submerged in a cooling medium. The sample temperature was reduced in 3°C increments at the top of the sample until the material stopped pouring. The sample no longer poured when the material in the test jar did not flow when held in a horizontal position for 5 s. The temperature of the cooling medium was chosen based on the expected pour point of the material. Samples with pour points that ranged from +9 to − 6, − 6 to − 24 and − 24 to − 42°C were placed in baths of temperatures − 18, − 33 and − 51°C, respectively. The pour point was defined as the coldest temperature at which the sample still poured. All pour points were determined in duplicate and the average values are reported.
Cloud point
Cloud points were determined in accordance with Method D-2500-99 (American Society for Testing Materials, 1999) to an accuracy of ± 1°C. The cloud points were determined by placing a test jar with 50 ml of the sample into a cylinder submerged into a cooling medium. The sample temperature was reduced in 1°C increments until any cloudiness was observed at the bottom of the test jar. The temperature of the cooling medium was chosen based on the expected cloud point of the material. Samples with cloud points that ranged from room temperature to 10, 9 to − 6, and − 6 to − 24, − 24 to − 42°C were placed in baths of temperatures 0, − 18, − 33 and − 51°C, respectively. All cloud points were determined in duplicate and the average values are reported.
Viscosity and viscosity index
Viscosity measurements were made using calibrated Cannon–Fenske viscometer tubes purchased from Cannon Instrument Co. (State College, PA, USA). Viscosity measurements were made in a Temp-Trol (Precision Scientific, Chicago, IL, USA) viscometer bath set at 40.0 and 100.0°C. Viscosity and viscosity index (VI) were calculated using American Society for Testing Materials Methods D 445-97 (1997) and D 2270-93 (1998), respectively. All viscosity measurements were run in duplicate and the average values are reported.
Oxidative stability
Pressurized-differential scanning calorimetry (P-DSC) analyses were conducted with a TA Instruments (New Castle, DE, USA) model DSC 2910 fitted with an HP 2910 model high-pressure DSC cell (maximum 7 MPa). A model 5000 personal computer-based controller was used for data acquisition and determination of oxidation onset temperature (OT). Purge gas outside the cell was low-pressure nitrogen. All scans were conducted with the cell pressurized with oxygen to 3000 ± 50 kPa (440 ± 7 psig). A spring-action purge valve was fitted to the exhaust line to keep the cell at constant pressure during heating. P-DSC analyses were conducted using hermetically sealed aluminium pans with an ~0.5-mm-diameter pinhole punched in the top cover to allow direct contact between the sample and pressurized oxygen. Samples were analysed simultaneously with an identical empty pan. OT data reported in this work are means determined from replicate scans on three fresh samples.
In dynamic (positive gas flow) mode, the cell was pressurized and then sealed off. After the cell was equilibrated at 25°C, the inlet valve was opened wide and the outlet valve cracked slightly open to allow a steady flow of oxygen through the cell. Oxygen flow rate was set manually to 100 ± 10 ml/min and monitored by a calibrated gas flow meter connected downstream from the cell outlet valve. Once oxygen flow was established and pressure restabilized, the cell was equilibrated at 30°C and then heated with a ramp rate of 10°/min to a terminal temperature of 300°C. Sample mass for dynamic mode scans was 1.50 mg.
Acid value (AV) and free fatty acids (FFA)
A 751 GPD Titrino from Metrohm Ltd (Herisau, Switzerland) was used for measurements. AVs and percentage of FFA were determined by the official AOCS Method Te 2a-64 with ethanol substituted for methanol to increase the solubility of the estolide ester during the titration (Firestone, Reference Firestone1994). All AVs were run in duplicate and the average values are reported.
Gardner colour
Gardner colour was measured on a Lovibond 3-Field Comparator from Tintometer Ltd (Salisbury, England) using AOCS Method Td 1a-64. The Gardner colour scale is from 1 to 18 with 1 containing the least amount of colour and 18 with the maximum amount of colour. In many cases, the Gardner colour of materials can be susceptible to the interpretation of the recorder, thus the + and − notation was employed to designate samples that did not match one particular Gardner colour.
