Introduction
Deficiency of iron and zinc micronutrients in calcareous soils of arid and semi-arid regions of the world's cereal-growing area poses a major threat to grain yield (Graham and Welch, Reference Graham and Welch1996; Graham et al., Reference Graham, Senadhira, Beebe, Iglesias and Monasterio1999; Cakmak, Reference Cakmak2002; Yousfi et al., Reference Yousfi, Wissal, Mahmoudi, Abdelly and Gharsalli2007) and nutritional quality of the major staple food crops (Zvi et al., Reference Zvi, Saranga, Yazici, Fahima, Ozturk and Cakmak2008). Over two billion people of the world suffer from micronutrient malnutrition (Welch and Graham, Reference Welch and Graham2004; Bouis, Reference Bouis2007) caused particularly by deficiency of iron and zinc in their diets (Poletti et al., Reference Poletti, Gruissem and Sautter2004; White and Broadley, Reference White and Broadley2005). Poor growth of children, lower work productivity, and increased incidence of morbidity and mortality among pregnant women due to iron deficiency anaemia, especially in developing countries, are some of the serious implications of the micronutrient malnutrition on human health (Demment et al., Reference Demment, Young and Sensenig2003; Holtz and Brown, Reference Holtz and Brown2004). Among cereals, wheat alone contributes 28% of the world's edible dry matter and up to 60% of daily calorie intake in several developing countries, thus emphasizing the need for biofortification of wheat for the micronutrients. Among various approaches of combating malnutrition, biofortification of cereals (Welch and Graham, Reference Welch and Graham2004; Distelfield et al., Reference Distelfeld, Cakmak, Peleg, Ozturk, Yazici, Budak, Saranga and Fahima2007) through the development of cultivars with efficient system for iron and zinc mobilization from soils, their uptake, translocation and sequestration in grains is the most cost effective (Graham and Rengel, Reference Graham, Rengel and Robson1993; Cakmak et al., Reference Cakmak, Yilmaz, Ekiz, Torun, Erenoglu and Braun1996).
In order to acquire more iron and zinc from the problematic soils, graminaceous plants release mugineic acid (MA) family of phytosiderophores (PS) from their roots (Takagi, Reference Takagi1976; Marschner et al., Reference Marschner, Römheld and Kissel1986; Higuchi et al., Reference Higuchi, Suzuki, Nakanishi, Yamaguchi, Nishizawa and Mori1999; Ueno and Ma, Reference Ueno and Ma2009). Enhanced production and release of PS by the roots of graminaceous plants have been observed under both iron- and zinc-deficient conditions (Zhang et al., Reference Zhang, Römheld and Marschner1989; Cakmak et al., Reference Cakmak, Gulut, Marschner and Graham1994; Kobayashi et al., Reference Kobayashi, Suzuki, Inoue, Itai, Takahashi, Nakanishi, Satoshi and Nishizawa2005). Differential efficiency for iron and zinc solubilization and mobilization from the soil has been observed among Triticum (Tolay et al., Reference Tolay, Erenoglu, Römheld, Braun and Cakmak2001) and graminaceous species (Römheld and Marschner, Reference Römheld and Marschner1990). In case of wheat, only 2′-deoxymugineic acid (DMA) is secreted (Kobayashi et al., Reference Kobayashi, Nakanishi, Takahashi, Mori and Nishizawa2008), whereas in barley, four types of MAs, i.e. DMA, MA, 3-epihydroxy-2′-deoxymugineic acid (epi-HDMA) and 3-epihydroxymugineic acid (epi-HMA), are secreted under micronutrient deficiency, thus making barley more tolerant to iron deficiency (Mori and Nishizawa, Reference Mori and Nishizawa1987; Ma et al., Reference Ma, Taketa, Chang, Iwashita, Matsumoto, Takeda and Nomoto1999). In barley, the genes for hydroxylation of DMA to MA have been localized on the long arm of chromosome 4H, whereas another gene for hydroxylation at C-3 position from MA to epi-HMA or DMA to epi-HDMA was found to be located on the long arm of chromosome 7H (Ma et al., Reference Ma, Taketa, Chang, Iwashita, Matsumoto, Takeda and Nomoto1999). Secale cereale is also capable of producing three PS DMA, MA and HMA (Mori et al., Reference Mori, Nishizawa and Fujigaki1990; Ma and Nomoto, Reference Ma and Nomoto1994). Transgenic rice with iron deficiency syndrome gene (IDS3) of barley produced MA in addition to DMA, and it had shown 1.4–1.3 times higher grain iron and zinc contents than the non-transgenic rice seeds (Masuda et al., Reference Masuda, Suzuki, Morikawa, Kobayashi, Nakanishi, Takahashi, Saigusa, Satoshi and Nishizawa2008). Therefore, the development of plant cultivars with higher efficiency of uptake of iron and zinc from soil, their translocation within plants and higher storage in seeds are the various steps for effective micronutrient biofortification.
