Introduction
Fucosyltransferases catalyse the transfer of l-fucose (6-deoxy-galactose) from a donor (Guanosine diphosphate (GDP)-fucose) substrate to an acceptor molecule. The acceptor substrate may vary according to the fucosylation reaction such as the transfer of fucose to a core N-acetylglucosamine sugar as in the case of N-linked glycosylation or to a protein as in the case of O-linked glycosylation catalysed by an O-fucosyltransferase (Ma et al., Reference Ma, Simala-Grant and Taylor2006). Fucosylation reactions catalysed by fucosyltransferases and GDP-fucose transporter are highly important in mammalian biology. Thirteen fucosyltransferase genes have been identified in the human genome and divided into five groups based on the site of fucose addition: α-1,2 (FUT1 and FUT2), α-1,3/4 (FUT3, FUT4, FUT5, FUT6, FUT7 and FUT9), α-1,6 (FUT8), O-fucosyltransferases 1 and 2 (PoFUT1 and PoFUT2), and two additional α-1,3-fucosyltransferase genes (FUT10 and FUT11) (Ma et al., Reference Ma, Simala-Grant and Taylor2006). Among the several fucosyltransferases found in mammals, most are localized in the golgi apparatus, but some O-fucosyltransferases have recently been shown to be localized in the endoplasmic reticulum. O-Fucose may be associated with cancer biology because protein O-fucosyltransferase (PoFUT) 1 and PoFUT2 have been shown to target Notch and ADAMTS, superfamily proteins that regulate carcinogenesis and cancer progression (Ricketts et al., Reference Ricketts, Dlugosz, Luther, Haltiwanger and Majerus2007). GDP-fucose PoFUT1 is an enzyme responsible for the addition of fucose sugars in O-linkage to serine or threonine residues between the second and third conserved cysteine residues in epidermal growth factor (EGF)-like repeats on the Notch protein. This enzyme is an inverting glycosyltransferase producing fucose-α-O-serine/threonine and absolutely essential for Notch function, as has been shown in knockout experiments (Ma et al., Reference Ma, Simala-Grant and Taylor2006).
In plants, xyloglucan, the principal hemicellulose of dicotyledonous angiosperms, has a terminal fucosyl residue that modulates the extensibility of cell walls and determines plant morphology and growth (Levy et al., Reference Levy, York, Stuike-Prill, Meyer and Staehelin1991). The combination of a glycosynthase with the Arabidopsis thaliana xyloglucan-specific fucosyltransferase 1 (glycosyltransferase family GT37, EC 2.4.1.69) has allowed the production of fucogalactoxyloglucans of the type found in primary plant cell walls (Perrin et al., Reference Perrin, DeRocher, Bar-Peled, Zeng, Norambuena, Orellana, Raikhel and Keegstra1999). Though not absolutely required, they provide adaptive conformations for the formation of cellulose–xyloglucan networks (Levy et al., Reference Levy, Maclachlan and Staehelin1997). A key component that has been characterized in the association between cellulose and xyloglucan is the l-fucose-containing trisaccharide side chain (Levy et al., Reference Levy, York, Stuike-Prill, Meyer and Staehelin1991, Reference Levy, Maclachlan and Staehelin1997). However, comparatively far little is known about the structural and functional characteristics of plant fucosyltransferases. Furthermore, the available information is limited to a few model plants and needs to be translated for their counterparts from other plants of economic significance. Therefore, this paper reports some proteomic characteristics of a fucosyltransferase derived from Silybum marianum, a medicinal plant highly valued for its most prominent hepatoprotective activity.
Materials and methods
Plant material
S. marianum cultivated variety CIM-LIV (genotype cv-CIM-LIV, conserved in the CIMAP Gene Bank) was raised and flower petals were used for the isolation and purification of enzyme.
Enzyme isolation
We have recently established methods for the purification and characterization of enzymes involved in the glycosylation of natural products from medicinal plants such as Withania somnifera (Mishra et al., Reference Mishra, Sangwan and Sangwan2013a) and Andrographis paniculata (Mishra et al., Reference Mishra, Sangwan and Sangwan2013b). A β-glucosidase was purified from the petals of S. marianum using the ammonium sulphate precipitation method, followed by enrichment using Sephacryl-200, Q-Sepharose and S-Sepharose (Mishra et al., Reference Mishra, Sangwan and Sangwan2013c). The final enzyme preparation was separated using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), and polypeptide bands that were obtained were subjected to proteomic analysis.
