Introduction
Sweetpotato, Ipomoea batatas (L) Lam. (Convolvulaceae), is an important food crop primarily grown for storage roots, but leaves and shoots also are consumed as a leafy vegetable in many countries (Woolfe, Reference Woolfe1992; Islam, Reference Islam2006). Sweetpotato leaves are more nutritious than storage roots or common leafy vegetables (Islam et al., Reference Islam, Yoshimoto, Ishiguro and Yamakawa2003; Sun et al., Reference Sun, Mu, Xi, Zhang and Chen2014). They are an excellent source of fibre, carbohydrates, protein, vitamins, minerals, polyphenols and anthocyanins (Villareal et al., Reference Villareal, Tsou, Lin and Chiu1979; Ishida et al., Reference Ishida, Suzuno, Sugiyama, Innami, Tadokoro and Maekawa2000; Truong et al., Reference Truong, McFeeters, Thompson, Dean and Shofran2007). Sweetpotato leaves contain biologically active compounds with medicinal value (Islam, Reference Islam2006; Johnson and Pace, Reference Johnson and Pace2010; Mohanraj and Sivasankar, Reference Mohanraj and Sivasankar2014; Wang et al., Reference Wang, Nie and Zhu2016). Varieties with improved culinary qualities as greens have been developed (Ishiguro et al., Reference Ishiguro, Toyama, Islam, Yoshimoto, Kumagai, Kai, Nakazawa and Yamakawa2004).
Sweetpotato leaves and vines are used for animal feed in many countries and they are more nutritious than most forage grasses (Murugan et al., Reference Murugan, Paramasivam, Nedunchezhiyan, Nedunchezhiyan and Byju2012). Sweetpotato leaves can be harvested several times a year (Pace et al., Reference Pace, Dull, Phills, Bonsi and Forrester1988), and moderate defoliation minimally affects root yields (Chalfant et al., Reference Chalfant, Jansson, Seal and Schalk1990). Thus, ‘dual-purpose’ sweetpotato varieties have been developed that optimize yields of both roots and foliage (Claessens et al., Reference Claessens, Stoorvogel and Antle2009; Niyireba et al., Reference Niyireba, Ebong, Agili, Low, Lukuyu, Kirui, Ndirigwe, Uwimana, Kakundiye, Mutimura, Gahakwa and Gachuiri2013).
Ornamental sweetpotato cultivars have become important landscape plants in the USA because they are showy, fast growing, drought tolerant and have few disease or insect problems (Carey et al., Reference Carey, Whipker, Bradley and Buhler2012). Plant type and leaf characteristics such as shape, size and colour are important considerations when breeding for ornamental sweetpotatoes. In addition, sweetpotato leaves are a source for value-added products, such as natural food colourants (Hue et al., Reference Hue, Boyce and Somasundram2011, Reference Hue, Boyce and Somasundram2014) or antioxidant compounds (Liao et al., Reference Liao, Lai, Yuan, Hsu and Chan2011; Hue et al., Reference Hue, Boyce and Somasundram2012). However, most sweetpotato leaves are discarded after roots are harvested (Hue et al., Reference Hue, Boyce and Somasundram2014; Mussoline and Wilkie, Reference Mussoline and Wilkie2017).
Studies of the genetic and phenotypic diversity of sweetpotato are important for utilization of its diverse germplasm in breeding programmes. Modern genomic techniques for the study and improvement of sweetpotato have progressed rapidly in the past few years (Si et al., Reference Si, Du, Huo, He, Liu and Zhai2016). Sweetpotato exhibits a high degree of genetic polymorphism and great diversity in its morphological traits (Huamán, Reference Huamán1991; Tairo et al., Reference Tairo, Mneney and Kullaya2008; Veasey et al., Reference Veasey, Borges, Rosa, Queiroz-Silva, de Andrade Bressan and Peroni2008). With sweetpotato, as with other crops, it is of utmost importance to link genotypes with phenotypes in order to fully utilize these genetic resources (Bolger et al., Reference Bolger, Schwacke, Gundlach, Schmutzer, Chen, Arend, Oppermann, Weise, Lange, Fiorani, Spannagl, Scholz, Mayer and Usadel2017; Anonymous, 2018e). Unfortunately, efforts to acquire phenotypic data have lagged behind the application of genomic tools.
The USDA-ARS maintains an important germplasm collection at the Plant Genetics Resources Conservation Unit (PGRCU), listing 762 sweetpotato accessions (Anonymous, 2018d). However, this germplasm is not fully characterized, nor have all of the accessions been evaluated for useable agronomic traits (Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018a; Anonymous, 2018d). Leaf colour is one phenotypic characteristic that has received little attention, and subjective colour data are available for less than one-half of the PGRCU collection (Anonymous, 2018d). In addition, no quantitative colorimeter data for sweetpotato leaves were found in the world-wide literature on sweetpotatoes. Therefore, we quantified colour characteristics of sweetpotato leaves from the USDA-ARS collection.
Materials and methods
A total of 737 I. batatas accessions were obtained as in vitro cultures from the USDA, ARS, PGRCU, Griffin, GA, USA. After receipt of the cultures at the USDA, ARS, US Vegetable Laboratory (USVL), Charleston, SC, USA, the accessions were maintained at 16 h/d fluorescent lighting and 25–30°C until they could be removed from the culture tubes (25 × 150 mm, Durex™ borosilicate glass, VWR International, Radnor, PA, USA). After removal from the culture vessel, all tissue culture media was rinsed from each plantlet, which was then planted into a 15 cm diameter plastic pot filled with Metro Mix® 360 potting soil (Sun Gro® Horticulture, Agawam, MA, USA). After watering, the plantlet and pot were covered with a 15 cm × 15 cm polyethylene bag (2-mil clear-line single-track seal, F20606, Elkay Plastics, Commerce, CA, USA) to maintain relative humidity at about 100%. Pots were kept at ambient temperature and indirect sunlight for several days until the plantlet had become acclimated and then moved to a greenhouse. At that time, a 2 cm isosceles triangle piece was cut off the corner of each polyethylene bag to increase airflow and reduce the humidity surrounding the plantlets. These holes were enlarged as the plantlets became stronger, until eventually the polyethylene bags were removed, usually within a month.
In the spring of each season (2012–2014), plants from the greenhouse were transferred to a plant bed in the field. A single plant from each accession was planted into a 76 cm wide row covered with black plastic film. The plants were spaced about 3 m apart. When these plants had become large enough, rootless cuttings (approximately 30 cm long) were planted into the field.
Field plots were maintained at the USVL for three growing seasons (2012–2014). Each sweetpotato accession was planted in two replications of single-row, five-plant plots arranged in a randomized complete block design. Sweetpotatoes were spaced 30 cm apart within rows, and rows were 1 m apart. Plots were staggered to minimize overlap of vines. In 2012, 102 accessions were planted on 26 July and 83 accessions were planted on 17 August. In 2013, 380 accessions were planted on 27 June, and in 2014 all 737 accessions were planted on 18 June. Local production practices were followed (Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018a), and when rainfall was not adequate during the growing season, supplemental irrigation was applied with overhead sprinklers. According to field history and recommended requirements, 10–10–10 fertilizer was applied on 12 July 2012 at the rate of 1121 kg/ha and on 13 June 2013 at a rate of 673 kg/ha. On 14 June 2014, 4–0–12 fertilizer was applied at a rate of 897 kg/ha. A pre-plant treatment of clomazone (Command® 3 ME, FMC Corporation, Philadelphia, PA, USA) was applied at the maximum recommended rate (4.7 l/ha) for weed control (Anonymous, 2018a).