Various wild progenitor and related species of cereals have been screened and found as the valuable sources of useful variability for several economic traits (Friebe et al., Reference Friebe, Jiang, Raupp, McIntosh and Gill1996; Kuraparthy et al., Reference Kuraparthy, Sood, Chhuneja, Dhaliwal, Kaur, Bowden and Gill2007a, Reference Kuraparthy, Chhuneja, Dhaliwal, Kaur, Bowden and Gillb). High variability for grain iron and zinc contents among wild Triticum species was reported by Monasterio and Graham (Reference Monasterio and Graham2000). A number of non-progenitor Aegilops species with two to three times higher grain iron and zinc contents than the durum and bread wheat cultivars were reported by Tiwari et al. (Reference Tiwari, Rawat, Neelam, Randhawa, Singh, Chhuneja and Dhaliwal2008) and Rawat et al. (Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009). This useful variability present in Aegilops species could further be utilized for the improvement of elite wheat cultivars.
This article deals with the studies on the production of PS in wheat and related non-progenitor Aegilops species under iron- and zinc-sufficient and -deficient conditions, and their uptake by seedling roots and shoots.
Materials and methods
Plant materials
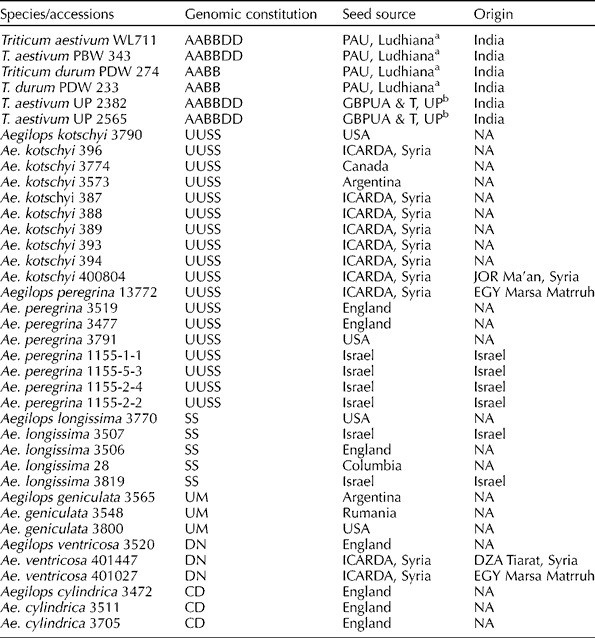
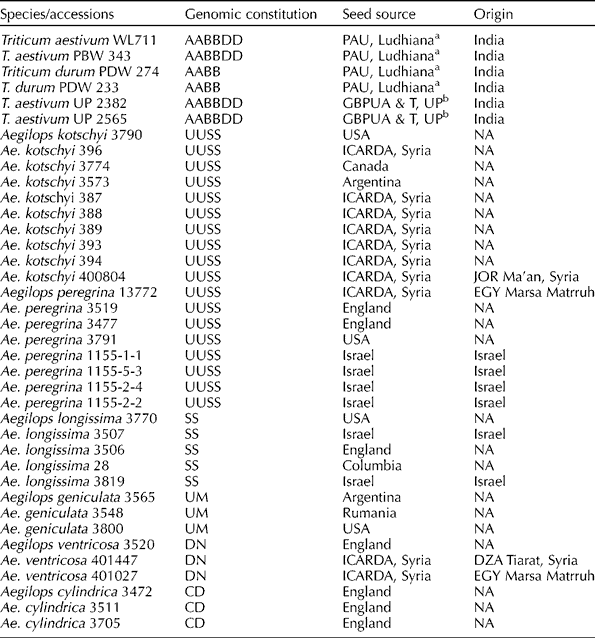
The experimental material comprised a total of 32 accessions of related Aegilops species, i.e. Aegilops kotschyi Boiss (10), Aegilops peregrina (Hack.) Maire et Weill (8), Aegilops ventricosa Tausch (3), Aegilops geniculata Roth (3), Aegilops longissima Schweinf & Muschl. (5) and Aegilops cylindrica Host (3), and six wheat and durum cultivars. The entire experimental material was obtained from the germplasm collection maintained at the Punjab Agricultural University, Ludhiana, India. Their genomic constitution and source of origin or collection are given in Table 1.
Table 1 Genomic constitution and source of plant materials used in this study
NA, not available.
a Punjab Agricultural University, Ludhiana, India.
b Govind Ballabh Pant University of Agriculture and Technology, Uttar Pradesh, India.
