Introduction
Mungbean is one of the important food grain legumes. It is a diploid species with 2n = 2x = 22 chromosomes and predominately a self-pollinated crop. Being short-duration grain legume, it is grown extensively in major tropical and subtropical countries of the world. India is the world's largest mungbean producer accounting for about 65% of the world's acreage and 54% of its global production. In India, the productivity of mungbean in farmer's fields is rather low (0.40 t ha−1) considering its potential in research stations (1.50 t ha−1).
The low productivity of mungbean is attributed to several diseases caused by fungus, bacteria and viruses. Among all the diseases, the one caused by mungbean yellow mosaic virus (MYMV) is the most destructive (Kang et al., Reference Kang, Yeam and Jahn2005). MYMV disease was first reported in India by Nariani (Reference Nariani1960). The MYMV disease is reported to occur only in Asian countries, viz., India, Sri Lanka, Bangladesh, Philippines, Pakistan, Myanmar, Thailand, Nepal, Indonesia, Malaysia and Taiwan (Karthikeyan et al., Reference Karthikeyan, Shobhana, Sudha, Raveendran, Senthil, Pandiyan and Nagarajan2014). It often assumes epiphytotic proportions in Northern plains, and central and Southern zones of India. MYMV disease is characterized by bright yellow mosaics on the leaves of infected plants. The symptoms start as small yellow specks along the veinlets and spread over the lamina; pods become thin and curl upwards (Karthikeyan et al., Reference Karthikeyan, Shobhana, Sudha, Raveendran, Senthil, Pandiyan and Nagarajan2014).
The MYMV disease is transmitted by whitefly, Bemisia tabaci Genn. (Hemiptera: Aleyrodidae) (Nariani, Reference Nariani1960). The whitefly transmits the virus in a persistent manner. It is a polyphagous insect and has an extremely wide host range infesting more than 500 species of plants belonging to 63 plant families (Greathead, Reference Greathead1986). It completes its life cycle in less than 2 weeks to more than 10 weeks depending upon temperature and host plant. Among the many biotypes recognized (Brown, Reference Brown2007; Prasanna et al., Reference Prasanna, Kanakala, Archana, Jyothsna, Varma and Malathi2015), the ‘B’ biotype (silver leaf whitefly) is the most conspicuous due to its wide distribution, attributed to its ability to colonize on many plant hosts and ability to transmit a number of geminiviral diseases.
The use of resistant varieties is considered as the most economical and eco-friendly method of reducing the production losses caused by MYMV disease. As an effective, safe, reliable and long lasting method of control, host plant resistance could form an important component of integrated management of MYMV disease. Most of the commercially grown cultivars are either susceptible or partially resistant to MYMV disease (Karthikeyan et al., Reference Karthikeyan, Shobhana, Sudha, Raveendran, Senthil, Pandiyan and Nagarajan2014; Sudha et al., Reference Sudha, Karthikeyan, Shoana and Nagarajan2015). Even the partially resistant cultivars are likely to become susceptible as a result of the breakdown of resistance attributable to the emergence of new isolates driven by high rates of mutation, recombination and re-assortment (Duffy and Holmes, Reference Duffy and Holmes2008; Lima et al., Reference Lima, Sobrinho, Gonza'lez, Rocha, Silva, Xavier, Silva, Duffy and Zerbini2012). Hence, there is a need for a continuous search for new sources of resistance. The objective of the study is to identify stable sources of resistance to MYMV disease.
Materials and methods
Material
The material for the study consisted of 14 mungbean genotypes, namely AVMU 1693, AVMU 1694, AVMU 1695, AVMU 1696, AVMU 1697, AVMU 1698, AVMU 1699, AVMU 16100, AVMU 16101, AVMU 16102, Harsha, KPS-2 and NM-94 obtained from The World Vegetable Center (WVC), Taiwan (formerly known as Asian Vegetable Research and Development Centre – AVRDC) and KKM-3, a high yielding variety released by the University of Agricultural Sciences (UAS), Bengaluru. India.