Determination of the hull/kernel ratio
Representative examples of each species having high, low and intermediate seed oil content values were utilized. For each accession, five intact seeds were divided into hull and kernel, and each portion weighed. This process was replicated four times (total of 20 seeds/accession) and the hull/kernel ratios calculated. Thirty-four accessions of C. moschata and 46 accessions of C. argyrosperma were thus analysed.
Statistical analysis
Pearson's coefficient analysis was performed to determine significant correlations. An analysis of variance was performed on the data, and means were separated using Tukey's studentized range Honestly Significant Difference (HSD) test. General statistical data were generated and analysed using SigmaPlot 11.2 and SAS.
Results
Physical properties of the seed oil standards
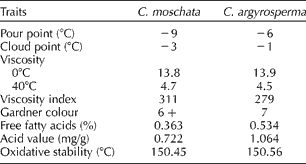
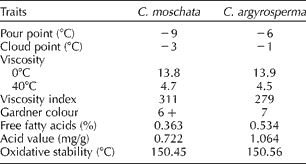
The physical characteristics of the oils from C. moschata (cv. Waltham Butternut) and C. argyrosperma (cv. green-striped cushaw), used as standards for the TD-NMR oil analysis, were generally similar (Table 1) to near-identical viscosities, VIs and colour. The pour point values of C. moschata and C. argyrosperma oils were − 9 and − 6°C, respectively. The oil of the C. moschata sample had a slightly lower pour point, cloud point, FFA value and AV when compared with C. argyrosperma. The P-DSC OT (as a measure of oxidative stability) for both samples was near 150°C. VIs for both samples were also similar at 311 and 279 for C. moschata and C. argyrosperma seed oils, respectively. Seed oil estimates (via TD-NMR) were 31.6 and 28.2% for cvs. green-striped cushaw and Waltham Butternut, respectively. The fatty acid profiles of both cultivars were likewise similar [Waltham Butternut: palmitic (17.7%), stearic (6.4%), oleic (29.2%) and linoleic (45.5%) vs. green-striped cushaw: palmitic (11.3%), stearic (7.9%), oleic (34.4%) and linoleic (45.4%)] but differed primarily in their concentration of palmitic acid.
Table 1 Physical properties of the seed oil from Cucurbita moschata cv. Waltham Butternut and Cucurbita argyrosperma cv. green-striped cushaw
Seed oil content and hull/kernel ratio
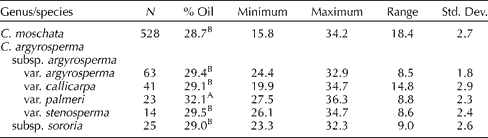
Seed oil content in the (entire) seed of 528 accessions of C. moschata and 166 accessions of C. argyrosperma is presented in Table 2. The mean oil content values for both species were similar at 28.7 ± /-2.7 and 29.8 ± /-2.6% for C. moschata and C. argyrosperma (all subspecies and varieties combined), respectively (P= 0.337). However, the seed oil content of C. argyrosperma subsp. argyrosperma var. palmeri (32.1%) was significantly higher than that of other C. argyrosperma subsp. argyrosperma varieties, C. argyrosperma subsp. sororia and C. moschata. Ranges in oil content within species were also similar [C. moschata − 15.8% (PI 442269) to 34.2% (PI 494850); C. argyrosperma − 19.9% (PI 511899) to 36.3% (PI 511987)]. The distributions of seed oil content were likewise similar for both species (Figs 1 and 2).
Table 2 Percentage of oil content and related general statistics on the seed of Cucurbita moschata and Cucurbita argyrosperma
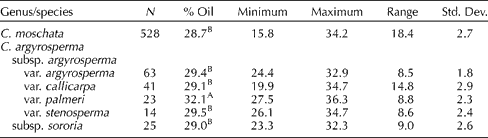
A,BMean values within columns followed by the same capital letters are not significantly different (α = 0.05) based on Tukey's studentized range (HSD) test.