A photographic record of each accession was made using a 10.1 mega-pixel digital camera with 3X optical zoom (Cybershot DSC-N2, Sony Corporation, Tokyo, Japan). Individual plants were photographed in the plant beds on 14 and 30 August 2012; 9–21 May and 5 and 17 June 2013; and 12, 20 and 28 May; 2–6, 11–12 and 23 June; and 11–17 July 2014. Whole field plots were photographed on 2, 9, 16, 23 and 30 August, 6, 13–14 and 20 September, and 4 and 15 October 2012; 12, 22–25 and 29–31 July, and 1–2 and 12–19 August 2013; and 1, 10–11, 21–22 and 30–31 July and 1 August 2014.
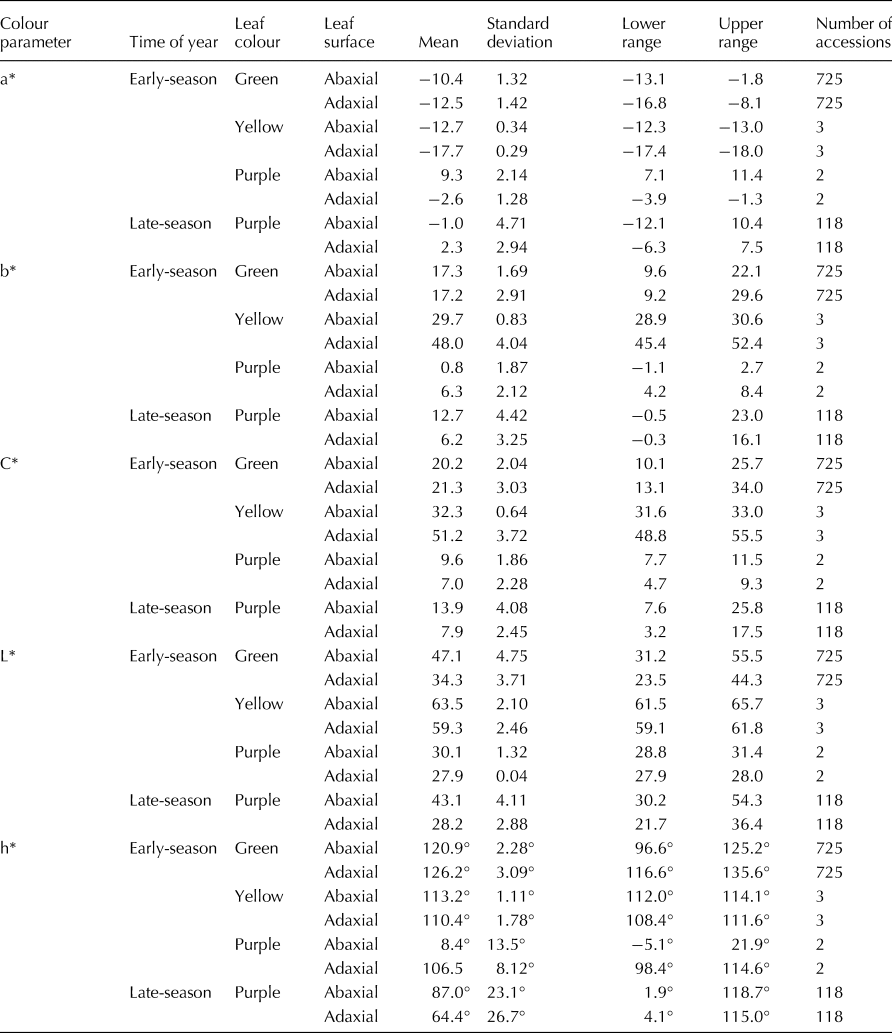
Early-season samples of mature leaves (fully expanded) were collected at 20–32 d after transplanting on 16–20 August 2012 (first planting), 6–11 September 2012 (second planting), 22–29 July 2013 and 9–16 July 2014. The number of accessions sampled each season were 185 (2012), 380 (2013) and 172 (2014). For each field replication, three representative examples of mature leaves were collected from each plot. Leaves were placed into a cooler with ice immediately after they were removed from the plants to prevent desiccation. After the leaves were brought into the laboratory, they were patted dry with a paper towel and then carefully spread out on a piece of graph paper (10 divisions per 2.54 cm) for a photographic record using the same digital camera (Cybershot DSC-N2) that was used to photograph the field plots. Both the adaxial (top) and abaxial (bottom) surfaces of the leaves were photographed under fluorescent lighting. These photographs were used later to determine subjective ratings for mature leaf colour and for abaxial leaf vein colour for each accession. Then, objective colour measurements of the abaxial and adaxial surfaces of these leaf samples were obtained using a tristimulus colorimeter, Konica Minolta Chroma Meter (CR-400 with 8 mm aperture and 0° viewing angle, Konica-Minolta, Inc., Tokyo, Japan) (Konica-Minolta, 2007a). The instrument was calibrated against a standard white reference tile provided by the instrument manufacturer. The instrument was positioned so that the aperture was within a centimetre of the leaf surface when the readings were taken. Care was taken to position the instrument above an area of leaf lamina without major veins or imperfections. The colorimeter was set so that it averaged three readings for each data point, and each reading measured 50.3 mm2 (8 mm diameter circular area) of the leaf surface. Data were recorded by means of Color Data Software CM-S100w SpectraMagic NX (Version 1.7) (Konica-Minolta, 2007b) using the CIE (Commission Internationale de l'Eclairage [International Commission on Illumination]) 1976 L*a*b* and CIE L*C*h colour spaces (McLaren, Reference McLaren1976; HunterLab, 2009a, b). CIE 1976 L*a*b* and CIE L*C*h are three-dimensional colour spaces where L* (lightness) represents the white to black axis, a* represents the red to green axis, and b* represents the yellow to blue axis. Hue angle (h*) was calculated as tan–1(b*/a*), and saturation chroma (C*) was calculated as square root (a*2 + b*2).
Purple leaf colour was subjectively rated for each field plot on 12, 22, 25, 29 July and 12 August 2013 (380 plant introductions (PIs)), and 2 and 13 June 2014 (all 737 PIs were grown in 2014). The following rating scale was used: 0 = all green leaves, 1 = some purple bronzing on new leaves, 2 = distinct purple on some small leaves, 3 = distinct purple on some larger leaves, 4 = many purple leaves, 5 = over 50% of leaves purple. In addition, late in the season in 2013 and 2014, purple leaves were collected from the field plots for 118 PIs that showed significant purple colouration in the field. Collections of purple leaves were done shortly before the plots were harvested on 22–25 October 2013 and 20–24 October 2014. Objective colour measurements of the abaxial and adaxial surfaces of the purple leaf samples were obtained with the tristimulus colorimeter.