PS production
Seeds of various Aegilops species and cultivars were surface sterilized with 1% sodium hypochlorite solution for 15 min, washed thrice with distilled water followed by soaking in distilled water overnight and germinated on a filter paper saturated with distilled water in the dark at 20°C. After 3–5 d, seedlings were transferred to Saran net floating on 0.5 mM CaCl2 solution at a pH of 5.6 in a plastic container. Seven-day-old seedlings were transferred to 10 l plastic trays with floating Styrofoam having 15 holes of 2 cm diameter with five seedlings in each hole, with continuous aeration of the nutrient solution containing half-strength Hoagland's solution (Ma and Nomoto, Reference Ma and Nomoto1993), which was converted later on to full strength.
Nutrient media composition
The nutrient media consisted of 0.7 mM K2SO4, 0.1 mM KCl, 2 mM Ca(NO3)2, 0.5 mM MgSO4, 0.1 mM KH2PO4, 1 μM H3BO3, 0.5 μM MnSO4, 0.2 μM CuSO4 and 0.01 μM (NH4)6Mo7O24. Nutrient media were supplemented with 0.5 μM ZnSO4 and 100 μM Fe-EDTA for zinc- and iron-sufficient conditions, respectively. For iron-deficient conditions, plants were grown in a nutrient solution without iron but with 0.5 μM ZnSO4, and for zinc-deficient conditions, the nutrient medium was supplemented with 100 μM Fe-EDTA without any zinc. Deionized water was used for the preparation of the nutrient media. Nutrient media were changed at 3-d intervals. Unadjusted pH of the nutrient media was found to vary between 6 and 7. All experiments were carried under controlled environmental condition (light/day) regime of 16/8 h at 23–25°C with relative humidity of 65–75%. Each experiment was performed in three replications of each of the cultivars and Aegilops accessions in a completely randomized design.
Collection of root exudates
Intact plants were removed from the nutrient media, and the roots were repeatedly washed in deionized water. The root exudates were collected in glass beakers having 20 ml of deionized water after 2 h of sunrise, and this was continued for 3 h. Collection was done in three replications of five plants each of wheat cultivars and related wild species. Exudates were collected on days 6, 11 and 17 after transfer of plants to iron-deficient conditions, while under zinc-deficient conditions, root exudate collections were made on days 10, 14 and 17 after transfer of plants to deficient conditions. To reduce microbial degradation of PS in the collections, Micropur was added to them, and they were stored at − 80°C till further processing. Exudate solutions were filtered through Whatman filter paper #42 prior to storage.
Determination of PS
The quantity of PS in the root exudates was determined indirectly by modified Fe-binding assay of Gries et al. (Reference Gries, Klatt, Crowley and Parker1995), which measures Fe–PS-binding capacity. Freshly prepared 0.5 ml of FeCl3 (1.0 mM) solution with a pH of 2.1 was added to 10 ml of the collected exudates, and shaken for 15 min followed by the addition of 1 ml of sodium acetate buffer (1 M, pH 7) while shaking for 10 min. The Fe–PS precipitates were filtered through Whatman filter paper #42 into 0.25 ml of 6 N HCl. Ferric irons were reduced by the addition of 0.5 ml of 8% hydroxylamine hydrochloride solution, and then heated at 60°C for 20 min. To this solution, 0.25 ml of 0.25% ferrozine and 1 ml of sodium acetate buffer (pH 4.7) were added. The concentration of ferrous ion was determined colorimetrically at 562 nm.
For determination of PS under zinc deficiency, Zn–CAS (Chrome Asurol S) method was used. For preparation of CAS, 6 ml of 10 mM hexadecyltrimethyl ammonium solution was added into 100-ml flask, followed by the addition of 1.5 ml of 1 mM ZnCl2 and 1.5 ml of 10 mM HCl. To the same flask, 7.5 ml of 2 mM aqueous CAS was also added, followed by the addition of a solution containing 4.2 g of anhydrous piperazine dissolved in 6.25 ml of 12 N HCl (pH 5.6). The final volume was made to 100 ml after the addition of 5-sulphosalicylic acid (4 mM). Determination of PS was done by the addition of 0.5 ml of aliquot of PS exudate and 0.5 ml of Zn–CAS solution. Change in colour from blue to orange was observed, and absorbance was recorded at 630 nm.
Determination of iron and zinc concentrations in shoots and roots of Aegilops species and wheat and durum cultivars
After the experiments under nutrient-sufficient and -deficient conditions, shoots and roots were collected from the plants, and washed several times with deionized water. Samples were dried at 70°C overnight and digested in a mixture of two parts of concentrated nitric acid and one part of perchloric acid (Zarcinas et al., Reference Zarcinas, Cartwright and Spouncer1987). Digestion was continued till a white residue was obtained, and iron and zinc concentrations were determined using Atomic Absorption Spectrophotometer (Avanta Grade M; GBC, Adelaide, SA, Australia). A minimum of five replications of chemical analysis were done for each of the cultivars and accessions of Aegilops species.