Proteomic analysis
The SDS–PAGE revealed two polypeptides with native molecular weights (MWs) of 67.6 and 74.1 kDa. The 67.6 kDa polypeptide alone corresponds to the β-glucosidase (Mishra et al., Reference Mishra, Sangwan and Sangwan2013c), while the 74.1 kDa polypeptide is a co-purifying protein. The co-purifying polypeptide was further analysed for proteomic identification by peptide mass fingerprinting using a Voyager-DE STR mass spectrometer (Applied Biosystems, Stockholm, Sweden). Briefly, a Coomassie-stained protein band (74.1 kDa, Fig. 1(a), upper band) was destained and digested in-gel with trypsin (Promega, SDS Biosciences, Falkenberg, Sweden). MALDI–TOF mass spectroscopy was carried out using an α-cyano-4-hydroxycinnamic acid (G2037A) matrix (Agilent Technologies, Stockholm, Sweden). The mass fragment data were subjected to database searches on an in-house Mascot server licensed to Umeå University by Matrix Science using the current version of the NCBInr database. The obtained fragment sequences were subjected to BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) analysis for the identification of the polypeptide and its signature motifs through Domain Enhanced Lookup Time Accelerated BLAST.
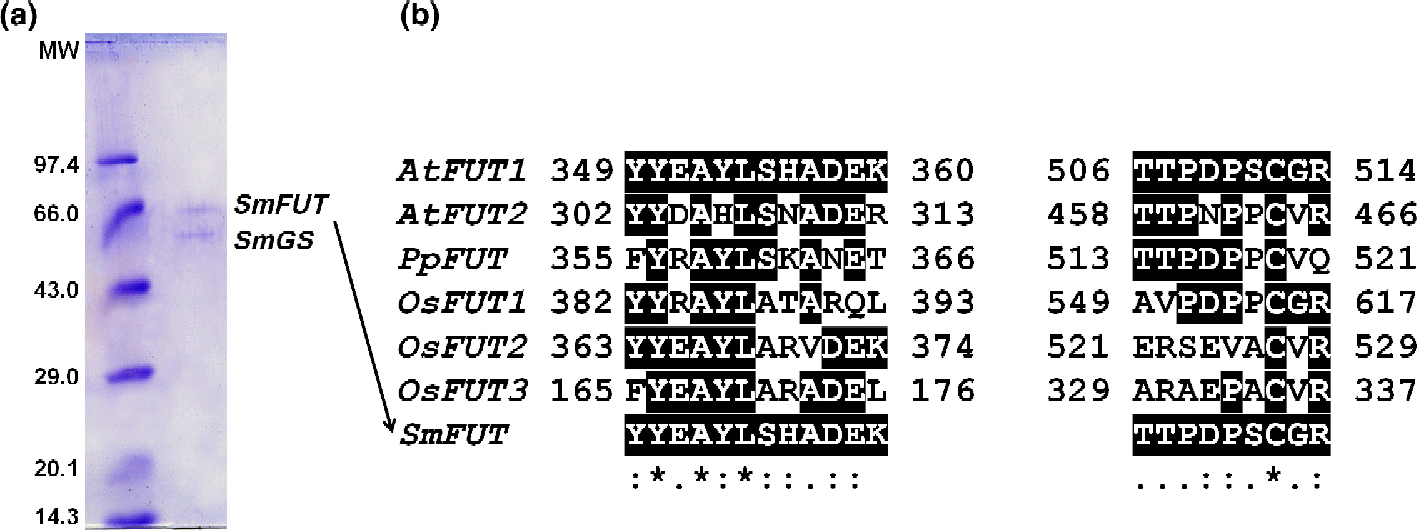
Fig. 1 (a) Sodium dodecyl sulphate–polyacrylamide gel electrophoresis carried out for Silybum marianum fucosyltransferase. Molecular weight (MW), standard MW marker (kDa) (Biogene, Cambridge, UK). (b) Sequence alignment of fucosyltransferase protein sequences: AtFUT1, Arabidopsis thaliana xyloglucan fucosyltransferase (Perrin et al., Reference Perrin, DeRocher, Bar-Peled, Zeng, Norambuena, Orellana, Raikhel and Keegstra1999); AtFUT2, A. thaliana putative fucosyltransferase (Sarria et al., Reference Sarria, Wagner, O'Neill, Faik, Wilkerson, Keegstra and Raikhel2001); PpFUT, Populus tremula× Populus alba α-1,2-fucosyltransferase (Costa et al., Reference Costa, Decou and Lhernould2007); OsFUT1, Oryza sativa putative xyloglucan fucosyltransferase (Sasaki et al., Reference Sasaki, Matsumoto and Katayose2002); OsFUT2, O. sativa Japonica cultivar putative xyloglucan fucosyltransferase (Matsumoto et al., Reference Matsumoto, Wu, Kanamori, Katayose, Fujisawa and Namiki2005); OsFUT3, O. sativa Japonica cultivar putative galactoside 2-α-l-fucosyltransferase (Matsumoto et al., Reference Matsumoto, Wu, Kanamori, Katayose, Fujisawa and Namiki2005); and SmFUT, S. marianum fucosyl transferase fragment. SmGS, S. marianum β-glucosidase; :, Conservative change; ., semi conservative change; *, no change.