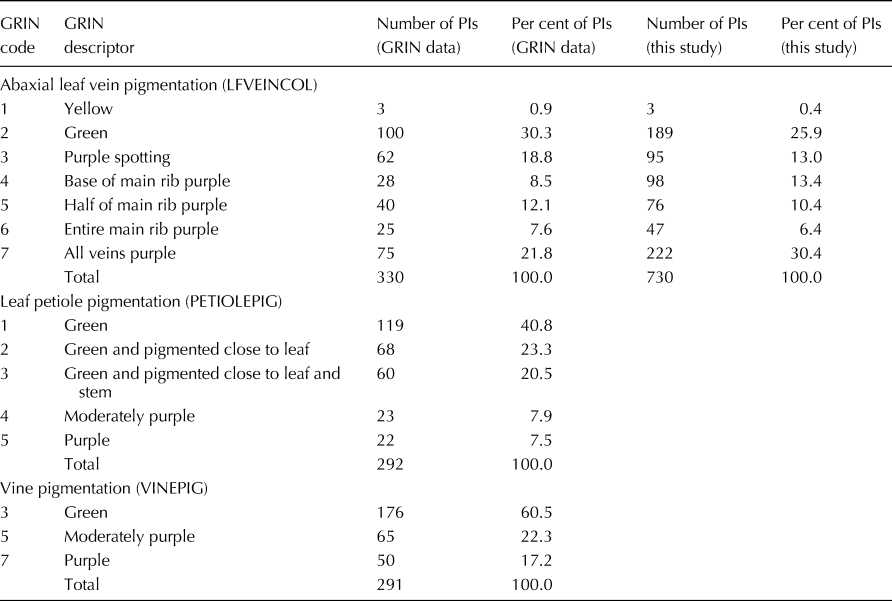
Much of the genetic diversity of sweetpotato is preserved in several collections of germplasm (Gregory, Reference Gregory1987; Roca et al., Reference Roca, Laliberté, Renoso, Rao and de Chavez2007). The USDA ARS sweetpotato collection used in the present study is part of the US National Plant Germplasm System, which also maintains the Germplasm Resources Information Network (GRIN) database for sweetpotatoes (Anonymous, 2018b, d). This database lists four subjective descriptors for leaf and vine colour: immature leaf colour (IMMLEAFCOL, 338 accessions), mature leaf colour (MATLEAFCOL, 337 accessions), abaxial leaf vein colour (LFVEINCOL, 330 accessions) and vine pigmentation (VINEPIG, 331 accessions) (Anonymous, 2018b, d). These descriptors are based on Huamán (Reference Huamán1991) and also are used in the Sweetpotato Ontology (Hualla et al., Reference Hualla, Simon, Mwanga, Soto and Montesinos2015). However, the largest sweetpotato germplasm collection in the world is at the International Potato Center (CIP) in Lima, Peru (Huamán et al., Reference Huamán, Aguilar and Ortiz1999), which currently lists 7365 I. batatas accessions, over 5500 of which are maintained in vitro (Anonymous, 2018c). Descriptive information is available for several of the accessions in the CIP collection. Leaf colour data from the present study were compared to information from both the GRIN and CIP databases.
Because leaf colorimeter readings were taken for each PI accession from only one of the three growing seasons, analyses were conducted to determine whether the colorimeter data sets for green leaves could be combined over years. The distributions of the colorimeter data sets (L*, a*, b*, C*, h* for both the abaxial and adaxial surfaces) were examined for kurtosis and skewness, and normalcy was determined by χ 2 analyses (Steel and Torrie, Reference Steel and Torrie1960). Then the homogeneity of variances for these data sets were determined using Levene's test (Levene, Reference Levene, Olkin, Ghurye, Hoeffding, Madow and Mann1960) to see if the data could be pooled over years. Paired t-tests were run to compare colour parameters for the adaxial versus abaxial leaf surfaces. The relationships between hue angle and lightness, and between hue angle and chroma were examined using regression analysis (SAS, 2009). Raw data and summary tables for individual sweetpotato PIs from this study are available in the US Public Domain at Ag Data Commons, USDA, ARS, National Agricultural Library (Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018b).
Results
Four PI accessions were determined to be duplicates; they were ‘Tainung 57’ (PI 531147 and PI 573295), ‘Wagabolige’ (PI 595888 and PI 633966), IITA TIS 9101 (PI 599385 and PI 606278) and ‘Helena’ (PI 606268 and PI 612696), with the first listed PI being used in this study. The leaves of NG7570 (PI 564142) showed severe bleaching symptoms throughout the season that apparently were due to clomazone injury (Harrison and Jackson, Reference Harrison and Jackson2011), so data from that accession were not used. Although some clomazone injury was apparent in several other PIs soon after transplantation (online Supplementary Table S1) (Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018b), these symptoms were absent by the time the leaf samples were taken for colour analyses. In addition, the leaves of ‘Topaz’ (PI 566659) and ‘W 51-19’ (PI 634424) showed severe deformation symptoms from virus infection in pots, plant beds and the field, so they also were deleted from these analyses, as virus infections are known to affect leaf colour and shape (Moyer and Salazar, Reference Moyer and Salazar1989). Therefore, data from only 730 PI accessions are reported.
Subjective colour ratings and colorimeter data for mature, early-season leaves showed that most accessions (725 of 730 PIs) had medium-to-dark green leaves (disregarding leaf vein colour) (Table 1). However, two PIs, ‘Vilca, Romero’ (PI 531126) and ‘Promesa’ (PI 531161), had totally purple leaves; and three PIs, ‘CN 1489-89’ (PI 556941), ‘CN 1367-2’ (PI 556947) and ‘Sulfur’ (PI 634402), had yellow or yellow-green (chartreuse) leaves (Fig. 1 and online Supplementary Fig. S1). This distribution of mature leaf colours is similar to what was reported in GRIN for 337 PIs under the descriptor MATLEAFCOL (Table 1) (Anonymous, 2018d). For the 725 accessions with primarily green leaves, analyses for normality indicated that there was moderate kurtosis for some of the parameters in 2013; however, none of the data sets were significantly skewed and the mean and median values were nearly identical in each case. For each of the colour parameters, analyses of homogeneity of variances indicated that variances were similar and that it was appropriate to pool data over years. The χ 2 analyses of the pooled data indicated that the combined data sets did not differ significantly from normal distributions.
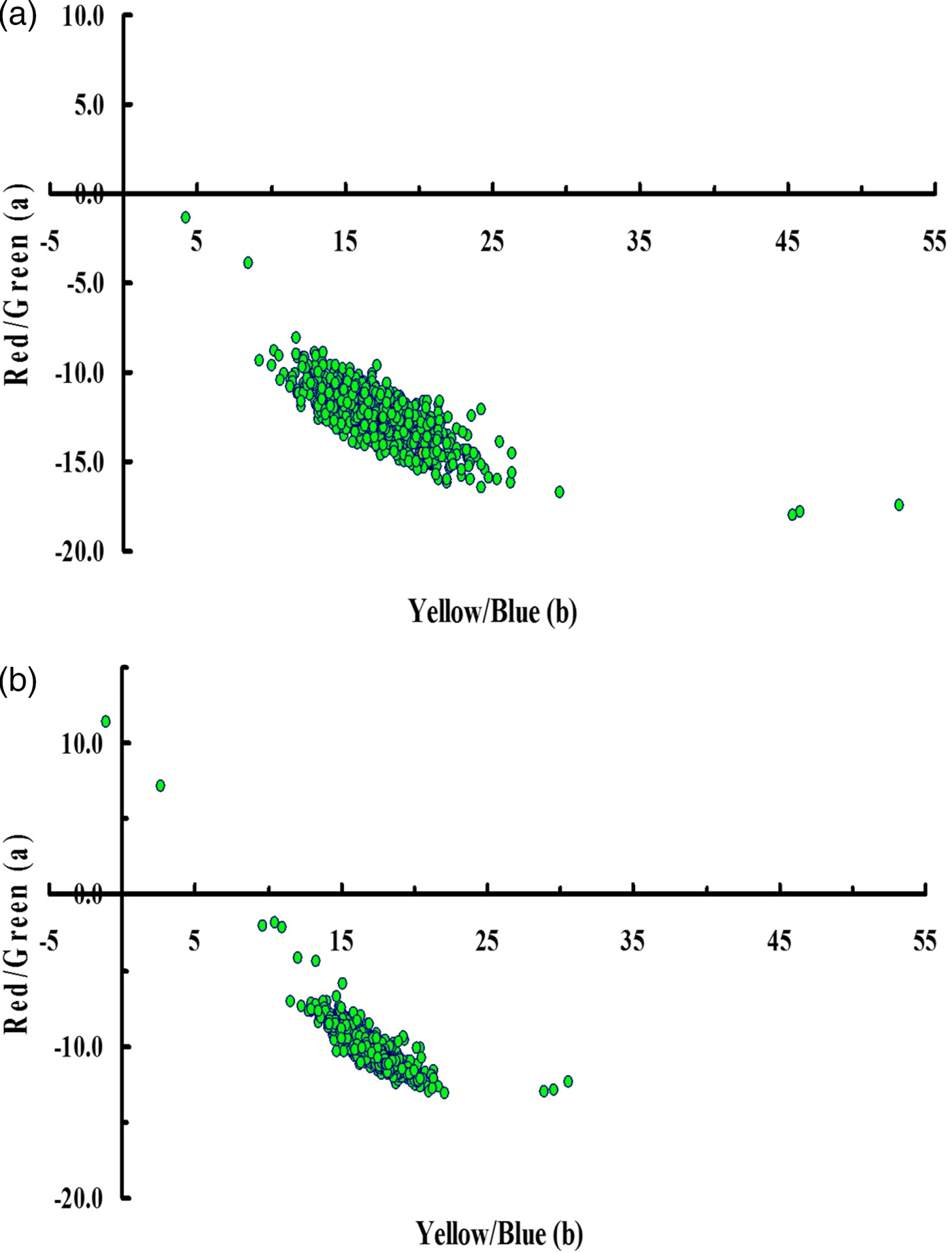
Fig. 1. Colour coordinates (a* = red-green axis and b* = yellow-blue axis) for the adaxial (a) and abaxial (b) surfaces of early-season, mature leaves from 730 sweetpotato plant introductions grown in field plots at Charleston, SC, USA, 2012-2014. The black data point (black circles) represent mean a* and mean b* values within each colour group.