Methods
Screening under natural infection condition
The genotypes were screened for responses to MYMV disease in an experimental plot located at the main research station, UAS, Bengaluru, India, under natural infection conditions. The seeds of each genotype were sown in a single row of 2.5 m length following randomized complete block design with three replications during 2017 summer (March–May), 2017 rainy (June–August) and 2018 summer (February–April) seasons. A susceptible check (Harsha) was sown after every four rows of test genotypes and all around the experimental plot to provide uniform disease inoculum to the test genotypes. Ten days after sowing, seedlings were thinned-out to maintain a spacing of 0.1 m between plants within a row and 0.45 m between rows. Recommended crop production practices were followed to raise a good crop. Each genotype consisted of 20 plants per replication. The genotypes were examined for the appearance of first symptoms typical of MYMV disease on the susceptible check. The disease severity in each of the 14 genotypes and replication was scored at 30, 45 and 55 d after sowing (DAS) using 1–6 scale developed by WVC and modified by Akhtar et al. (Reference Akhtar, Kitsanachandee, Srinives, Abbas, Asghar, Shah, Atta, Chatchawankanphanich, Sarwar, Ahmad and Sarwar2009) (Table 1). The disease scores averaged across three replications were used for statistical analysis. Based on the average disease scale, the per cent disease index (PDI) was calculated as the ratio of sum of numerical observations to the product of maximum disease scale and number of observations and expressed in per cent. The area under disease progressive curve (AUDPC) for each genotype was calculated by the trapezoidal integration of PDI estimated at 30, 45 and 55 DAS (Campbell and Madden, Reference Campbell and Madden1990).
where n = the number of assessment (at 30, 45 and 55 DAS); y = PDI and (t i+1−t i) = duration between two consecutive assessments (15 d).
Table 1. Description of symptoms, disease score and PDI and criteria of classification of genotypes into different responses groups (AVRDC Scale)

Screening under challenged inoculation in glasshouse
Low MYMV disease incidence and whitefly populations coupled with non-congenial weather conditions are likely to result in low/non-infection of MYMV, and thus, genotypes may escape from the disease occurrence (Vidaysky and Czosnek, Reference Vidaysky and Czosnek1998). To overcome such possibility, the genotypes were also screened under challenging conditions in glasshouse using artificial inoculation of MYMV (Pico et al., Reference Pico, Diez and Nuez1998). The indigenous non-viruliferous whiteflies B. tabaci Gennadius maintained on cotton were used for artificial inoculation of MYMV to genotypes. Whiteflies were starved for 2 h followed by acquisition access on MYMV-infected mungbean for 12 h. Then, viruliferous white flies were allowed to feed on 10–12 d old healthy mungbean plants for 24 h. About 10–15 viruliferous whiteflies were allowed to feed on each of the 20 plants of test genotypes for transmission of virus. Later, inoculated plants were sprayed with a systemic insecticide, imidacloprid 17.8% SL @ 0.05% to kill the whiteflies, and then the plants were kept in insect proof cages and maintained separately till the appearance of symptoms. The genotypes were scored for their responses to MYMV disease based on symptoms and classified them into different response groups using the scale and the criteria described in Table 1.
Detection of MYMV using polymerase chain reaction
The genotypes were screened for the presence/absence of MYMV to confirm if the symptoms in resistant and susceptible ones were caused due to MYMV only, using polymerase chain reaction (PCR) amplification of MYMV genome sequence complementary to MYMV coat protein (CP) gene-specific primers. The leaf samples were collected from all the 14 mungbean genotypes at 30 and 55 DAS along with KKM 3 (UASB released variety) from the field. DNA was extracted from leaf samples by modified C-TAB method (Doyle and Doyle, Reference Doyle and Doyle1987). The quality and quantity of DNA was checked using nano drop. The genomic regions of MYMV were amplified using MYMV CP gene-specific primers that amplify approximately 900 bp CP gene product. The amplified products were separated using agarose gel electrophoresis and their size was estimated by comparing with standard 1 kb ladder.