Statistical analysis
The data of PS-released, root and shoot iron and zinc concentrations were subjected to the computation of standard deviation and standard error. Student's t-test was applied for testing the significance of differences among means of cultivars and Aegilops species. Correlation between the concentrations of iron and zinc in roots and PS released under iron- and zinc-deficient conditions was calculated.
Results
Plant response to iron- and zinc-deficient conditions
Typical visual iron deficiency symptoms in the wheat cultivars were observed after 7 d of transfer of plants to iron-deficient conditions. Under iron deficiency, severe yellowing in the younger leaves of cultivars was observed compared with the Aegilops species. All the Aegilops species except Ae. longissima stayed green till 15 d of growth under iron-deficient conditions during which slight yellowing of the lower leaves was observed. Under zinc deficiency, only white necrotic spots on leaves were observed in cultivars at the end of the experiment, whereas no such deficiency symptoms were observed among the Aegilops species.
Release of PS under iron-sufficient and -deficient conditions
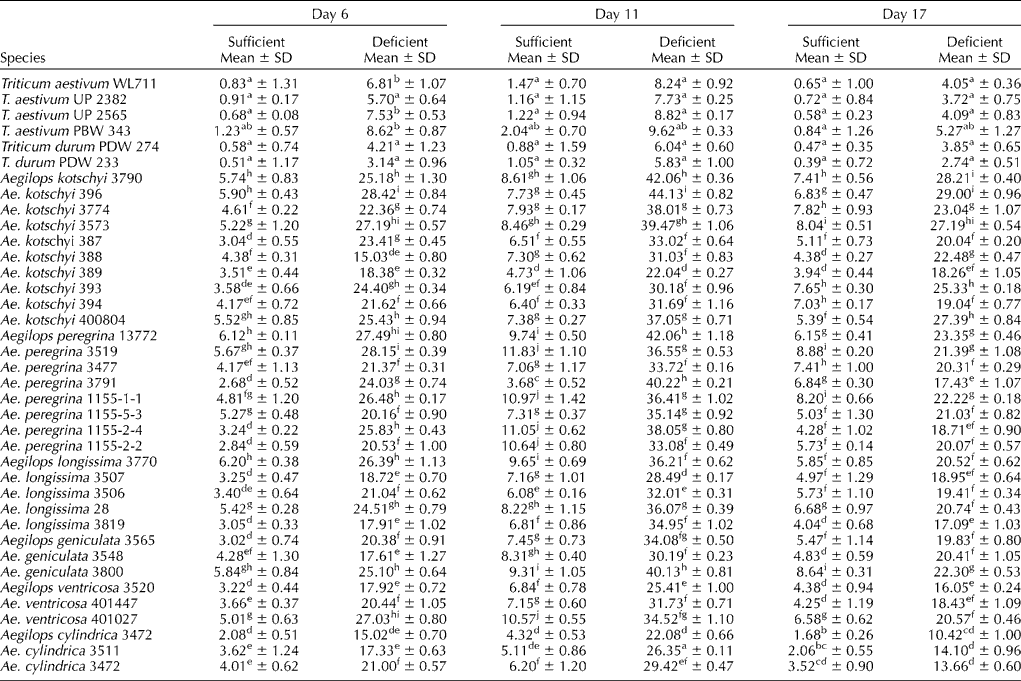
The amount of PS released by roots of wheat and durum cultivars and various accessions of different Aegilops species on different days in iron-sufficient and -deficient medium is given in Table 2. The amount of PS among wheat cultivars under sufficient conditions on day 6 varied from 0.58 to 1.23 μg of iron mobilized/g dry root weight, whereas it was 3.5 times higher among Aegilops species (2.08–6.20 μg of Fe mobilized/g dry root weight/3 h). The amount of PS was almost doubled by day 11, and then it decreased to the level observed on day 6 at the end of the experiment.
