Introduction
A great number of mutants have been generated and widely used in plant breeding programmes, which has successfully led to the release of more than 3200 new varieties in ~200 plant species (http://mvgs.iaea.org/). However, it was not until the beginning of this century that molecular genetic studies started shedding light on the nature of induced mutations. The integration of molecular and genomic techniques with induced mutagenesis, such as TILLING (Targeting Induced Local Lesions In Genome), and their applications have already been critically reviewed in the past few years (McCallum et al., Reference McCallum, Comai, Greene and Henikoff2000; Waugh et al., Reference Waugh, Leader, McCallum and Caldwell2006; Pathirana, Reference Pathirana2011; Sikora et al., Reference Sikora, Chawade, Larsson, Olsson and Olsson2011). The present review focuses on the molecular nature of mutants induced by various mutagens with the aim of helping researchers design better research strategies.
Chemically induced mutations
Alkylating agents such as ethyl methanesulphonate (EMS), 1-methyl-1-nitrosourea and 1-ethyl-1-nitrosourea are the most commonly used chemical mutagens in plants (Leitao, Reference Leitao, Shu, Forster and Nakagawa2012). EMS induces almost 100% GC>TA substitutions, while other mutagens generate >90% such mutations (McCallum et al., Reference McCallum, Comai, Greene and Henikoff2000; Greene et al., Reference Greene, Codomo, Taylor, Henikoff, Till, Reynolds, Enns, Burtner, Johnson, Odden, Comai and Henikoff2003; Cooper et al., Reference Cooper, Till, Laport, Darlow, Kleffner, Jamai, El-Mellouki, Liu, Ritchie, Nielsen, Bilyeu, Meksem, Comai and Henikoff2008; Sikora et al., Reference Sikora, Chawade, Larsson, Olsson and Olsson2011).
TILLING studies have also enabled the estimation of mutation frequencies in different crops (Table 1). Higher mutation frequencies are achieved with increasing ploidy; for example, one mutation per 30 kb has been attained in hexaploid wheat and oats, while the maximum has been one mutation per 89 kb in diploid Arabidopsis (Table 1). Mutation frequencies can be increased by the optimization of treatment doses or selection of chemical mutagens, as has been shown for Arabidopsis and rice (Greene et al., Reference Greene, Codomo, Taylor, Henikoff, Till, Reynolds, Enns, Burtner, Johnson, Odden, Comai and Henikoff2003; Till et al., Reference Till, Cooper, Tai, Colowit, Greene, Henikoff and Comai2007; Suzuki et al., Reference Suzuki, Eiguchi, Kumamaru, Satoh, Matsusaka, Moriguchi, Nagato and Kurata2008; Martín et al., Reference Martín, Ramiro, Martínez-Zapater and Alonso-Blanco2009). In practice, a higher mutation frequency may not always be effective because the proportion of non-synonymous mutations could become less as reported by Gottwald et al. (Reference Gottwald, Bauer, Komatsuda, Lundqvist and Stein2009). Each report has presented a great variation in mutation frequencies among different genes; for example, Gottwald et al. (Reference Gottwald, Bauer, Komatsuda, Lundqvist and Stein2009) reported mutation frequencies ranging from 1 per 870 kb for Mlo9 to 1 per 200 kb for HvHox1 in barley.
Table 1 Mutation frequencies and estimated number of mutations in a single M2 plant derived from chemical mutagenesis
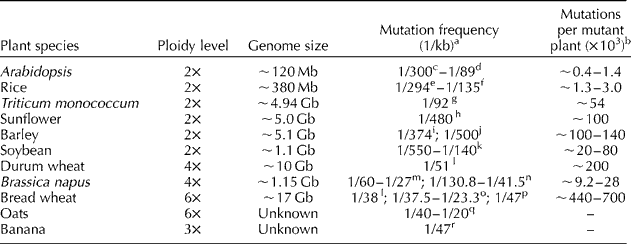
a Mutation frequency is estimated as the number of mutations per kb of DNA sequence. In most cases, the average is quoted, e.g. 1/300 reported by Greene et al. (Reference Greene, Codomo, Taylor, Henikoff, Till, Reynolds, Enns, Burtner, Johnson, Odden, Comai and Henikoff2003) and 1/89 reported by Martín (Reference Martín, Ramiro, Martínez-Zapater and Alonso-Blanco2009) for Arabidopsis; in a few cases, the range is given, e.g. 1/67–1/20 reported by Wang et al. (Reference Wang, Wang, Tian, King, Zhang, Long, Shi and Meng2008) in Brassica napus L. for information of variation.
b The number of mutations in a single plant is estimated by genome size × mutation frequency.
c Greene et al. (Reference Greene, Codomo, Taylor, Henikoff, Till, Reynolds, Enns, Burtner, Johnson, Odden, Comai and Henikoff2003).