Detection of MYMV using rolling circle amplification
If MYMV particles are fewer in host plants, standard PCR fail to detect them. Hence, in the present study, rolling circle amplification (RCA) was used to confirm the presence/absence of MYMV in only those genotypes where PCR failed to amplify MYMV CP gene priming regions. The RCA was carried out using bacteriophage Φ29 DNA polymerase included in the ‘Illustra TempliPhi 100 Amplification Kit’ (GE Healthcare, Proteogen Biosciences (India) Pvt. Ltd., Bengaluru). The RCA is an isothermal amplification method that produces microgram of DNA from picograms of DNA in a few hours (Jeske et al., Reference Jeske, Gotthardt and Kober2010; Richert-Pöggeler and Minarovits, Reference Richert-Pöggeler and Mináróvits2014; Bora et al., Reference Bora, Gogoi and Kalita2016). The DNA polymerase replicates MYMV in large numbers to facilitate their easy detection using standard PCR amplification of MYMV. The product obtained by RCA was subjected to PCR in order to detect a trace amount of MYMV in the RCA-subjected samples. MYMV CP-specific primer was used to amplify the RCA product with the expected amplicon of 900 bp size. Each reaction contained 12.5 µl of the master mix, 2 µl each of forward and reverse primers, 2 µl of RCA product and 6.5 µl of sterile distilled water. The MYMV viral DNA was initially denatured at 94 °C for 4 min and then amplified in a thermal cycler (BioRad) for 34 cycles. The PCR products were separated by electrophoresis on an agarose gel (1.5%) stained with ethidium bromide in 1X TAE buffer.
Similarity of MYMV CP gene sequence with that of other Geminiviruses
After successful confirmation of the presence of MYMV, the CP gene amplified from the host plants was sequenced in both directions (forward and reverse) using CP-specific primers with automated sequencing facility at Eurofins Genomics India Pvt. Ltd., Bengaluru. The sequences (Acc.no. MH885653) obtained from both forward and reverse reactions were aligned and joined together to get a full length sequence using ‘Basic Local Alignment Search Tool (BLAST)’ available at the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/). Sequences were compared with other respective viral sequences of the NCBI database using BLAST and multiple aligned using CLUSTALW2 multiple alignment tool (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The phylogenic neighbour-joining trees analysis was constructed using MEGA 7.0 software package. Robustness of trees was determined by bootstrap sampling of multiple sequence alignment with 1000 replications.
Biochemical basis of MYMV disease resistance
To explore the possible biochemical basis of resistance to MYMV disease, activities of enzymes such as peroxidase (POX) (Hartee, Reference Hartee, Peach and Tracey1955), polyphenol oxidase (PPO) (Mayer et al., Reference Mayer, Harel and Shaul1965) and phenylalanine ammonia lyase (PAL) (Ross and Senderoff, Reference Ross and Senderoff1992), and levels of total phenols (Sadasivam and Manickam, Reference Sadasivam and Manickam1996) were estimated in leaf samples of ‘AVMU 16101’, the MYMV-resistant genotype, and ‘Harsha’, the susceptible genotype.
Results
Responses of genotypes to MYMV under natural and challenged infection
The genotypes differed for their responses to infection by MYMV under natural infection conditions. Yellow specks, the typical initial symptoms of MYMV disease, appeared earlier in ‘Harsha’, the susceptible check, compared with those in other genotypes under natural infection conditions (Table 2). Yellow mosaic symptoms (YMS) covered 50% of the leaf area in susceptible genotypes ‘Harsha’ within 15 d after the appearance of initial symptoms. The appearance of initial symptoms delayed by at least 10 d in five genotypes namely AVMU1698, AVMU 1699, AVMU 16100, AVMU 16101 and KPS2 compared with those in susceptible check during all the three seasons of evaluation (Table 2).The estimates of PDI in these five genotypes remained lower than those in other test genotypes and were at least lower by 38% compared with those in susceptible check during all the three seasons of evaluation (Table 3). Further, the estimates of AUDPC were lower in these five genotypes compared with that in susceptible check as well as in other test genotypes across all the three seasons of evaluation under natural infection (Table S1). Under challenged infection also, the estimates of PDI were lower in these five genotypes compared with those in susceptible check and other test genotypes (Table 4). Based on the disease scale and the criteria (Table 1), five genotypes namely AVMU 1698, AVMU1699, AVMU16100, AVMU16101 and KPS2 were identified as resistant to MYMV disease. KKM 3, the variety released by UAS, Bengaluru, showed moderate resistance responses to MYMV disease.