Fig. 1 Frequency histogram depicting the distribution of seed oil content in the 528 genebank accessions of Cucurbita moschata.

Fig. 2 Frequency histogram depicting the distribution of seed oil content in the 166 genebank accessions of Cucurbita argyrosperma.
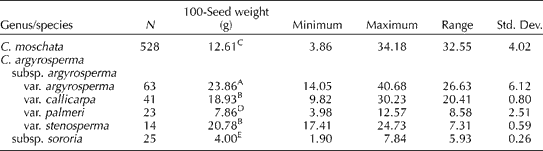
In our sampling of the 34 accessions of C. moschata and the 46 accessions of C. argyrosperma, the means for the percentage of hull were 22.7 ± 5.2 and 25.5 ± 4.2% for these species, respectively. A significant difference was detected between these means (P= 0.013). Seed oil content was positively correlated with the percentage of hull (as the percentage of total seed weight) in both C. moschata (R 2= 0.585) and C. argyrosperma (R 2= 0.386), respectively. The percentage of total seed weight attributable to the kernel varied within and between (P= 0.011) species. The average percentage of total seed weight attributable to the kernel ranged from 62.0 to 85.3% (mean 77.3%) in C. moschata and from 65.1 to 80.4% (mean 74.5%) in C. argyrosperma. No correlation was found between 100-seed weight and oil content for either C. moschata (R 2= 0.065) or C. argyrosperma (R 2= 0.051). Significant differences in the 100-seed weight values of the various taxa examined were also detected (Table 3).
Table 3 One hundred-seed weight (mean) and related general statistical data on the seed of Cucurbita moschata and Cucurbita argyrosperma genebank accessions
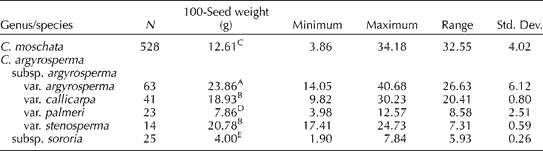
A,B,C,D,EMean values within columns followed by the same capital letters are not significantly different (α = 0.05) based on Tukey's studentized range (HSD) test.
Fatty acids
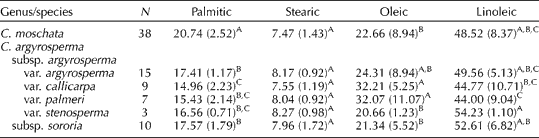
The mean concentrations of individual fatty acids in the seed of C. moschata are indicated in Table 4. The mean values of individual fatty acids in the seed of C. moschata (n= 38) were as follows: palmitic 20.7%; stearic 7.5%; oleic 22.7%; linoleic 48.5%. Among these same materials, low and high values (and ranges) for each were as follows: linoleic 24.7–61.7% (37.0%); oleic 10.0–53.8% (43.8%); palmitic 13.6–27.8% (14.2%); stearic 3.7–9.5% (5.8%); arachidonic 0.2–0.6% (0.4%). Average concentrations of palmitic acid were significantly higher in the seed of C. moschata when compared with C. argyrosperma (Table 4). Published values for individual fatty acids present in C. moschata seed, as previously cited, fall within the ranges reported here, though the ranges presented in this study are larger than those in earlier reports.
Table 4 Principal fatty acids (mean %(±SD)) in the seed of genebank accessions of Cucurbita moschata and Cucurbita argyrosperma
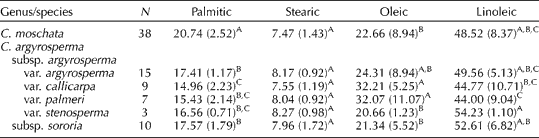
A,B,CMean values within columns followed by the same capital letters are not significantly different (α = 0.05) based on Tukey's studentized range (HSD) test.
