Introduction
Plants have developed defence mechanisms to survive in various stressful environments. Plants encounter abiotic and biotic stresses such as drought, salinity, freezing, nutrient deficiency and pathogen infection. The ability to survive in the face of stress determines, in part, the geographical distribution of species as well as their growth habits and life cycles. Therefore, plants have developed complicated mechanisms involving morphological, physiological and biochemical processes to cope with these stresses.
The WRKY transcription factors in plants, characterized by the WRKYGQK amino-acid sequence at the N-terminal domain and by a zinc-finger-like motif, are important regulators of stress-related genes in plants. This group is one of the largest families of transcriptional regulators in Arabidopsis (>70 genes) and in rice (>100 genes) (Chi et al., Reference Chi, Yang, Zhou, Zhou, Fan, Yu and Chen2013). WRKY transcription factors have been classified into three groups: groups I–III, depending on the number and structure of the WRKY zinc-finger motifs (Eulgem et al., Reference Eulgem, Rushton, Robatzek and Somssich2000). WRKY transcription factors physically interact with other proteins, such as 14-3-3-proteins, calmodulin, histone deacetylases, mitogen-activated protein (MAP) kinase, resistance proteins, VQ-domain proteins and other WRKY transcription factors (Park et al., Reference Park, Lee, Yoo, Moon, Choi, Kang, Lee, Kim, Kang, Chung, Lim and Cho2005; Kim et al., Reference Kim, Lai, Fan and Chen2008; Chang et al., Reference Chang, Curran, Woolsey, Quilici, Cushman, Mittler, Harmon and Harper2009; Popescu et al., Reference Popescu, Popescu, Bachan, Zhang, Gerstein, Snyder and Dinesh-Kumar2009; Rushton et al., Reference Rushton, Somssich, Ringler and Shen2010; Cheng et al., Reference Cheng, Zhou, Yang, Chi, Zhou, Chen, Wang, Fan, Shi, Zhou, Yu and Chen2012; Chi et al., Reference Chi, Yang, Zhou, Zhou, Fan, Yu and Chen2013). They influence a range of biological activities including development and defence signalling in both monocotyledonous and dicotyledonous plants, including rice and Arabidopsis (Eulgem and Somssich, Reference Eulgem and Somssich2007).
VQ-domain proteins in Arabidopsis
VQ-domain proteins have a region consisting of 57 amino acids with the highly conserved ‘FXXXVQX(L/V/F)TG’ motif and physically interact with WRKY transcription factors (Cheng et al., Reference Cheng, Zhou, Yang, Chi, Zhou, Chen, Wang, Fan, Shi, Zhou, Yu and Chen2012). In Arabidopsis, 34 Arabidopsis thaliana VQ (AtVQ) genes have been identified, each with the conserved VQ motif. The smallest, AtVQ1, protein contains 430 amino acids. There is little sequence homology among the AtVQ proteins except for the short VQ motif (Cheng et al., Reference Cheng, Zhou, Yang, Chi, Zhou, Chen, Wang, Fan, Shi, Zhou, Yu and Chen2012). To date, six AtVQ proteins have been functionally analysed and five of them, AtVQ9, AtVQ14, AtVQ16, AtVQ21 and AtVQ23, have been found to interact with WRKY transcription factors (Table 1). AtVQ15/AtCAMBP25, known as the calmodulin-binding protein, was the first to be reported as an AtVQ protein, although it was not called a VQ-domain protein at the