Table 2. Appearance of yellow mosaic virus disease symptoms in mungbean genotypes

a Susceptible check.
DAS, days after sowing.
Table 3. Estimates of per cent disease index across three seasons

a Based on PDI values at 55 DAS.
b Susceptible check.
MYMV, mungbean yellow mosaic virus; DAS, days after sowing; R, resistant; MR, moderately resistant; MS, moderately susceptible; S, susceptible.
Table 4. Disease severity of mungbean genotypes under glasshouse condition though whitefly (Bemisia tabaci)

MYB, mungbean yellow mosaic Bengaluru; R, resistant; S, susceptible; Acquisition Access Period (AAP) = 12 h; Inoculation Access Period (IAP) = 24 h; number of whiteflies per plant = 15–20.
Detection of MYMV in resistant genotypes through PCR and RCA-PCR
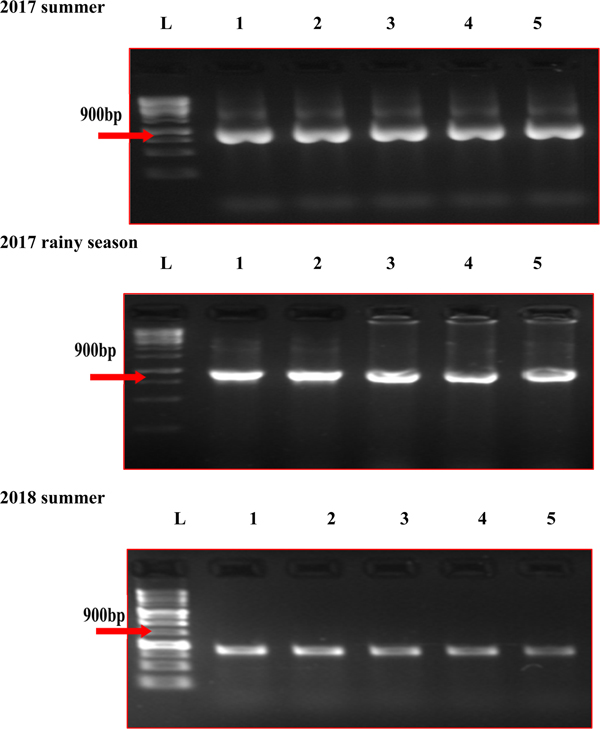
In the five MYMV disease-resistant genotypes, the presence of MYMV could not be detected through standard PCR, although it could be easily detected in susceptible check and other test genotypes (Fig. 1). However, MYMV was detected in all the five MYMV-resistant genotypes through RCA-PCR (Fig. 2). The sequence of PCR amplicon of CP gene priming regions of the five MYMV disease-resistant genotypes was similar to MYMV CP gene sequence Vigna I segment A deposited in NCBI data base by 99.60% and out-grouped with minimum similarity (40.1%) with horsegram yellow mosaic virus (HYMV) segment DNA B and complete sequence of French bean yellow mosaic virus isolates (AM932426.1) (Fig. S1).