Table 2 Phytosiderophore released (μg Fe mobilized/g dry root weight/3 h) from the roots of wheat cultivars and Aegilops species under iron-sufficient and -deficient conditions
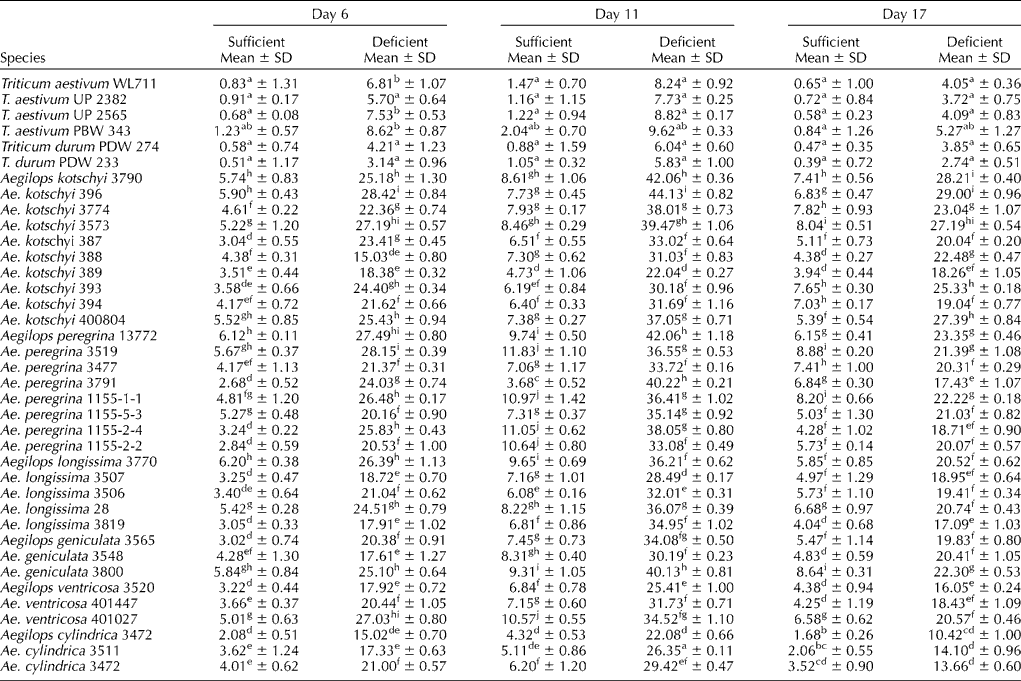
Note: Different lower case alphabets used as superscripts within each column indicate significant differences in the means of different accessions.
All the wheat cultivars and various accessions of Aegilops species had five to seven times higher PS released on day 6 under iron-deficient conditions compared with that released in the sufficient medium (Table 2), which increased to all time high on day 11. The rate of release of PS among Aegilops species from day 6 to 11 was much higher than that of the wheat cultivars, which was also subsequently reduced by day 17 at a rate lower than that of the wheat cultivars. The absolute amount of PS among the Aegilops species under all the conditions of the experiment remained several folds higher than that among wheat cultivars (Table 2). On an average, Ae. peregrina and Ae. kotschyi had higher absolute amount of PS released under all the conditions of the experiment. There were significant differences among various accessions of different Aegilops species for PS production under both iron-sufficient and -deficient conditions (Table 2).
Release of PS under zinc-sufficient and -deficient conditions
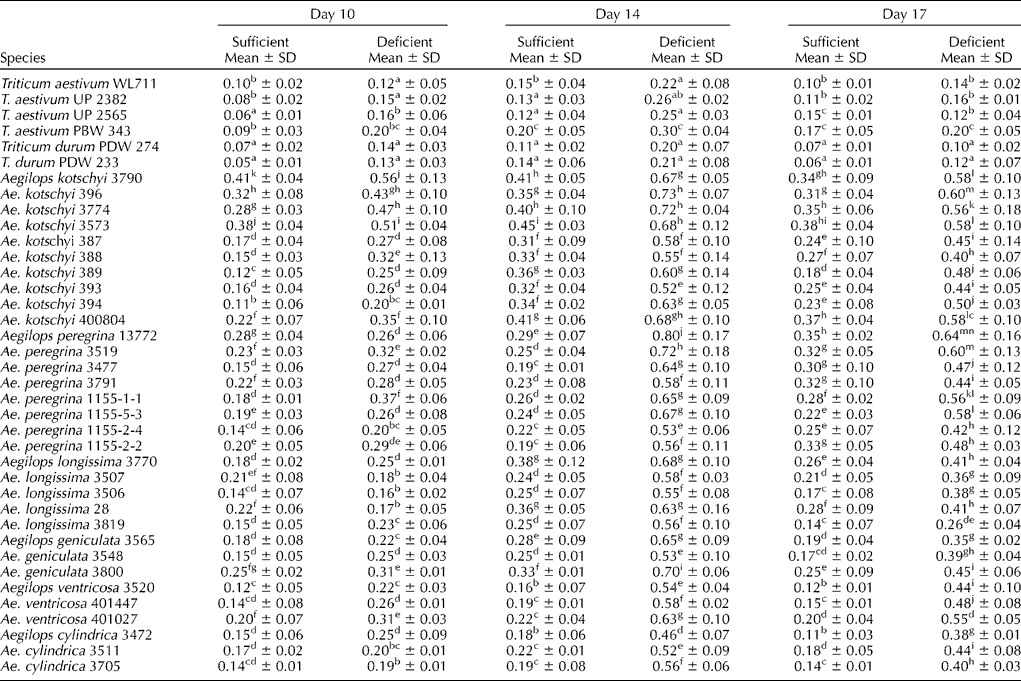
The response for PS release among wheat and Aegilops species under zinc-sufficient and -deficient conditions was almost similar to that for iron (Table 3). Even under zinc-sufficient conditions, two to three times higher release of PS was observed in all Aegilops species over wheat cultivars. The absolute amount of PS released under zinc-deficient conditions was, however, several folds lower than that released under iron-deficient conditions. The release of PS was, however, delayed by 4 d under zinc deficiency. There was hardly any increase in the release of PS under zinc-deficient conditions till day 6 (data not shown), which started increasing by day 10 with a maximum amount on day 14 followed by a slower reduction towards day 17 of the experiment (Table 3). The level of PS released under zinc-deficient conditions did not level off quickly as that released under iron-deficient conditions. There were significant differences among and within Aegilops species for PS release under zinc-deficient conditions also.