The mean concentrations of individual fatty acids in the seed of C. argyrosperma are also indicated in Table 4. Linoleic acid was the predominant fatty acid present in the seed of C. argyrosperma, reaching both a maximum (62.3%) and a minimum (25.4%) in subsp. argyrosperma var. callicarpa (range 36.8%). Concentrations of palmitic acid were highest (19.0%) in var. argyrosperma (range 4.9) and lowest (12.1%) in var. callicarpa (range 6.8). Concentrations of stearic acid were similar among all taxa (including C. moschata), and concentrations of oleic acid reached a maximum of 49.0% and a minimum of 12.7% in the seed of var. callicarpa. Concentrations of oleic acid and linoleic acid were significantly higher in the seed of C. argyrosperma subsp. argyrosperma var. palmeri and C. argyrosperma subsp. argyrosperma var. stenosperma, respectively. However, we acknowledge that the number of accessions examined for each taxon varied widely (i.e. var. argyrosperma, n= 15; var. stenosperma, n= 3).
Discussion
Pour point values observed for C. moschata and C. argyrosperma were similar to each other and similar to or identical with pour point values observed for canola ( − 9°C), soyabean ( − 9°C), safflower ( − 6°C) and meadowfoam (+3°C) seed oils (Asadauskas and Erhan, Reference Asadauskas and Erhan1999). Cloud point (phase separation) values for C. moschata ( − 3°C) and C. argyrosperma ( − 1°C) were similar to those of canola ( − 2°C) and sunflower ( − 1.0°C) oils (Knothe et al., Reference Knothe, Gerpen and Krahl2005). Though the lower temperature properties of these and other vegetable oils make them less attractive for use as industrial lubricants, they have qualities such as high VI that make them ideal as industrial fluids. Petroleum oil typically has a VI of 90–100, significantly lower than that of soyabean oil (VI = 222). Since the viscosity of an oil with a higher VI changes less than that of an oil with a lower VI for a given change in temperature, for the purposes of use as an industrial fluid, a higher VI is desirable. The high VI values observed in the present study suggest that these oils have potential as industrial fluids.
The seed oils from C. moschata and C. argyrosperma were similar in colour, being somewhat darker than peanut oil which typically has a Gardner colour value of ~4 (Dean et al., Reference Dean, Davis, Sanders and Gunstone2011). AV is a measure of the FFA present in the oil. It is used as a measure of oil quality, lower values being preferable to higher. Fokou et al. (Reference Fokou, Achu, Kansci, Ponka, Fotso, Tchiegang and Tchouanguep2009) reported a range in the acid index values from 1.77 to 7.81 mg KOH/g oil (mean 4.31 ± 2.69) for four varieties of C. moschata grown in Cameroon. Chemical refining could be used to improve the AV, FFA and colour values (Evangelista, 2005). In general, P-DSC OTs range from 143 to 178°C for most vegetable oils (Adhvaryu et al., Reference Adhvaryu, Erhan, Liu and Perez2000). The cucurbits examined in this study had a P-DSC OT (~150°C) similar to that of cotton seed oil (149.9°C). Oxidative stability is affected by various factors such as the percentage of FFA present and the process used to remove the oil. Many seeds have natural antioxidants that provide resistance to oxidation. The relatively high oxidative stability of oils of C. moschata and C. argyrosperma is indicative of their potential for use in cooking and/or industrial applications. Inter- and intra-specific variability for these and other physical characteristics is to be expected, and it would be premature to suggest that the values presented are fully representative of the taxa examined.