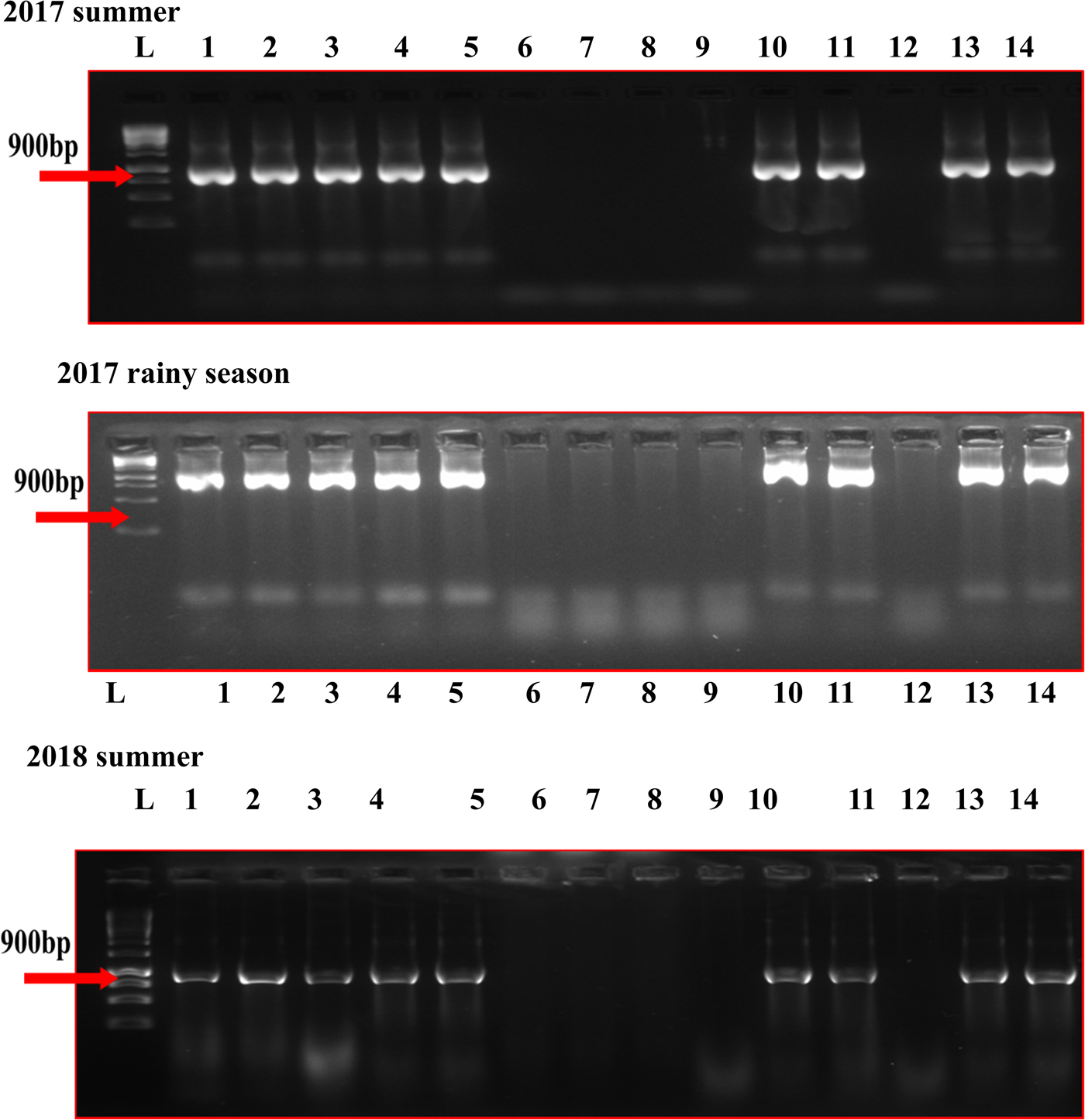
Fig. 1. CP gene analysis of MYMV in mungbean genotypes by PCR in different seasons. L: Ladder (1kb), (1) AVMU-1693, (2) AVMU-1694, (3) AVMU-1695, (4) AVMU-1696, (5) AVMU-1697, (6) AVMU-1698, (7) AVMU-1699, (8) AVMU-16100, (9) AVMU-16101