time (Perruc et al., Reference Perruc, Charpenteau, Ramirez, Jauneau, Galaud, Ranjeva and Ranty2004). The results of a functional study indicate that AtVQ15 is a negative regulator of osmotic stress responses. In a study, transgenic plants that overexpressed AtVQ15 were found to be hypersensitive to osmotic stress during seed development (Perruc et al., Reference Perruc, Charpenteau, Ramirez, Jauneau, Galaud, Ranjeva and Ranty2004). The second reported AtVQ protein, AtVQ21, is MAP kinase 4 substrate 1, which interacts with MAP kinase 4. The AtVQ21 protein has been reported to form complexes with AtWRKY25 and AtWRKY33, which were members of the Group I WRKY family (Andreasson et al., Reference Andreasson, Jenkins, Brodersen, Thorgrimsen, Petersen, Zhu, Qiu, Micheelsen, Rocher, Petersen, Newman, Bjørn Nielsen, Hirt, Somssich, Mattsson and Mundy2005; Qiu et al., Reference Qiu, Fiil, Petersen, Nielsen, Botanga, Thorgrimsen, Palma, Suarez-Rodriguez, Sandbech-Clausen, Lichota, Brodersen, Grasser, Mattsson, Glazebrook, Mundy and Petersen2008). AtVQ14, also called HAIKU1, regulates endosperm growth and seed development through its interaction with AtWRKY10, called MINI3 (Wang et al., Reference Wang, Xie, Chen, Tang, Yang, Ye, Liu, Lin, Xu, Xiao and Zhang2010). Recently, AtVQ23 and AtVQ16, also called sigma factor-binding proteins 1 and 2 (SIB1 and SIB2), have been identified and shown to interact with AtWRKY33, an important WRKY transcription factor involved in plant disease resistance to necrotrophic pathogens (Xie et al., Reference Xie, Li, Guo, Dong, Zhang, Fu, Ren, Peng and Xia2010; Lai et al., Reference Lai, Li, Wang, Cheng, Fan, Yu and Chen2011). These proteins complex with AtWRKY33 through the recognition of the C-terminal WRKY domain and increase the DNA-binding activity of AtWRKY33. Support for the role of these proteins as dual activators of AtWRKY33 comes from a study in which resistance to Botrytis cinerea, a necrotrophic pathogen, was found to be compromised in AtVQ23 and AtVQ16 mutants, but enhanced in transgenic plants overexpressing AtVQ23 (Lai et al., Reference Lai, Li, Wang, Cheng, Fan, Yu and Chen2011). However, Xie et al. (Reference Xie, Li, Guo, Dong, Zhang, Fu, Ren, Peng and Xia2010) reported that AtVQ23-overexpressing plants exhibit resistance to only Pseudomonas syringae and not to B. cinerea. More recently, AtWRKY8 has been reported to interact with AtVQ9, which is localized in the nucleus, and this interaction has been found to decrease DNA binding to W-box repeats in target genes. Even though the AtVQ9 gene has been found to be highly expressed during salt treatment, AtVQ9 mutant plants exhibit enhanced tolerance to salt stress, and AtVQ9 may be a negative regulator of the AtWRKY8-mediated signalling response (Hu et al., Reference Hu, Chen, Wang, Zhang, Wang and Yu2013).
Table 1 Genes of VQ-domain proteins involved in stress responses
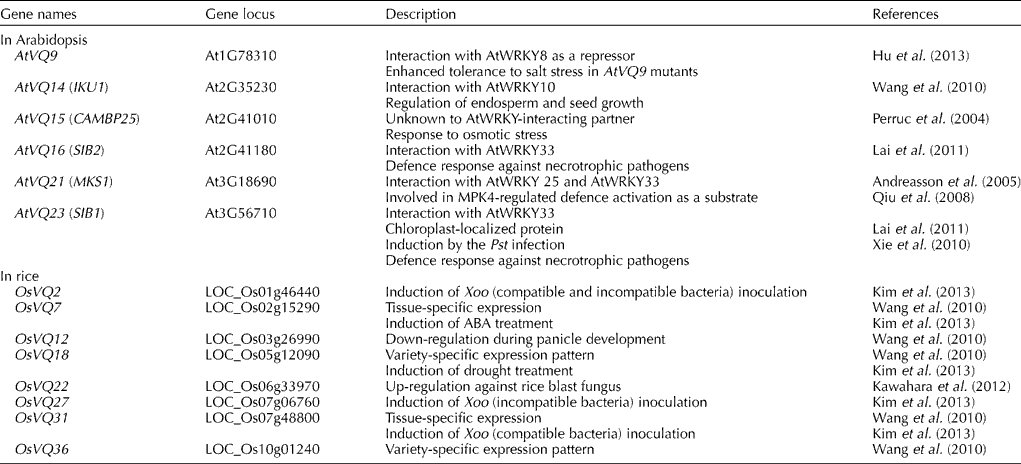
AtVQ; Arabidopsis thaliana VQ; IKU1, HAIKU1; CAMBP25, calmodulin-binding protein 25; SIB1 and SIB2, sigma factor-binding proteins 1 and 2; MKS1, mitogen-activated protein kinase 4 substrate 1; Pst, Pseudomonas syringae pv. tomato; OsVQ, Oryza sativa VQ; Xoo, Xanthomonas oryzae.