Table 3 Phytosiderophore released (activity units of PS/g dry root weight/3 h) from the roots of wheat cultivars and Aegilops species under zinc-sufficient and -deficient conditions
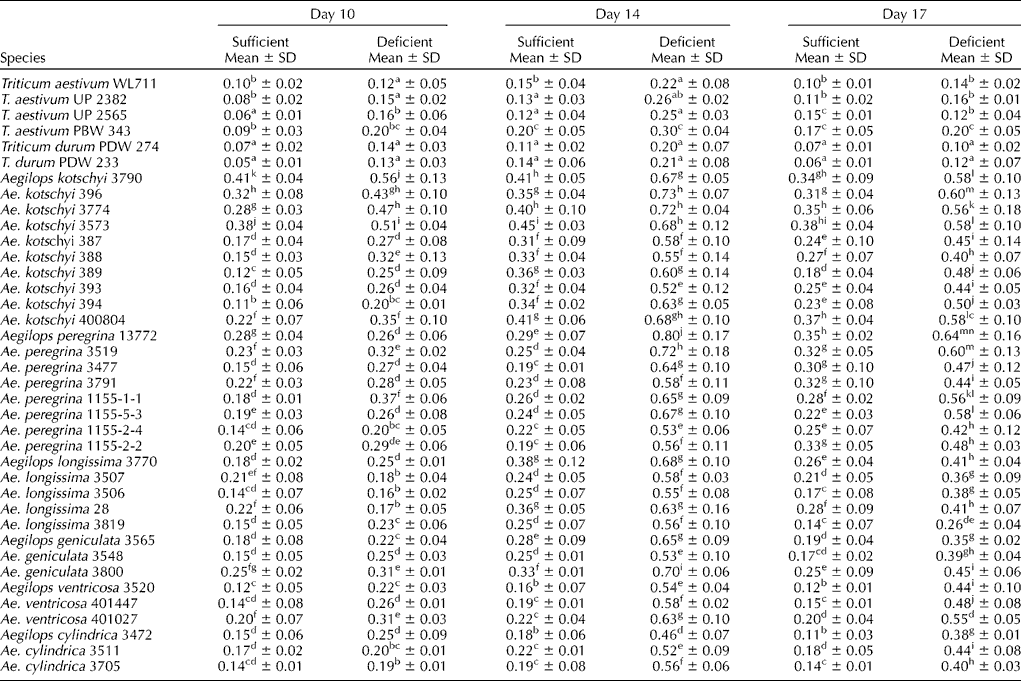
Note: Different lower case alphabets used as superscripts within each column indicate significant differences in the means of different accessions.
Iron and zinc contents in shoots and roots of wheat cultivars and Aegilops species
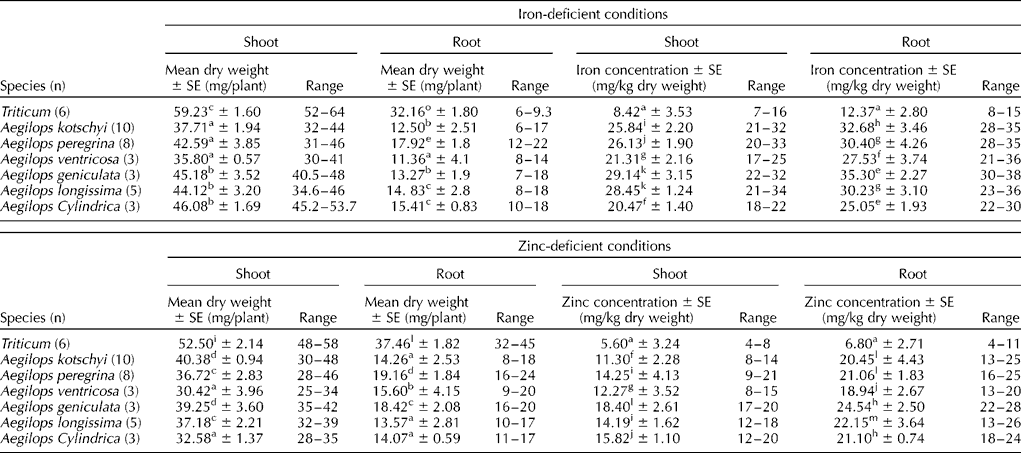
All the Aegilops species had significantly lower (two to three times) mean and range of root dry weight per plant compared with that of the wheat cultivars (Table 4) under both iron- and zinc-deficient conditions. The mean shoot dry weight per plant of Aegilops species was nearly two-third of that of the wheat cultivars. Various Aegilops species had significantly high (two to three times higher) iron concentration under iron-deficient conditions and zinc content under zinc-deficient conditions in both shoots and roots after 17 d of growth (Table 4), suggesting higher efficiency of Aegilops roots for nutrient uptake and their translocation to the shoots. The roots of both wheat cultivars and Aegilops species had invariably higher iron and zinc contents than the shoots.