, (10) AVMU-16102, (11) Harsha, (12) KPS-2, (13) NM-94, (14) KKM-3
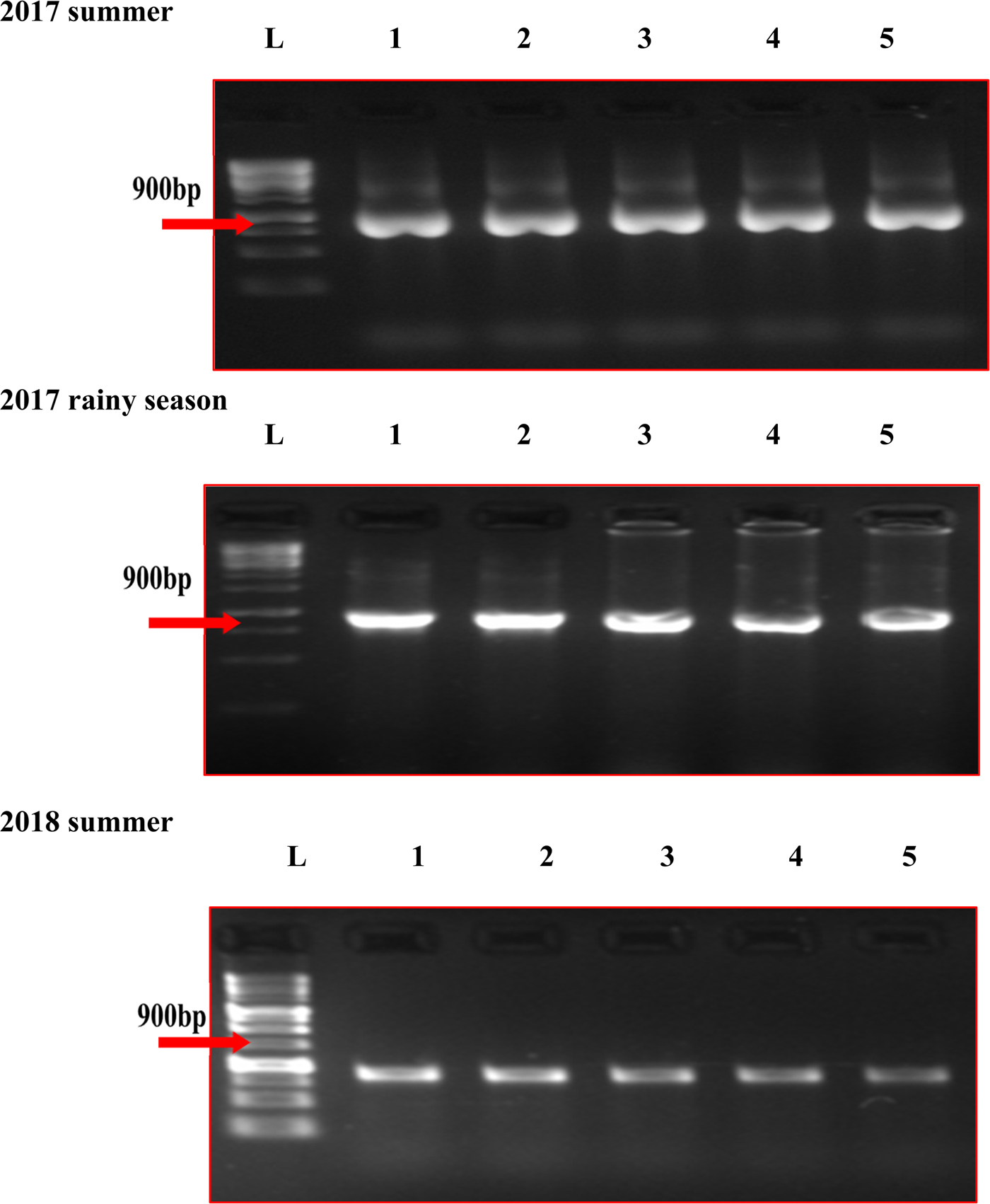
Fig. 2. RCA-PCR analysis of MYMV in mungbean genotypes in different seasons. L: Ladder (1kb), (1) AVMU-1698, (2) AVMU-1699, (3) AVMU-16100, (4) AVMU-16101, (5) KPS-2.