Table 1. Number and per cent of plant introductions (PIs) for immature leaf colour (IMMLEAFCOL) and mature leaf colour (MATLEAFCOL) for the 730 PIs in this study and 338 PIs reported in the Germplasm Resources Information Network (GRIN) (Anonymous, 2018d)

The mean values for the red-green coordinate (a*) of the 725 PIs with green leaves were −12.5 for the adaxial surface and −10.4 for the abaxial surface of mature, field-grown leaves (Table 2, Fig. 1). For the green leaves of these same 725 PIs, the mean values for the blue-yellow coordinate (b*) were 17.2 for the adaxial surface and 17.3 for the abaxial surface. Values for hue angle (h*) for the abaxial surface of early-season mature green leaves were tightly clustered around a mean value of 120.9°; while h* values for the adaxial leaf surface were less tightly grouped around a mean of 126.2° (Fig. 2(a)). A paired t-test showed that these mean h* values were significantly different (t = 46.0, df = 729, P < 0.0001) between the adaxial and abaxial leaf surfaces. Interestingly, this difference in hue angle values between the abaxial and adaxial surfaces was due almost entirely to a significant difference in values for the red-green coordinate (a*) (t = 47.3, df = 724, P < 0.0001), as there was no significant difference (t = 0.47, df = 724, P = 0.642) in values for the blue-yellow coordinate (b*). Thus, in general, the adaxial leaf surface was slightly ‘greener’ (lower a*) than the abaxial leaf surface of green sweetpotato leaves in this study.
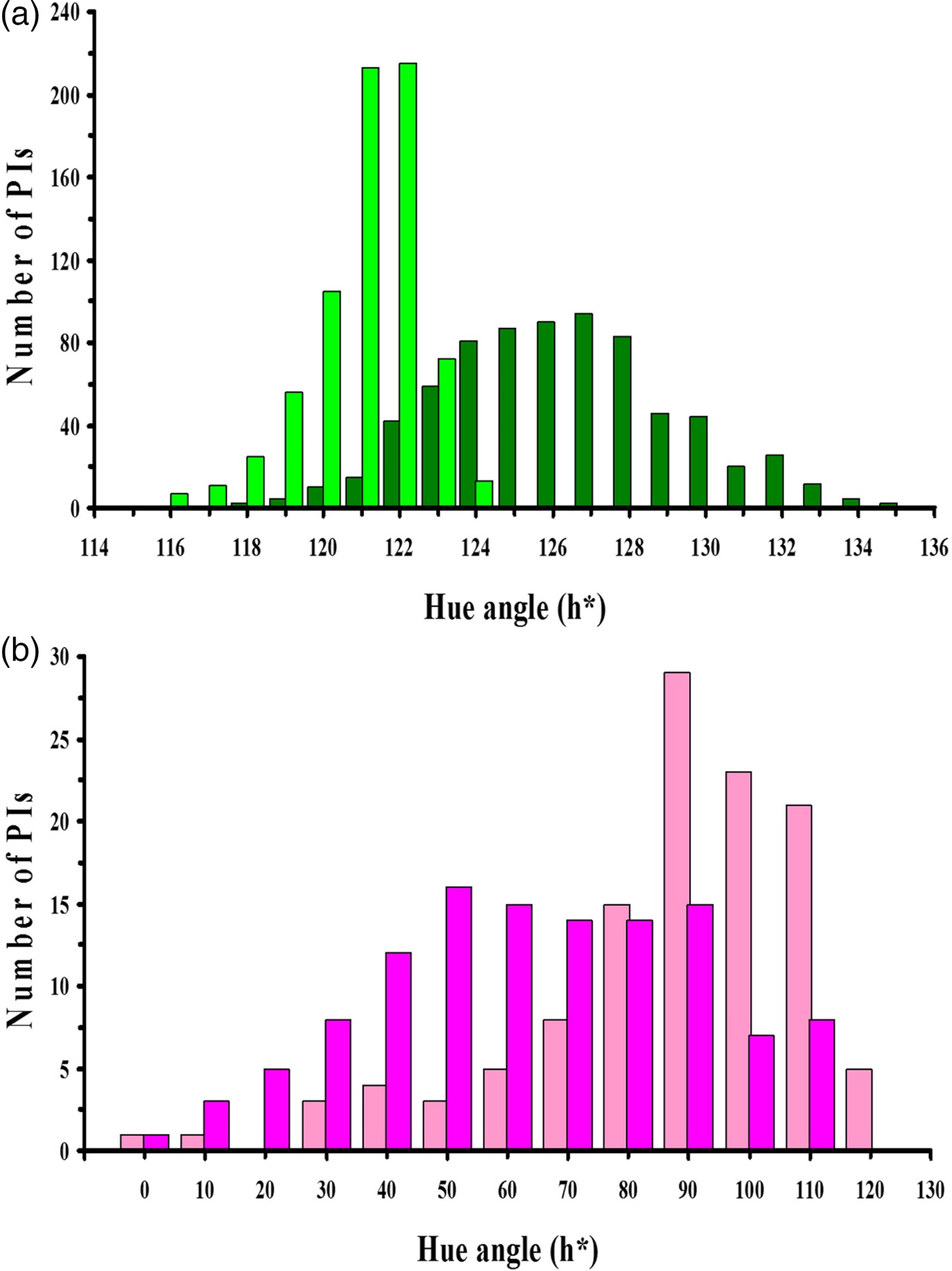
Fig. 2. Frequency distribution of hue angles (h*) for the adaxial (dark green) and abaxial (light green) surfaces for early-season, mature green leaves of 725 sweetpotato plant introductions (PIs) (a); and the adaxial (dark purple) and abaxial (light purple) surfaces for the late-season, mature purple leaves of 118 sweetpotato PIs (b) grown in field plots at Charleston, SC, USA, 2012-2014.
Table 2. Mean values, standard deviations and ranges of colour parameters for mature leaves of 730 sweetpotato PIs

The mean values for colour saturation (Chroma, C*) of mature green leaves were 21.3 for the adaxial leaf surface and 20.2 for the abaxial leaf surface of green leaves (Table 2). The mean values for lightness (L*) were 34.3 for the adaxial leaf surface and 47.1 for the abaxial leaf surface. According to a paired t-test, the mean value of C* for the adaxial surface was significantly higher (t = 12.13, df = 724, P < 0.0001) than it was for the abaxial surface of green leaves. Reciprocally, the mean value of L* for the adaxial surface was significantly lower (t = 166.9, df = 724, P < 0.0001) than it was for the abaxial surface of green leaves. There was a significant negative correlation between hue angle (x) and chroma (y) (y = −0.58x + 95.0, R 2 = 0.35, n = 725) for the adaxial leaf surface of green leaves, however this correlation was positive, but weak for the abaxial surface (y = 0.36x–23.6, R 2 = 0.16, n = 725). Likewise, there were non-significant correlations between hue angle (x) and lightness (y) for both the adaxial (y = 0.06x + 26.8, R 2 = 0.003, n = 725) and abaxial (y = 0.76x–44.7, R 2 = 0.13, n = 725) surfaces of green leaves. Thus, in general, the abaxial surface was brighter (higher L*) but slightly less colour-saturated (lower chroma, C*) than the adaxial leaf surface of green sweetpotato leaves in this study.