d Martín et al. (Reference Martín, Ramiro, Martínez-Zapater and Alonso-Blanco2009).
e Till et al. (Reference Till, Cooper, Tai, Colowit, Greene, Henikoff and Comai2007).
f Suzuki et al. (Reference Suzuki, Eiguchi, Kumamaru, Satoh, Matsusaka, Moriguchi, Nagato and Kurata2008).
h Kumar et al. (Reference Kumar, Boualem, Bhattacharya, Parikh, Desai, Zambelli, Leon, Chatterjee and Bendahmane2013).
i Talamè et al. (Reference Talamè, Bovina, Sanguineti, Tuberosa, Lundqvist and Salvi2008).
j Gottwald et al. (Reference Gottwald, Bauer, Komatsuda, Lundqvist and Stein2009).
k Cooper et al. (Reference Cooper, Till, Laport, Darlow, Kleffner, Jamai, El-Mellouki, Liu, Ritchie, Nielsen, Bilyeu, Meksem, Comai and Henikoff2008).
m Wang et al. (Reference Wang, Wang, Tian, King, Zhang, Long, Shi and Meng2008).
n Harloff et al. (Reference Harloff, Lemcke, Mittasch, Frolov, Wu, Dreyer, Leckband and Jung2012).
o Dong et al. (Reference Dong, Dalton-Morgan, Vicent and Sharp2009).
p Chen et al. (Reference Chen, Cheng, Ma, Wu, Liu, Zhou, Chen, Ma, Bi, Zhang, Guo, Wang, Lei, Wu, Lin, Liu, Liu and Jiang2013).
q Chawade et al. (Reference Chawade, Sikora, Bräutigam, Larsson, Vivekanad, Nakash, Chen and Olsson2010).
r Jankowicz-Cieslak et al. (Reference Jankowicz-Cieslak, Huynh, Brozynska, Nakitandwe and Till2012).
The number of mutations existing in a single M2 plant can be estimated based on genome size and mutation frequency. For example, a single Arabidopsis M2 plant may have 400 mutations, on average, while a bread wheat plant can have as many as 0.7 million mutations (Table 1). As most mutations are not desirable, it is essential that they be cleared before a mutant could be practically used as a new variety.
The majority of chemically induced mutations seem to be either silent (synonymous) or missense, with each accounting for slightly less than 50% (Fig. S1, available online). Nonsense mutations that produce knockout mutants are often less than 5% (Fig. S1, available online).
Most data on mutation profiles are obtained from seed-propagated crops. A recent TILLING study on banana has revealed that a high mutation frequency could also be achieved (Table 1). Of note is that up to 15% of the mutations were nonsense mutations (Fig. S1, available online) (Jankowicz-Cieslak et al., Reference Jankowicz-Cieslak, Huynh, Brozynska, Nakitandwe and Till2012). It may reflect that less harmful mutations are eliminated during vegetative propagation than during seed propagation.
Physically induced mutations
A number of physical mutagens have proven to be efficient for inducing mutations in plants (Mba et al., Reference Mba, Afza, Shu, Shu, Forster and Nakagawa2012). Unlike chemical mutagens, physical radiations have quite different properties and can induce different types of mutations.
γ-Radiation
The most commonly used physical mutagen in plant breeding is γ-radiation. In rice, for example, more than 450 new varieties have been bred. By amplification and sequencing of target genes in induced mutants, Morita et al. (Reference Morita, Kusaba, Iida, Yamaguchi, Nishio and Nishimura2009) identified 22 mutations. In addition, individual forward genetics studies have also identified dozen more mutations. Altogether, a total of 66 γ-ray-induced mutations with the nature of DNA lesions are summarized in Table S1 (available online).
Fast neutrons (FNs)
FNs have long been used for both forward and reverse genetic studies in plants. The identification of a ~460 kb FN-induced deletion enabled the cloning of a key gene involved in nodulation in legume crops (Men et al., Reference Men, Laniya, Searle, Iturbe-Ormaetxe, Gresshoff, Jiang, Carroll and Gresshoff2002; Searle et al., Reference Searle, Men, Laniya, Buzas, Iturbe-Ormaetxe, Carroll and Gresshoff2003). In Arabidopsis, a survey of FN-induced mutations indicated that the deletion size ranged from 5 to 35 kb (Li and Zhang, Reference Li and Zhang2002). Using the reverse genetics approaches such as ‘Deleteagene’ (Li et al., Reference Li, Song, Century, Straight, Ronald, Dong, Lassner and Zhang2001) and De-TILLING (Rogers et al., Reference Rogers, Wen, Chen and Oldroyd2009), mutations with deletions of 0.8–12 kb in Arabidopsis and rice and of 0.42–1.72 kb in Medicago truncatula have been identified. Although these early studies have suggested that FNs induce large deletions, a recent study has demonstrated that single-base substitutions and other types of mutations also exist (Belfield et al., Reference Belfield, Gan, Mithani, Brown, Jiang, Franklin, Alvey, Wibowo, Jung, Bailey, Kalwani, Ragoussis, Mott and Harberd2012).