Bio-chemical constituents in MYMV-resistant and susceptible genotypes
The activities of enzymes such as POX (0.34 ΔAbs min−1 g−1), PPO (0.05ΔAbs min−1 g−1) and PAL (0.58 µ moles of transcinnamic acid min−1 g−1) and levels of total phenols (3.01 mg g−1) were significantly higher in MYMV-resistant genotype, AVMU 16101, compared with those in susceptible check, Harsha (0.096 ΔAbs min−1 g−1, 0.018ΔAbs min−1 g−1, 0.21 µ moles of transcinnamic acid min−1 g−1, 1.97 mg g−1 and 0.41 mg g−1, respectively).
Discussion
Substantial differences in responses of genotypes to MYMV disease suggested successful infection of MYMV under both natural and challenged inoculation. Delayed appearance of initial symptoms, and lower estimates of PDI and AUDPC under natural infection and lower PDI under challenged infection indicated and confirmed resistance responses of AVMU-1698, AVMU-1699, AVMU-16100, AVMU-16101 and KPS-2 to MYMV disease. Several researchers have identified either moderately resistant or resistant mungbean genotypes to MYMV disease under natural and/or both natural and challenged inoculation in glasshouse conditions. To quote a few, Akhtar et al. (Reference Akhtar, Sarwar, Abbas, Asghar, Sarwar and Shah2011) could identify 35 mungbean genotypes (from among 162 genotypes sampled from eight different geographic regions) moderately resistant to Mungbean Yellow Mosaic India Virus (MYMIV) disease under natural infection in field condition and challenged inoculation in glasshouse conditions. Gupta and Mishra (Reference Gupta and Mishra2014) reported resistance response of 54 mungbean genotypes to MYMV disease under natural infection. In a recent study, Farooq et al. (Reference Farooq, Naila, Muhammad, Muhammad, Rahila, Shabir, Muhammad and Nabeela2018) identified seven (among 100) mungbean cultivars moderately resistant to MYMV disease.
Most often, mungbean is infected by begomoviruses causing yellow mosaic disease (YMD) in soybean, horsegram, mungbean and French bean. YMS are produced on the leaves of these pulses due to infection by any one or combination of these viruses. Similarly, the whitefly vector, B. tabaci, can acquire more than one virus under field condition and transmit to the healthy plants which could induce YMS. Bemisia tabaci cryptic species Asia II 1 was found dominant in Northern India, whereas Asia II 8 was found predominant in Southern India (Nair et al., Reference Nair, Götz, Winter, Giri, Boddepalli, Sirari, Bains, Taggar, Dikshit, Aski and Boopathi2017). We used indigenous B. tabaci for transmission of MYMV.
In the present study, to confirm that YMS are produced in both susceptible and resistant genotypes due to infection by MYMV only, the MYMV CP gene-specific primers were used to amplify CP gene priming regions of MYMV. Successful amplification of MYMV CP gene priming regions using PCR in susceptible, resistant and other genotypes provided evidence for the appearance of YMS attributable to infection by MYMV only. Several researchers such as Brown (Reference Brown2007), Ashwathnarayana et al. (Reference Ashwathnarayana, Shankarappa, Prameela, Raghavendra, Keshava murthy and Rangaswamy2005), Obaiah et al. (Reference Obaiah, Bhaskara Reddy, Eswara Reddy and Siva Prasad2014) and Deepa et al. (Reference Deepa, Govindappa, Kulkarni, Kenganal and Biradar2017) have used primers designed to amplify the conserved region of begomo viruses' CP gene to confirm that MYMV disease is caused by begomoviruses in a wide range of crop plants.