The values of a* for the three PIs with yellow (or chartreuse) leaves averaged −17.7 for the adaxial surface and −12.7 for the abaxial surface of early-season, mature field-grown leaves (Table 2). For these same PIs, the mean values for b* were 48.0 for the adaxial surface and 29.7 for the abaxial surface. The mean values for h* for the yellow leaves were 110.4° for the adaxial and 113.2° for the abaxial leaf surface. C* averaged 51.2 for the adaxial leaf surface and 32.3 for the abaxial leaf surface; while L* range averaged 59.3 for the adaxial leaf surface and 63.5 for the abaxial surface of yellow leaves.
The mean values of a* for 118 PIs with purple leaves sampled late in the season were 2.3 for the adaxial surface and −1.0 for the abaxial leaf surface (Table 2, online Supplementary Fig. S2). For these same 118 PIs, b* averaged 6.2 for the adaxial leaf surface and 12.7 for the abaxial leaf surface. C* averaged 7.9 for the adaxial leaf surface and 13.9 for the abaxial leaf surface; while L* averaged 28.2 for the adaxial surface and 43.1 for the abaxial leaf surface of purple leaves. Values of h* were scattered over a large range for both the adaxial (4.1–115.0°, ![]() $\bar{x}$ = 64.4°) and abaxial surfaces (1.9–118.7°,
$\bar{x}$ = 64.4°) and abaxial surfaces (1.9–118.7°, ![]() $\bar{x}$ = 87.0°) of late-season purple leaves (Fig. 2(b)). Although statistically significant, there was only a very weak correlation between hue angles of the abaxial (x) and adaxial (y) leaf surfaces for purple (y = −0.22x + 82.3, R 2 = 0.04, n = 120) leaves (combined for two early-season ratings and 118 late-season accessions). Similar to green leaves, there were significant differences (paired t-test) between the adaxial and abaxial purple leaf surfaces for h* (t = 5.93, df = 119, P < 0.0001), a* (t = 8.08, df = 119, P < 0.0001), C* (t = 20.2, df = 119, P < 0.0001) and L* (t = 47.6, df = 119, P < 0.0001). However, unlike green leaves, there also was a significant difference between the adaxial and abaxial purple leaf surfaces for b* (t = 20.4, df = 119, P < 0.0001). There was a weak, but significant, negative correlation between hue angle (x) and colour intensity (chroma) (y) for the adaxial surface of purple leaves (y = −0.02x + 9.3, R 2 = 0.06, n = 120). In contrast, there was a moderately strong positive correlation between hue angle and colour intensity (y) for the abaxial surface of purple leaves (y = 0.12x + 3.59, R 2 = 0.54, n = 120). In addition, there was a strong positive correlation (y = 0.16x + 29.2, R 2 = 0.83, n = 120) between hue angle and lightness for the abaxial surface, but a much weaker negative correlation (y = −0.036x + 30.5, R 2 = 0.12, n = 120) between these parameters for the adaxial surface.
$\bar{x}$ = 87.0°) of late-season purple leaves (Fig. 2(b)). Although statistically significant, there was only a very weak correlation between hue angles of the abaxial (x) and adaxial (y) leaf surfaces for purple (y = −0.22x + 82.3, R 2 = 0.04, n = 120) leaves (combined for two early-season ratings and 118 late-season accessions). Similar to green leaves, there were significant differences (paired t-test) between the adaxial and abaxial purple leaf surfaces for h* (t = 5.93, df = 119, P < 0.0001), a* (t = 8.08, df = 119, P < 0.0001), C* (t = 20.2, df = 119, P < 0.0001) and L* (t = 47.6, df = 119, P < 0.0001). However, unlike green leaves, there also was a significant difference between the adaxial and abaxial purple leaf surfaces for b* (t = 20.4, df = 119, P < 0.0001). There was a weak, but significant, negative correlation between hue angle (x) and colour intensity (chroma) (y) for the adaxial surface of purple leaves (y = −0.02x + 9.3, R 2 = 0.06, n = 120). In contrast, there was a moderately strong positive correlation between hue angle and colour intensity (y) for the abaxial surface of purple leaves (y = 0.12x + 3.59, R 2 = 0.54, n = 120). In addition, there was a strong positive correlation (y = 0.16x + 29.2, R 2 = 0.83, n = 120) between hue angle and lightness for the abaxial surface, but a much weaker negative correlation (y = −0.036x + 30.5, R 2 = 0.12, n = 120) between these parameters for the adaxial surface.
Nearly one-half (46%) of the 730 PIs received a subjective rating of zero for purple leaf colouration in field plots late in the seasons of 2012 and 2013 (Fig. 3). These 336 PIs showed no evidence of purple colouration throughout the season. Another 46% of the PIs had an average rating of two or less, indicating only purple colouration on the immature leaves. However, the remaining 58 PIs (8%) showed extensive purple colouration on mature leaves late in the season, with two PIs (PI 531126, ‘Vilca, Romero’; and PI531161, ‘Promesa’) having an average rating of 5 with over 50% of the leaves being totally purple.

Fig. 3. Frequency distribution of subjective rating for purple leaf colouration for 730 sweetpotato plant introductions grown in field plots at Charleston, SC, USA, 2012-2014. Plants were rated 0-5, with 0 = all green leaves, 1 = some purple ‘bronzing’ on new leaves, 2 = distinct purple colouration on small leaves, 3 = distinct purple colouration on some larger leaves, 4 = many purple leaves and 5 = over 50% of the leaves distinctly purple.
The distribution of ratings for abaxial leaf vein pigmentation is shown in Table 3. About a quarter of the PIs (178) had all of their leaf veins either yellow or green, a quarter of the PIs (188) had all of their leaf veins purple, and roughly half of the PIs (364) had lesser levels of purple pigmentation on their abaxial leaf veins (Table 3). This distribution for leaf vein pigmentation is similar to the one published in GRIN for the descriptor LFVEINCOL (Anonymous, 2018d) (Table 3). Although not rated in the present study, the GRIN system also contains data for petiole pigmentation (PETIOLEPIG, 292 PIs) and vine pigmentation (VINEPIG, 291 PIs) (Anonymous, 2018d). In those data sets, only 15.4% of the leaf petioles were classified as moderately purple or purple; whereas 39.5% of vines were classified as moderately purple or purple (Table 3).