The mean seed oil content values reported here are similar to those reported by Bemis et al. (Reference Bemis, Berry, Kennedy, Woods, Moran and Deutschman1967) who noted an average of 33.5% oil in the (apparently entire) seed of C. moschata (cv. Butternut), 31.2% in the seed of cv. Seminole Pumpkin (C. argyrosperma) and 37.4% in the seed of Cucurbita mixta (syn. C. argyrosperma) cv. Tucson 2. The literature contains a variety of other reports on oil content in C. moschata and, to a lesser extent, C. argyrosperma. However, data on the seed oil content of individual subspecies and varieties of C. argyrosperma do not appear to be available. Many previous studies of seed oil content have utilized only kernels (decorticated seed) in their analyses, while others have examined the entire seed. Decorticated seed of C. moschata yielded 46.1% oil as determined by Lal et al. (Reference Lal, Datta and Madaan1983). Teotia et al. (Reference Teotia, Ramakrishna Berry and Kaur1989) reported an oil yield of 46.2% in the kernels of C. moschata, whereas Al-Khalifa (Reference Al-Khalifa1996) reported an oil content of 43% in the seeds of this species. Fokou et al. (Reference Fokou, Achu, Kansci, Ponka, Fotso, Tchiegang and Tchouanguep2009) reported an average oil content in the decorticated seed of four varieties of C. moschata as 50.8 (41.9–54.4)%, while kernals of Egyptian varieties of C. moschata yielded 44.5% oil (El-Aziz and El-Kalek, Reference El-Aziz and El-Kalek2011).
In addition to the values reported by Bemis et al. (Reference Bemis, Berry, Kennedy, Woods, Moran and Deutschman1967) for decorticated seed, the entire seed of C. moschata (four varieties) and C. argyrosperma (three varieties) examined by Applequist et al. (Reference Applequist, Avula, Schaneberg, Wang and Khan2006) yielded a range from 31.2 to 39.2% oil and from 30.6 to 43.0% in each of these species, respectively. In that study, seeds of cv. green-striped cushaw (C. argyrosperma subsp. argyrosperma var. callicarpa), one of the few cultivated varieties of C. argyrosperma, were found to contain 41.1% oil. We found the entire seed of this cv. to contain 31.2% oil. Stevenson et al. (Reference Stevenson, Eller, Wang, Jane, Wang and Inglett2007) reported on the oil content in the seed of both C. moschata and C. argyrosperma. The values ranged from 29.1 to 43.3% and from 36.0% to 40.1% for C. moschata and C. argyrosperma, respectively. In general, published values for seed oil content in C. moschata were much higher than the 22.1% reported by Sae-lim et al. (2008) but somewhat similar to the 35.7% oil present in the entire seed of a Korean variety of C. moschata (Kim et al., Reference Kim, Kim, Kim, Choi and Lee2012). The range of oil content values reported in the present study is similar to the values reported by Madaan et al. (Reference Madaan, More, Lal and Seshadri1982) in a study of 91 accessions of Cucumis melo L. Madaan and Lal (Reference Madaan and Lal1984) also reported that approximately 75% of the total seed weight of C. moschata was attributable to the kernel and ~25% to the hull. The range of values observed in the present study was considerably smaller than the range of values reported by Madaan et al. (Reference Madaan, More, Lal and Seshadri1982) for a large number of C. melo accessions in which the percentage of the total seed weight attributable to the kernel ranged from 25.0 to 74.0%. In a subsequent study of various Cucurbita spp. (Madaan and Lal, Reference Madaan and Lal1984), the percentage of total seed weight attributable to the kernel ranged from 54.5 to 75.5%.