Ion beam radiations (IBRs)
IBRs differ from γ-rays in their linear energy transfer (LET). Different LETs could be achieved by adjusting the type of ions and their energy (Abe et al., Reference Abe, Ryuto, Fukunishi, Shu, Forster and Nakagawa2012); for example, carbon ions (12C6+) can have different LETs (Fig. 1). IBRs seem to produce more large deletions (>1 kbp) and complex mutations than single-base substitutions and short deletions. Deletions up to 225 kbp, inversions of fragments of ~3.4 Mbp and (reciprocal) translocations have been reported in Arabidopsis mutagenized using carbon ion beams (Shikazono et al., Reference Shikazono, Suzuki, Kitamura, Watanabe, Tano and Tanaka2005).

Fig. 1 Types and profiles of mutations induced by γ-rays in rice and fast neutrons (FNs) and ion beams in Arabidopsis. C (I), C (II) and C (III) represent carbon ion (12C6+) beams with linear energy transfer (LET) of 22.5–30.0, 113 and 290 keV/μm, respectively. Ar represents argon ion (40Ar17+) beam with a LET of 290 keV/μm. Data on γ-rays were extracted from Table S1 (available online) with a reference list; data on FNs were extracted from Belfield et al. (Reference Belfield, Gan, Mithani, Brown, Jiang, Franklin, Alvey, Wibowo, Jung, Bailey, Kalwani, Ragoussis, Mott and Harberd2012) and those on C (I) from Kazama et al. (Reference Kazama, Hirano, Saito, Liu, Ohbu, Hayashi and Abe2011), on C (II) from Shikazono et al. (Reference Shikazono, Suzuki, Kitamura, Watanabe, Tano and Tanaka2005) and on C (III) and Ar from Hirano et al. (Reference Hirano, Kazama, Ohbu, Shirakawa, Liu, Kambara, Fukunishi and Abe2012).
Comparison of mutation profiles
Analysis of the above-mentioned mutations indicated that all radiations could produce deletions and other types of DNA lesions, though the former predominate in most cases (Fig. 1(a)). Base substitutions, previously assumed to be rare, indeed represent the majority in FN-induced mutations (58.2%) (Fig. 1(a)) as revealed by whole-genome resequencing (Belfield et al., Reference Belfield, Gan, Mithani, Brown, Jiang, Franklin, Alvey, Wibowo, Jung, Bailey, Kalwani, Ragoussis, Mott and Harberd2012). The size of deletions ranges from 1 bp to half million bp (Fig. 1(b)), with short ones ( < 5 bp) predominating in all cases (Fig. 1(c)). In addition, insertions up to 7 kbp have also been reported even in γ-ray-induced mutants (Chen et al., Reference Chen, Cheng, Ma, Wu, Liu, Zhou, Chen, Ma, Bi, Zhang, Guo, Wang, Lei, Wu, Lin, Liu, Liu and Jiang2013; Zhao et al., Reference Zhao, Cui, Xu, Tan, Fu, Liu, Poirier and Shu2013). Complex mutations, such as inversions and translocations, which are often combined with deletions or insertions (Hirano et al., Reference Hirano, Kazama, Ohbu, Shirakawa, Liu, Kambara, Fukunishi and Abe2012), have more frequently been identified in ion beam-mutagenized plants (Fig. 1(a)). Furthermore, carbon ions with greater LETs seem to produce more large-sized deletions/complex mutations (Hirano et al., Reference Hirano, Kazama, Ohbu, Shirakawa, Liu, Kambara, Fukunishi and Abe2012).
Perspectives
Substantial progress in molecular elucidation of mutations induced by various mutagens during the past decade has immediate implications for the selection of a proper mutagen for a particular research purpose. For example, IBRs should be a nice choice for deleting clustered genes, while chemical mutagens are better suited to induce missense mutations, which might be gain-of-function mutations, e.g. tolerance to herbicides. The dense background mutations indicate, on the other hand, that it would take more generations than previously taught in textbooks to develop a stable mutant line. No whole-genome analysis has been carried out for plants mutagenized by any mutagen except FNs; therefore, we have to be cautious while interpreting the differences in mutation profiles. In this regard, genome resequencing studies should also be carried out for obtaining holistic mutation profiles in plants mutagenized using chemical mutagens as well as γ-rays and IBRs.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262114000318
Acknowledgements
This work was financially supported by the Natural Science Foundation of China through research contract no. 11275171 and in part supported by the Special Fund for Agro-scientific Research in the Public Interest (201103007) and the 8812 Program of Zhejiang Province.