Table 3. Total number and per cent of PIs categorized for each descriptor for abaxial leaf vein pigmentation (LFVEINCOL), leaf petiole pigmentation (PETIOLEPIG) and vine pigmentation (VINEPIG) for this study and as reported in the Germplasm Resources Information Network (GRIN) (Anonymous, 2018d)

Discussion
Visual perception of leaf colour is inherently subjective, as it depends on the light conditions in which the leaves are being observed and on the observer (Pathare et al., Reference Pathare, Opara and Al-Said2013). Colour charts, such as the Munsell and the Royal Horticultural Society (RHS) colour charts, have been used extensively to record colours of plant materials (Tucker et al., Reference Tucker, Maciarello and Tucker1991). However, these charts also rely on our subjective colour perceptions, and they can be time consuming and difficult to use (Voss, Reference Voss1992). Therefore, less subjective, quantitative methods for describing plant colours have been developed. Colorimetry has been used to quantify the colours of plant materials (McGuire, Reference McGuire1992; Pathare et al., Reference Pathare, Opara and Al-Said2013; Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018a). Colorimetry is a system to reduce light spectral data to human perception of colour using three-dimensional physical coordinates, such as the CIE 1931 XYZ, CIE L*C*h or CIE 1976 L*a*b* colour space tristimulus values (Wyszecki and Stiles, Reference Wyszecki and Stiles1982). The eyes of humans possess three independent channels for conveying colour information, and these are derived from the three types of retinal cone cells that have different absorption spectra (Hunter and Harold, Reference Hunter and Harold1987). People with normal vision see different colours mediated by interactions among these three types of colour-sensing cone cells (trichromatic colour vision) (Wyszecki and Stiles, Reference Wyszecki and Stiles1982; Hunter and Harold, Reference Hunter and Harold1987). Voss (Reference Voss1992) was able to correlate colorimeter readings with values from the RHS colour chart. Unlike other models, colour perception is uniform in the L*a*b* colour space, meaning that ‘the Euclidean distance between two colours corresponds approximately to the colour difference perceived by the human eye’ (Hunt, Reference Hunt1991). As such, the L*a*b* colour space has been used successfully in several horticultural studies (Ameny and Wilson, Reference Ameny and Wilson1997; Arias et al., Reference Arias, Lee, Logendra and Janes2000; Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018a). Because light reflected from sweetpotato leaves is measurable both in intensity and in wavelength (Pathare et al., Reference Pathare, Opara and Al-Said2013), the use of these quantitative methods were applied in the present study. We conclude that the L*a*b* and CIE L*C*h colour spaces are appropriate to characterize the leaf colour of sweetpotato accessions, as they provide consistent quantitative values that are easy to analyse and interpret. Although Huamán (Reference Huamán1991) did not list colorimeter values as a descriptor for leaf or storage root colour, we believe colorimetry data provide a reliable, objective measure of colour parameters that should be included in the Sweetpotato Ontology (Anonymous, 2018e).
The primary pigments responsible for colouration in higher plants are chlorophylls (green), carotenoids (yellow, orange), anthocyanins (red, blue, purple) and betalains (red); and each of these classes of pigments, except betalains, are found in sweetpotatoes (Ameny and Wilson, Reference Ameny and Wilson1997; Hue et al., Reference Hue, Boyce and Somasundram2011). Leaf colours are determined by the spectral properties of these pigments, which can be measured directly using high-resolution spectroscopy (Ustin et al., Reference Ustin, Gitelson, Jacquemoud, Schaepman, Asner, Gamon and Zarco-Tejada2009). In addition, quantitative levels of leaf pigments can be measured through chemical analyses, although those methods are often costly and time consuming (Islam et al., Reference Islam, Yoshimoto, Terahara and Yamakawa2002a).
The leaves of higher plants are green due to chlorophyll that allows plants to maximum use of the visible light spectrum during photosynthesis. Therefore, it is not surprising that for the 337 sweetpotato accessions currently described in the GRIN system under the descriptor, mature leaf colour (MATLEAFCOL), 333 (98.8%) had green leaf lamina (Anonymous, 2018b, d). Indeed, for the present study, which included those 337 PIs plus 388 additional PIs, the predominant colour for healthy, mature, non-senescent sweetpotato leaves is green (725 of 730 PIs = 99.3%). This is similar to what is listed for the CIP collection in Peru, where most of the I. batatas accessions (3017 of 3050 = 98.9%) are listed as having predominately green mature leaves (Anonymous, 2018c). However, for both the CIP and USDA-ARS collections, there are many accessions that have mature green leaves with purple leaf veins or some purpling at the leaf edges (Anonymous, 2018c). Thus, we were careful to take our colorimeter readings from an area of uniform leaf lamina, thus avoiding major leaf veins. Consequently, the colour parameters observed for green leaves in the present study are tightly grouped around the mean values with low variances (Fig. 1). This is in contrast to the data we reported previously for the periderm and stele of storage roots for these same accessions where there was much more variation in colour parameters (Jackson et al., Reference Jackson, Harrison, Jarret and Wadl2018a).
Although the primary colour of healthy, mature, non-senescent sweetpotato leaves is green, there are a few genotypes with yellowish leaves. Yellow leaf colours are primarily due to carotenoids that include the xanthophylls (lutein and zeaxanthin) and carotenes (α-carotene, β-carotene and lycopene) (Menelaou et al., Reference Menelaou, Kachatryan, Losso, Cavalier and LaBonte2006; Khoo et al., Reference Khoo, Prasad, Kong, Jiang and Ismail2011). Only one accession, ‘CN 1367-2’ (PI 556947 from Taiwan), of 337 PIs in GRIN is described as having mature, fully yellow leaves, and one accession, ‘Sulfur’ (PI 634402 from the USA), is described as having mature yellow-green (chartreuse) leaves (Anonymous, 2018d). Our examinations of these PIs confirm these earlier observations, but we also identified a third accession, ‘CN 1489-89’ (PI 556941 from Taiwan), with mature, fully yellow leaves (Fig. 1 and online Supplementary Fig. S1). However, no leaf colour data are noted in GRIN for this accession (Anonymous, 2018d). Only one accession, ‘CN 1367-2’ (PI 556947), is noted in GRIN as having a fully yellow immature leaf colour (IMMLEAFCOL), but 28 accessions were categorized as having yellow-green immature leaves. However, the colour of immature leaves was not measured in the present study. These findings are similar to those of materials in the CIP collection, where five of 3050 accessions (0.2%) have been categorized as having mature yellow-green leaves, and 29 of 2930 accessions (1.0%) have been categorized as having yellow-green immature leaves (Anonymous, 2018c).
Leaf colour can be an extremely important characteristic in ornamental sweetpotatoes. There are currently several ornamental cultivars of sweetpotato having yellow, light-green or variegated green/yellow leaves. Examples of these ornamental cultivars include ‘Margarita’ (Armitage and Garner, Reference Armitage and Garner2001), ‘Sweet Caroline Light Green’ (Pecota et al., Reference Pecota, Yencho and Pierce2004b), ‘Sweet Caroline Sweetheart Light Green’ (Yencho et al., Reference Yencho, Pecota and Hancock2008a), ‘Sweet Caroline Green Yellow’ (Yencho and Pecota, Reference Yencho and Pecota2008b) and ‘CH-1’ (Weng et al., Reference Weng, Chien, Jiang, Shih and Chen2011). However, these ornamental cultivars are not maintained in the USDA-ARS sweetpotato germplasm collection at PGRCU.
Leaf senescence and environmental stresses can lead to chlorophyll degradation and leaf yellowing (Huang et al., Reference Huang, To, Yap, Chiang, Suen and Chen2001), but this is fundamentally different from the normal yellow leaf colours of the cultivars discussed above. Stress conditions such as nutrient deficiencies, plant growth regulators, water deficits, wounding, inadequate light, excess UV, pollutants (e.g. ozone) or other environmental factors can all affect levels of pigments in leaves (Ravi and Saravanan, Reference Ravi, Saravanan, Nedunchezhiyan and Byju2012). For example, Rodríguez-Deflin et al. (Reference Rodríguez-Delfín, Posadas, León-Velarde, Mares and Quiroz2011) reported that total chlorophyll increased with increased salinity but decreased under water stress conditions. In addition, sweetpotato leaves infected with viruses or other foliar diseases may have lower levels of chlorophyll and a reduced photosynthetic rate, or they may exhibit chlorosis (yellowing), spotting (mosaic), vein-clearing or other abnormal leaf colour characteristics compared with healthy leaves (Gibson et al., Reference Gibson, Mpembe, Alicai, Carey, Mwanga, Seal and Vetten1998; Clark et al., Reference Clark, Ferrin, Smith and Holmes2013). Feeding by sucking insects, such as whiteflies and aphids, weakens sweetpotato plants by removing nutrients necessary for plant growth and maintenance, and can lead to changes in leaf appearance, such as yellowing, necrosis, chlorosis, discolouration, stunting, wilting and an acceleration of senescence (Quisenberry and Ni, Reference Quisenberry, Ni, van Emden and Harrington2007). Thus, leaf colour parameters can be used as a measure of plant health, and this study gives a base line for the range of colour parameters found for healthy sweetpotato leaves. In addition, it is very important that leaf colour characteristics for phenotyping be measured on healthy, non-senescent leaves.