Successful detection of MYMV through RCA-PCR suggested latent infection of MYMV in resistant genotypes and it is likely that the resistant genes present in these genotypes would have restricted the multiplication of MYMV resulting in the non-appearance of YMS. The restricted multiplication of MYMV in these five genotypes, and hence their resistance response could be attributed to increased activity of POX, PPO and PAL, and enhanced levels of total phenols. Several studies have indicated increased activities of PAL and PPO when plants are challenged with pathogens (Zeier et al., Reference Zeier, Delledonne, Mishina, Severi, Sonoda and Lamb2004; Niranjanraj et al., Reference Niranjanraj, Sarosh and Shetty2006). Enhanced activity of PAL leads to alternate processes such as significant lignifications and production of phenolic compounds which in turn offer defence against diseases (Zeier et al., Reference Zeier, Delledonne, Mishina, Severi, Sonoda and Lamb2004; Umesha, Reference Umesha2006). POX is also known to play a significant role in lignification and suberification of plant cell walls which restrict the movement of viruses from cell to cell and thus preventing the spread of the disease (Bowles, Reference Bowles1990). Literature is abundant and shows that increased activities of POX, PPO and PAL and enhanced levels of total phenols impart resistance to viral diseases in several crops. To mention a few, Kumar et al. (Reference Kumar, Singh, Dalal and Kumar2017) reported a significant role of POX, total phenol, flavanoids and tannins in conferring resistance to apical leaf curl virus disease in potato. The level of phenolic compounds, total soluble proteins and malondialdehyde and the activities of PAL, POX, catalase, proteases, superoxide dismutase and PPO were significantly higher in cotton leaf curl burewala virus resistant genotypes compared with those in susceptible genotypes (Zeeshan et al., Reference Zeeshan, Akhtar, Hameed, Sarwar, Imran-Ul-Haq and Khan2014). In yardlong bean, Lovely et al. (Reference Lovely, Radhadevi and Umamaheswaran2017) reported the role of PAL activity in imparting resistance to black eye cowpea mosaic virus disease.
A very high degree of similarity of MYMV CP gene-specific primer-binding sequence amplified through RCA-PCR with Vigna segment A MYMV CP gene sequence deposited in NCBI database provided a further line of evidence for resistance responses of AVMU 1698, AVMU 1699, AVMU 16100, AVMU 16101 and KPS 2 to YMD. In an attempt to study the similarity of the sequence of CP genes of begomoviruses infecting different pulses, Maheshwari et al. (Reference Maheshwari, Panigrahi and Angappan2014) reported that CP gene sequences of viruses causing YMD in blackgram, cowpea and green gram were similar to those of MYMV-Tamil Nadu isolates. In a similar study, Manjunatha et al., (Reference Manjunatha, Noorulla, Anjaneya reddy, Archana and Manjunath2015) reported that CP gene sequence of virus infecting pigeonpea was similar to HYMV by 98% and to MYMV by 87%. Geographical confinement of species of the yellow mosaic virus is validated as MYMIV strain is more prevalent in northern, central and eastern regions of India, whereas, MYMV is predominant in the southern regions (Shahakar et al., Reference Shahakar, Renuka and Pater2018).
The five MYMV disease-resistant genotypes identified in the present study could be used as potential donors to develop MYMV disease-resistant cultivars. Considering that resistance to MYMV disease is controlled by a single dominant gene (Sandhu et al., Reference Sandhu, Brar, Sandhu and Verma1985) or a single recessive gene (Reddy and Singh, Reference Reddy and Singh1995; Saleem et al., Reference Saleem, Haris and Malik1998), or two independent recessive genes (Verma and Singh, Reference Verma and Singh1988; Ammavasai et al., Reference Ammavasai, Phogat and Solanki2004) or two complementary recessive genes (Shukla and Pandya, Reference Shukla and Pandya1985), DNA marker-assisted introgression of resistant genes from donors to elite agronomic background is likely to be more effective to develop MYMV disease-resistant mungbean cultivars.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262119000121
Acknowledgement
Authors acknowledge World Vegetable Centre, Taiwan, Regional centre, ICRISAT campus, Hyderabad for providing mungbean genotypes and Directorate of Research, University of Agricultural Sciences, Bengaluru, India for providing financial support to conduct the research.