Table 4 Shoot and root dry matter production, and iron and zinc concentrations in the shoots and roots of wheat cultivars and Aegilops species grown for 17 d in iron- and zinc-deficient media
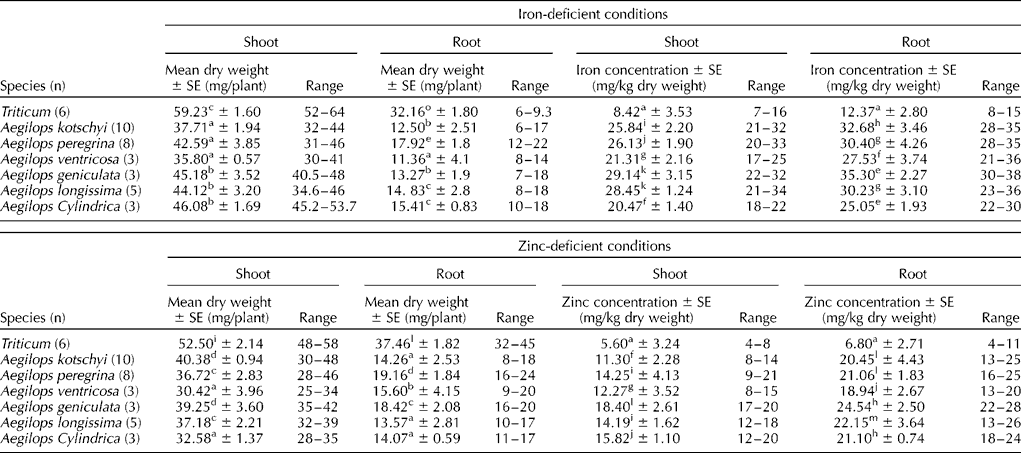
Note: Different lower case alphabets used as superscripts within each column indicate significant differences in the means of different species for shoot and root dry matter production and micronutrient concentrations.
Correlation between root iron and zinc concentrations and MA released
Significant correlation between means of root iron concentration (day 11) and PS released (day 17) was observed (r = 0.94) under iron-deficient conditions. High correlation (r = 0.91) between means of root zinc concentration (on day 17) and PS released (on day 14) was also observed under zinc-deficient conditions.
Discussion
Marked differences in the appearance of chlorosis and necrosis were observed between wheat cultivars and Aegilops species. Both wheat and durum cultivars showed leaf yellowing after 7 d under the deficient conditions, whereas comparatively delayed development of the deficiency symptoms was observed among Aegilops species. Differential sensitivity to iron and zinc deficiency among and within cereal species has been observed by various workers (Marschner et al., Reference Marschner, Römheld and Kissel1986). In young rice plants, susceptibility to chlorosis was attributed to lower amount of DMA secreted by their roots under micronutrient-deficient conditions (Mori et al., Reference Mori, Nishizawa, Hayashi, Chino, Yoshimura, Ishihara, Chen and Hadar1991). Higher iron and zinc contents in the grains of Aegilops species (Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006) compared with Triticum species probably provided the micronutrients to the growing seedlings for a longer time. Rawat et al. (Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009) also reported higher concentration of iron and zinc in the flag leaves of Aegilops species compared with Triticum aestivum cv. WL711. Alternatively, this could be due to higher translocation rate of these minerals from roots to shoots in Aegilops species compared with the wheat cultivars. The higher amount of both iron and zinc in the shoots and roots of Aegilops species compared with that of the wheat cultivars even under deficient conditions provides unequivocal support to the fact that Aegilops species possess an efficient system for the uptake and translocation of micronutrients to the leaves and ultimately to grains.