Results and discussion
While purifying and characterizing a β-glucosidase from the petals of S. marianum (Mishra et al., Reference Mishra, Sangwan and Sangwan2013c), we obtained a final enzyme preparation containing two polypeptides – 74.1 and 67.6 kDa (Fig. 1(a)). The native MW data of the enzyme and proteomic analysis indicated that the lower-MW polypeptide (67.6 kDa) alone constituted the β-glucosidase (Mishra et al., Reference Mishra, Sangwan and Sangwan2013c), while the higher-MW polypeptide (74.1 kDa) was a contaminant of the enzyme preparation. The contaminating polypeptide was analysed for the identification of protein by peptide mass fingerprinting.
Protein sequences that were obtained were subjected to protein BLAST at the NCBI. Of the 15 mass fragments, peptide sequences assigned to those that matched considerably to the known sequences are listed in Table 1. The sequences of two peptide fragments (YYEAYLSHADEK and TTPDPSCGR, designated as motif 1 and motif 2, respectively) from the 74.1 kDa polypeptide exhibited strong homology with A. thaliana xyloglucan fucosyltransferase (accession number NP_178421, gb|AEC05676.1) located at locus NP_178421 containing a total of 558 amino acids (Lin et al., Reference Lin, Kaul, Rounsley, Shea, Benito and Town1999). On multiple sequence alignment of the homologues using ClustalX, the two fragments of the 74.1 kDa polypeptide from the petals of S. marianum (designated as fucosyltransferase, SmFUT) were found to have complete (100%) sequence homology with A. thaliana xyloglucan fucosyltransferase (AtFUT1) (Perrin et al., Reference Perrin, DeRocher, Bar-Peled, Zeng, Norambuena, Orellana, Raikhel and Keegstra1999) and sequence homology of 67% with A. thaliana putative fucosyltransferase (AtFUT2) (Sarria et al., Reference Sarria, Wagner, O'Neill, Faik, Wilkerson, Keegstra and Raikhel2001). The α-1,2-fucosyltransferase (PpFUT) from a hybrid variety of Populus (Populus tremula× Populus alba) (Costa et al., Reference Costa, Decou and Lhernould2007) also had good sequence homology with Silybum fucosyltransferase (SmFUT). Similarly, the fragments from SmFUT had strong sequence resemblance to a putative xyloglucan fucosyltransferase and a Japonica cultivar putative xyloglucan fucosyltransferase (OsFUT1 and OsFUT2) as well as a putative galactoside 2-α-l-fucosyltransferase (OsFUT3) from Oryza sativa (Sasaki et al., Reference Sasaki, Matsumoto and Katayose2002; Matsumoto et al., Reference Matsumoto, Wu, Kanamori, Katayose, Fujisawa and Namiki2005). Additionally, a peptide motif LLPEVDTLVERSR of SmFUT exhibited high score similarity with the Arabidopsis counterpart gb|AEC05676.1 (Table 1).
Table 1 Matching peptide mass fragments obtained from the Silybum marianum petal 71.4 kDa polypeptide

Most interestingly, hydrophilic amino-acid residues were predominant in both motifs: nine of 12 residues and eight of nine residues for motif 1 and motif 2, respectively (Fig. 1(b)) We observed that any variation in the sequence of these motifs was either conservative or semi-conservative across all the plant fucosyltransferases identified so far. Motif 1 had a highly conserved peptide sequence of Tyr, Ala and Lys, YxAxL. Considering the catalytic nature of the hydrolytic transfer of fucose moiety from a donor to an acceptor, the motif could be directly involved in the nucleophile-initiated acid–base catalysis. The other conservations in motif 1 and motif 2 could be an approximate conformational requirement of the protein and/or active site for the catalytic site functionality. However, these are most likely speculated for testing through the structural models or catalytic traits of position-specific muteins. In any case, these motifs provide reliable signatures for fucosyltransferase genes from diverse plant sources/tissues and targets for functional aspects of conservation (Perrin et al., Reference Perrin, DeRocher, Bar-Peled, Zeng, Norambuena, Orellana, Raikhel and Keegstra1999; Sarria et al., Reference Sarria, Wagner, O'Neill, Faik, Wilkerson, Keegstra and Raikhel2001; Sasaki et al., Reference Sasaki, Matsumoto and Katayose2002; Matsumoto et al., Reference Matsumoto, Wu, Kanamori, Katayose, Fujisawa and Namiki2005; Costa et al., Reference Costa, Decou and Lhernould2007). The biosynthetic potential of cloned and overexpressed fucosyltransferases could be substantially broadened if non-natural donor sugars could also be transferred with the same stereochemical fidelity as the parent fucose. Thereby, investigation of their catalytic characteristics could be important from a potential biotransformation perspective.
Acknowledgements
S.K.M. is grateful to the CSIR for providing the Senior Research Fellowship grant to him. The authors are grateful to the NWP09 CSIR network project for providing financial grant. The authors are also grateful to the Director CSIR-CIMAP for providing academic support.