Purple colour is expressed in storage roots, vines and leaves of sweetpotatoes (Islam et al., Reference Islam, Yoshimoto, Terahara and Yamakawa2002a; Luo et al., Reference Luo, Zhou, Yang, Wang and Zhang2018), and several purple-fleshed varieties have been developed (Tanaka et al., Reference Tanaka, Ishiguro, Oki and Okuno2017). This purple colouration is due to anthocyanin pigments, with cyanidin derivatives being more common in sweetpotato leaves than peonidins (Islam et al., Reference Islam, Yoshimoto, Terahara and Yamakawa2002a, Reference Islam, Yoshimoto, Yahara, Okuno, Ishiguro and Yamakawab). Anthocyanins are water-soluble vacuolar flavonoid pigments synthesized via the phenylpropanoid pathway (Gould et al., Reference Gould, Davies and Winefield2009). When the sugars produced by the chlorophyll cannot be deposited in roots, stalks and new leaves, sugars are converted to anthocyanins, which are red to purple in colour. They impart red, blue or purple colouration in higher plants, however some anthocyanin derivatives are colourless (Ojong et al., Reference Ojong, Njiti, Guo, Gao, Besong and Barnes2008). It is believed that anthocyanins function as photo-protectants by absorbing UV-B and by acting as light screens to modulate the amount of light absorption (Close and Beadle, Reference Close and Beadle2003). Thus, anthocyanins affect both the quantity and quality of light reaching the chloroplasts (Steyn et al., Reference Steyn, Wand, Holcroft and Jacobs2002). Anthocyanins may also play a more direct role in plant defence by serving as a visual warning signal to potential herbivores (Lev-Yadun and Gould, Reference Lev-Yadun, Gould, Winefield, Davies and Gould2008).
Of the 337 accessions in GRIN with data for the descriptor mature leaf colour (MATLEAFCOL), only two PIs (0.6%) are noted as totally purple. We identified the same two accessions, ‘Vilca, Romero’ (PI 531126 from Peru) and ‘Promesa’ (PI531161 from Peru), as having early-season mature purple leaves (Fig. 1 and online Supplementary Fig. S1). The CIP sweetpotato collection notes nine of 3050 accessions (0.3%) as having totally purple leaves, although 19 other accessions (0.6%) were categorized as having lesser levels of purple leaf pigmentation (Anonymous, 2018c). There are several purple-leaf ornamental cultivars that are not listed in the USDA or the CIP sweetpotato germplasm collections. Some of the more popular ones are ‘Blackie’, ‘Black Heart’ (also known as ‘Ace of Spades’), ‘Midnight Lace’ (Carey et al., Reference Carey, Whipker, Bradley and Buhler2012), ‘Sweet Caroline Purple’ (Pecota et al., Reference Pecota, Yencho and Pierce2004a), ‘Sweet Caroline Bewitched Purple’ (Yencho and Pecota, Reference Yencho and Pecota2008a) and ‘Sweet Caroline Sweetheart Purple’ (Yencho et al., Reference Yencho, Pecota and Hancock2008b). Many new ornamental varieties with a wide range of leaf colours and shapes are now available, including those with bronze/copper (Pecota et al., Reference Pecota, Yencho and Pierce2004c) and red (Pecota et al., Reference Pecota, Yencho and Hancock2007; Yencho and Pecota, Reference Yencho and Pecota2008c) leaf colours, and new ornamental varieties are being developed (Carey et al., Reference Carey, Whipker, Bradley and Buhler2012).
In many plant species, purple leaf colouration is transient, and anthocyanins are found more frequently in young, expanding leaves and in old, senescing leaves that are more susceptible to photo-inhibition (Karageorgou and Manetas, Reference Karageorgou and Manetas2006). This is true for sweetpotatoes, where many genotypes express purple colouration in their new leaves, although for most PIs, the purple colour dissipates as the leaves mature through mid-season. Although the colour of immature leaves was not measured quantitatively during the present study, we observed many PIs having young leaves with purple colouration. Fifty-one accessions are noted in GRIN as having purple immature leaf colour (IMMLEAFCOL). This in planta anthocyanin degradation has been reported for other species (Oren-Shamir, Reference Oren-Shamir2009). Sweetpotato leaves may still contain anthocyanins, but when the levels of these pigments become low, they are masked by the green pigment of chlorophyll and the leaves appear green (Islam et al., Reference Islam, Yoshimoto, Terahara and Yamakawa2002a). Thus, sweetpotato leaves may contain anthocyanins at levels that are too low to be visible to the naked eye (Carvalho et al., Reference Carvalho, Cavaco, Carvalho and Duque2010). Interestingly, one accession, ‘Promesa’ (PI 531161) (online Supplementary Fig. S1), had the opposite pattern with the young leaves being green and the mature leaves being purple.
We recently published on the genetic diversity of the USDA-ARS sweetpotato collection (Wadl et al., Reference Wadl, Olukolu, Branham, Jarret, Yencho and Jackson2018). In that study, 417 USDA sweetpotato accessions originating from eight broad geographical regions (Africa, Australia, Caribbean, Central America, Far East, North America, Pacific Islands and South America) were identified and the genetic diversity was determined using a genotyping-by-sequencing protocol (GBSpoly) for 32,784 segregating single nucleotide polymorphisms (SNPs). This study (Wadl et al., Reference Wadl, Olukolu, Branham, Jarret, Yencho and Jackson2018) concluded that although there was a high degree of mixed ancestory, the accessions could be clustered geographically based on genetic distance. In the present study, we also looked to see if there was clustering or other relationships between the colour parameters we measured and geographical origin. Using GRIN information (Anonymous, 2018d), we were able to assign a geographic region of origin to the 725 accessions that had primarily green leaves. However, analysis of variance showed that there were no significant relationships between any of the colour parameters (L*, a*, b*, C* and h*) and geographical origin.
Growth stage (Hue et al., Reference Hue, Boyce and Somasundram2011) and environmental factors (Islam et al., Reference Islam, Jalaluddin, Garner, Yoshimoto and Yamakawa2005; Mortley et al., Reference Mortley, Burrell, Bonsi, Hill and Morris2009) affect the expression of pigment levels in sweetpotato leaves. Late in the season, many accessions again showed purple leaf colouration as anthocyanin production was increased. This may be due to changes in environmental factors, such as low temperatures (Oren-Shamir and Levi-Nissim, Reference Oren-Shamir and Levi-Nissim1997), photoperiod (Carvalho et al., Reference Carvalho, Cavaco, Carvalho and Duque2010), nutrient deficiencies (Ravi and Saravanan, Reference Ravi, Saravanan, Nedunchezhiyan and Byju2012; Clark et al., Reference Clark, Ferrin, Smith and Holmes2013) and water stress (Close and Beadle, Reference Close and Beadle2003), that can affect production of anthocyanins (de Pascual-Teresa and Sanchez-Ballesta, Reference de Pascual-Teresa and Sanchez-Ballesta2008; Oren-Shamir, Reference Oren-Shamir2009). During leaf senescence, chlorophylls are broken down leading to leaf yellowing (Huang et al., Reference Huang, To, Yap, Chiang, Suen and Chen2001) and the expression of other pigments such as anthocyanins (Carvalho et al., Reference Carvalho, Cavaco, Carvalho and Duque2010). Anthocyanin production is maximized at moderate temperatures and full sun (Islam et al., Reference Islam, Jalaluddin, Garner, Yoshimoto and Yamakawa2005). However, it appears that there are genotypic differences in levels of this expression within the sweetpotato germplasm collection. While many PIs showed increased purple foliage colouration as the season progressed, other PIs were unaffected and remained green until plants were harvested in the fall.