Under nutrient-deficient conditions, much higher rate of release of PS was found in both wheat cultivars and Aegilops species compared with that found under the sufficient conditions. The phenomenon of significant release of PS by graminaceous species (Strategy II plants) under deficiency of iron, zinc and other micronutrients has been reported by various workers (Zhang et al., Reference Zhang, Römheld and Marschner1989; Mori et al., Reference Mori, Nishizawa, Hayashi, Chino, Yoshimura, Ishihara, Chen and Hadar1991; Cakmak et al., Reference Cakmak, Gulut, Marschner and Graham1994; Kanazawa et al., Reference Kanazawa, Higuchi, Nishizawa, Fushiya and Mori1995; Higuchi et al., Reference Higuchi, Watanabe, Takahashi, Kawasaki, Nakanishi, Nishizawa and Mori2001). Aegilops species released higher amounts of PS under all conditions throughout the experiment.
In Aegilops species, nearly two to four times higher amount of PS was released under iron and zinc deficiency compared with Triticum species. Even under nutrient-sufficient conditions, all Aegilops species showed higher rate of release of PS than the wheat cultivars. Sufficient variability within and among Aegilops species for PS release has been observed. Variation in the rate of PS release by Triticum and Aegilops species with different genomes under deficiency of iron and zinc has been reported by Tolay et al. (Reference Tolay, Erenoglu, Römheld, Braun and Cakmak2001). Variation in zinc efficiency among hexaploid wheat, hexaploid oats, diploid rye, triticale, Aegilops and wheat-alien chromosome addition lines has been documented by Schlegel et al. (Reference Schlegel, Cakmak, Torun, Eker, Tolay, Ekiz, Kalayci and Braun1998). Greater sensitivity of durum wheat to zinc deficiency was attributed to its low capacity for uptake of adequate zinc under deficient conditions, and also to less secretion of zinc-mobilizing PS to the rhizosphere from the roots (Cakmak et al., Reference Cakmak, Yilmaz, Ekiz, Torun, Erenoglu and Braun1996; Rengel and Graham, Reference Rengel and Graham1996). In addition to the amount of PS released by the roots, the diversity of MAs also affects the ability of the plants to tolerate micronutrient deficiency (Mori and Nishizawa, Reference Mori and Nishizawa1987). In rice, wheat, maize and sorghum, only DMA is secreted, making these plants more susceptible to micronutrient deficiency (Kobayashi et al., Reference Kobayashi, Nakanishi, Takahashi, Mori and Nishizawa2008; Masuda et al., Reference Masuda, Suzuki, Morikawa, Kobayashi, Nakanishi, Takahashi, Saigusa, Satoshi and Nishizawa2008). In barley, in addition to DMA, 3-HMA and 3-epi-HMA are secreted, thus making it more efficient in uptake of micronutrients under low mineral availability (Mori et al., 1987; Singh et al., Reference Singh, Chino, Nishizawa, Ohata, Mori, Randall, Delhaize, Richarads and Munns1993; Ma et al., Reference Ma, Taketa, Chang, Iwashita, Matsumoto, Takeda and Nomoto1999). It is possible that more than one type of MA are secreted by Aegilops species also, as a result of which they have higher absolute amount of PS enabling them to sustain the deficiency of micronutrients for longer time compared with wheat.
The amount of PS released under iron deficiency was much higher compared with that released under zinc deficiency. Tolay et al. (Reference Tolay, Erenoglu, Römheld, Braun and Cakmak2001) also reported lower release of PS in Triticum species and Aegilops tauschii under zinc deficiency. There may be some other zinc transporters in addition to Zn–PS complex under zinc-deficient conditions. High correlation between seedling root zinc concentration and PS released under zinc deficiency in spite of its lower absolute amount is indeed intriguing.
Thus, high genetic diversity in non-progenitor Aegilops species for high grain iron and zinc concentrations (Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Rawat et al., Reference Rawat, Tiwari, Singh, Randhawa, Singh, Chhuneja and Dhaliwal2009) may be attributed to their diverse and efficient PS release systems for micronutrient uptake, which could be used for biofortification of wheat through interspecific hybridization. The use of some accessions of Aegilops species with higher PS release under iron- and zinc-sufficient and -deficient conditions and higher grain micronutrient content and concentration for introgression of useful variability in wheat is in progress.
Acknowledgements
The Department of Biotechnology, Government of India, is acknowledged for supporting the work through a project, ‘Biofortification of wheat for enhanced iron and zinc content by conventional and molecular breeding’. The authors thank the Head, Institute Instrumentation Centre, IIT Roorkee, and Mr R. Juyal for their help in chemical analyses. The authors also thank Dr Bhupinder Singh of Indian Agricultural Research Institute for his help in MA estimation. The authors are thankful to Dr Parveen Chhuneja of Punjab Agricultural University, Ludhiana, for meticulous checking of the manuscript.