VQ-domain proteins in rice
Accumulating evidence suggests that Oryza sativa WRKY (OsWRKY) genes (OsWRKY6, OsWRKY12, OsWRKY13, OsWRKY30, OsWRKY45, OsWRKY53, OsWRKY71 and OsWRKY89) are also major regulators of the transcriptional activation of defence-related genes in rice (Liu et al., Reference Liu, Bai, Qian, Wang, Chen and Chu2005, Reference Liu, Bai, Wang and Chu2007; Qiu et al., Reference Qiu, Xiao, Ding, Xiong, Cai, Cao, Li, Xu and Wang2007; Shimono et al., Reference Shimono, Sugano, Nakayama, Jiang, Ono, Toki and Takatsuji2007; Wang et al., Reference Wang, Hao, Chen, Hao, Wang, Lou, Peng and Guo2007; Hwang et al., Reference Hwang, Yie and Hwang2011; Lee et al., Reference Lee, Ko, Cha, Park, Ahn and Hwang2013). However, the VQ-domain proteins of rice have not been studied to the same extent as those of Arabidopsis. Thirty-nine Oryza sativa VQ (OsVQ) genes have been identified in the rice genome and found to have the conserved VQ motif, except two VQ-domain proteins (OsVQ37 and OsVQ39) (Kim et al., Reference Kim, Kwon, Choi, Lee, Ahn, Park, Bae, Lee and Hwang2013). The putative OsVQ proteins contain 77 (OsVQ2) to 439 (OsVQ35) amino-acid residues. Although expression patterns have been examined for all these OsVQ genes, their functions have not been assessed (Table 1).
Recently, using the rice Affymetrix GeneChip Arrays with 39 tissues of two rice indica varieties (Minghui 63 and Zhenshan 97), the expression profiles of OsVQ genes have been examined during development (Wang et al., Reference Wang, Xie, Chen, Tang, Yang, Ye, Liu, Lin, Xu, Xiao and Zhang2010). Notably, two genes, OsVQ7 and OsVQ31, displayed tissue-specific expression at the late stage of leaf development, while variety-specific expression patterns were observed for the OsVQ18 and OsVQ36 genes. The expression of the OsVQ12 gene was gradually down-regulated during panicle development (Wang et al., Reference Wang, Xie, Chen, Tang, Yang, Ye, Liu, Lin, Xu, Xiao and Zhang2010). In addition, according to the RNA-Seq analysis of Xanthomonas oryzae (Xoo)-infected rice, the OsVQ22 gene was highly expressed following infection with the rice blast fungus, Magnaporthe oryzae (Kawahara et al., Reference Kawahara, Oono, Kanamori, Matsumoto, Itoh and Minami2012).
Additionally, we studied 39 OsVQ-domain proteins in rice and analysed the phylogenetic relationship between the VQ domains of Arabidopsis and those of rice. We also examined the expression profiles of OsVQ genes during biotic stress, specifically upon inoculation with the bacterial pathogen Xoo, and exposure to the abiotic stresses abscisic acid and drought (Kim et al., Reference Kim, Kwon, Choi, Lee, Ahn, Park, Bae, Lee and Hwang2013). From these initial studies, we hope to be able to elucidate the biological functions of the VQ-domain proteins in rice.
Concluding remarks
Over the past decade, a number of proteins that interact with WRKY transcription factors have been identified, using various approaches such as yeast two-hybrid screening and co-immunoprecipitation assays. Some of the proteins, including VQ-domain proteins, may activate or suppress the expression of target genes through their compatible interaction with WRKY transcription factors. The study of the chemical and/or physical interactions between WRKY transcription factors and VQ-domain proteins may permit the identification of the specific mechanisms that regulate DNA-binding activity and transcription efficiency during environmental stresses. Eventually, understanding the interaction between VQ-domain proteins and WRKY transcription factors may help obtain information on the molecular mechanisms of WRKY transcription factors in the regulation of stress-induced signal transduction.
Acknowledgements
This work was supported by two grants (PJ007850 and PJ008574) from the Rural Development Administration to Dr D.-J. Hwang.