While an extensive amount of information has been accumulated regarding the long-chain fatty acids in the seeds of pumpkins (Cucurbita spp.; Hopkins, Reference Hopkins, Bates, Robinson and Jeffrey1990), information specific to C. moschata is somewhat less abundant. Bemis et al. (Reference Bemis, Berry, Kennedy, Woods, Moran and Deutschman1967) noted the occurrence of four fatty acids in the seed of C. moschata cv. Butternut (palmitic 19.0%, stearic 7.0%, oleic 40.0% and linoleic 34.0%) and cv. Seminole Pumpkin (palmitic 17.0%, stearic 7.0%, oleic 50.0% and linoleic 26.0%). Al-Khalifa (Reference Al-Khalifa1996) noted that linoleic acid was the predominant fatty acid in C. moschata (53.2%) followed by oleic (26.2%), palmitic (13.1%) and stearic (6.0%). Sae-lim et al. (2008) found the fatty acids in C. moschata seed to be 56.0% linoleic, 20.4% oleic, 13.4% palmitic and 9.9% stearic – similar to soyabean (46.9% linoleic, 26.0% oleic, 10.5% palmitic and 3.4% stearic). Fokou et al. (Reference Fokou, Achu, Kansci, Ponka, Fotso, Tchiegang and Tchouanguep2009) reported an average fatty acid composition (four varieties) of C. moschata as 49.5% linoleic, 19.6% oleic, 19.2% palmitic and 9.2% stearic with traces (1.7%) of arachidonic (C20:0) acid. In more recent reports, Applequist et al. (Reference Applequist, Avula, Schaneberg, Wang and Khan2006) reported average fatty content values (four varieties) in C. moschata of 55.0% linoleic, 19.2% oleic, 15.8% palmitic and 6.9% stearic. Raharjo et al. (Reference Raharjo, Nurliana and Mastjeh2011) reported that the predominant fatty acids associated with seed phospholipids were oleic and palmitic in phosphatidylcholine and phosphatidylethanolamine, and oleic and linoleic in phosphatidylserine. Kim et al. (Reference Kim, Kim, Kim, Choi and Lee2012) identified four fatty acids in the seed of C. moschata, these being linoleic (35.7%), oleic (31.3%), palmitic (12.8%) and stearic (7.3%). Fatty acid composition may be subject to certain environmental effects, as alluded to by Applequist et al. (Reference Applequist, Avula, Schaneberg, Wang and Khan2006). Thus, the ranges established for individual subspecies and varieties may be subject to modification.
Fewer published reports are available on the fatty acid content in the seed of C. argyrosperma, compared with C. moschata. Bemis et al. (Reference Bemis, Berry, Kennedy, Woods, Moran and Deutschman1967) reported a fatty acid profile in the seed of C. mixta cv. Tucson 2 as 34.0% linoleic, 46.0% oleic, 12.0% palmitic and 8.0% stearic. Applequist et al. (Reference Applequist, Avula, Schaneberg, Wang and Khan2006) reported average values (three varieties) of C. argyrosperma as 55.0% linoleic, 19.2% oleic, 15.8% palmitic and 6.9% stearic. Previously published values for individual fatty acids present in C. argyrosperma seed fall within the ranges reported here. However, the literature does not contain previous reports of fatty acid composition in the seed of C. argyrosperma at the level of individual subspecies or varieties. Hence no comparison with previously published data is possible.
Reports in the literature regarding seed oil and fatty acid composition (and other characteristics such as medicinal properties) associated with ‘pumpkins’ and pumpkin seed oil do not always clearly identify the taxonomic classification of the plant materials utilized. Several of the reports on the medicinal properties associated with the use of pumpkin seed oil, as cited by Stevenson et al. (Reference Stevenson, Eller, Wang, Jane, Wang and Inglett2007), were conducted utilizing a commercially available product marketed as ‘Pepon’ (Mepaco-Medifood, Egypt). The literature on this product notes that ‘Pepon’ contains ‘pumpkin seed oil, which is composed mainly from many oils’. As noted previously, the term ‘pumpkin’ is commonly used to refer to C. pepo, C. moschata, C. argyrosperma, C. maxima and possibly fruit of other taxa (Stevenson et al., Reference Stevenson, Eller, Wang, Jane, Wang and Inglett2007; Ferriol and Pico, Reference Ferriol, Pico, Prohens and Nuez2008). Reports on seed oil content that do not identify the taxa from which the oils were obtained, or indicate whether entire or decorticated were utilized, can be difficult to interpret in relation to the published literature and can confound efforts to establish ranges.
Acknowledgements
We acknowledge the expert technical assistance of Ms Amber Durham (USDA/ARS, Peoria, IL – testing of oil physical properties), Ms Sally Belflower (USDA/ARS, Tifton, GA – FAME analysis), Ms Sarah Moon (USDA/ARS, Griffin, GA – extraction of fatty acids), Mr Chris Tatum (University of Georgia, Griffin – TD-NMR analysis of seed oil content) and Mr Jerry Davis (University of Georgia, Griffin – statistical analysis).