The nutritional and health benefits of sweetpotato leaves are well documented (Islam, Reference Islam2006; Yoshimoto et al., Reference Yoshimoto, Kurata, Okuno, Ishiguro, Yamakawa, Tsubata, Mori and Takagaki2006; Johnson and Pace, Reference Johnson and Pace2010; Sun et al., Reference Sun, Mu, Xi, Zhang and Chen2014). For example, total phenolic content of sweetpotato leaves has been correlated with free radical scavenging activity (Islam et al., Reference Islam, Yoshimoto, Ishiguro and Yamakawa2003), which is known to have antioxidant, antimicrobial, anti-carcinogenic, anti-diabetic and anti-inflammatory effects (Yoshimoto et al., Reference Yoshimoto, Kurata, Okuno, Ishiguro, Yamakawa, Tsubata, Mori and Takagaki2006; Lee et al., Reference Lee, Park, Ahn, Kim, Chung, Jeong and Bang2007; Karna et al., Reference Karna, Gundala, Gupta, Shamsi, Pace, Yates, Narayan and Aneja2011). However, the nutritive and medicinal values of the leaves and shoots of several sweetpotato cultivars have been found to be quite variable (Islam et al., Reference Islam, Yoshimoto, Yahara, Okuno, Ishiguro and Yamakawa2002b; Truong et al., Reference Truong, McFeeters, Thompson, Dean and Shofran2007; Sun et al., Reference Sun, Mu, Xi, Zhang and Chen2014). It has been shown that leaf colour affects the nutritional quality of sweetpotato leaves (Chen et al., Reference Chen, Li, Lin, Hsu, Shieh and Liu2005; Chao et al., Reference Chao, Huang and Hsieh2013). Acylated anthocyanins from sweetpotato have important anti-oxidant and anti-inflammatory properties that are associated with health benefits in humans (Mohanraj and Sivasankar, Reference Mohanraj and Sivasankar2014; Luo et al., Reference Luo, Zhou, Yang, Wang and Zhang2018). Purple sweetpotato leaves not only contain higher levels of anthocyanins (primarily cyanidin) than green leaves, they also possess higher levels of other antioxidants with free-radical scavenging activity, such as polyphenols and flavonoids, and they have a relatively high ORAC (Oxygen Radical Absorbance Capacity) and TEAC (Trolox Equivalent Antioxidant Capacity) values (Chao et al., Reference Chao, Lin, Lin, Liu, Hsu, Yang and Lai2014). Consumption of purple sweetpotato leaves modulates various immune functions in humans (Chen et al., Reference Chen, Li, Lin, Hsu, Shieh and Liu2005).
Leaf colour may affect host-plant preference by insect pests, or the incidence of insect-transmitted diseases in plants (Prokopy and Owens, Reference Prokopy and Owens1983). For hemipteran pests, yellow or green leaves are preferred over darker purple, red or brown leaves (Prokopy and Owens, Reference Prokopy and Owens1983). In addition, it has been shown that herbivore feeding damage is correlated with leaf colouration. Darker leaves receive less damage because darker coloured pigments, such as anthocyanins, may serve as a signal to repel herbivores (Lev-Yadun and Gould, Reference Lev-Yadun, Gould, Winefield, Davies and Gould2008). Other studies have shown that anthocyanins can directly affect insect feeding and development (Johnson et al., Reference Johnson, Berhow and Dowd2008), or play an indirect role in pest resistance by acting as phyto-protectants of other defence compounds in the leaves (Gould, Reference Gould2004). However, levels of phenolic compounds also are higher in darker pigmented leaves, and many phenolic compounds have insecticidal properties (Karageorgou and Manetas, Reference Karageorgou and Manetas2006). However, to date, there have been no comprehensive studies on the importance of leaf colour on disease or insect infestations in sweetpotatoes per se.
Sweetpotato leaves have been investigated as a source of natural food colourants (Hue et al., Reference Hue, Boyce and Somasundram2014). Anthocyanins, lutein, β-carotene and other carotenoids can be extracted from sweetpotatoes (Akoetey et al., Reference Akoetey, Britain and Morawicki2017). Lutein potentially could replace the artificial food colourant tartrazine (FD&C Yellow 5) (Hue et al., Reference Hue, Boyce and Somasundram2014; Akoetey et al., Reference Akoetey, Britain and Morawicki2017). Anthocyanins are relatively unstable compounds and must be handled carefully to retain their colour (de Pascual-Teresa and Sanchez-Ballesta, Reference de Pascual-Teresa and Sanchez-Ballesta2008). In addition, the use of anthocyanins and polyphenols from sweetpotato leaves as natural antioxidant food additives has been investigated (Hue et al., Reference Hue, Boyce and Somasundram2012).
Whereas, leaf colouration is relatively unimportant when breeding for improved sweetpotato storage root characteristics, knowledge of leaf colour can serve several useful purposes. For instance, this study provides baseline data for breeders to ascertain whether new breeding lines fall within the normal range of acceptable leaf colours. Leaf colour is likely to be quite important when breeding cultivars for use as a green vegetable, as levels of important nutrients and anti-oxidative compounds are associated with leaf pigmentation (Chen et al., Reference Chen, Li, Lin, Hsu, Shieh and Liu2005; Chao et al., Reference Chao, Huang and Hsieh2013; Luo et al., Reference Luo, Zhou, Yang, Wang and Zhang2018). As described above, leaf colour can be an indicator of plant health. In addition, leaf colour is a critical consideration when breeding for ornamental sweetpotatoes.
The lack of detailed phenotyping data limits the use of the genetic resources from the genebanks of many crops globally (Dwivedi et al., Reference Dwivedi, Scheben, Edwards, Spillane and Ortiz2017). The applied value of a germplasm collection is increased when its plant materials are fully characterized, evaluated and properly documented. In this study, we have characterized a collection of sweetpotato germplasm for an important leaf descriptor, mature leaf colour. Deviations from the normal ranges of the evaluated colour parameters could be used to identify plants that are senescing or are under stress from inadequate nutrition, insect feeding, diseases or environmental factors. The ability to utilize automated imaging platforms and computer-assisted analysis tools have been developed and are being used to detect slight to severe deviations when plants are subjected to abiotic and biotic stressors (Simko et al., Reference Simko, Jimenez-Berni and Sirault2017). Plant colour also can be analysed using digital imaging software to generate colour space parameters (Humplík et al., Reference Humplík, Lazár, Husiíková and Spíchal2015), and our photographic record of each sweetpotato PI also might be useful in this regard. The leaf colour information provided in our study serves as baseline for the normal ranges of colour parameters for healthy, mature leaves among a diverse set of genotypes from the USDA sweetpotato collection. The USDA sweetpotato germplasm collection includes many PIs that might be of use in a breeding programme aimed at developing sweetpotatoes with specific foliage characteristics, either for ornamental purposes or as a green vegetable for animal or human consumption. Leaf colour characteristics would be an important consideration during these breeding efforts because of the association of leaf colour parameters with nutritional or aesthetic characteristics.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262119000042.
Acknowledgements
The authors thank Ty Phillips, Sarah Moon and Merrelyn Spinks for their excellent technical support. This project was partially funded for 2 years by the USDA, REE, ARS, Office of National Programs, Crop Production and Protection as Germplasm Evaluation Project No. 6659-22000-024-00D, ‘Evaluating the Integrity of the Sweetpotato Germplasm Collection’.