Introduction
Cowpea [Vigna unguiculata (L.) Walp.] is a major staple grain legume widely cultivated in most tropical regions around the world, especially in West Africa. Its importance is attributed to its tolerance to drought, nitrogen fixing ability, adaptability to different cropping systems, and nutritional and economic values, which help, in particular, small-scale farmers who have limited resources (Coulibaly and Lowenberg-DeBoer, Reference Coulibaly, Lowenberg-DeBoer, Fatokun, Tarawali, Kormawa and Tamo2002).
Cowpea is a primary source of protein for the ever-growing population of both rural and urban dwellers in West Africa. As a legume in general, its protein content is approximately twice that of cereals, and its amino acid (AA) profile, rich in lysine (Lys) and tryptophan (Trp), complements those of cereals, which are rich in sulphurous AAs (Nielsen et al., Reference Nielsen, Brandt and Singh1993). Cowpea is also an excellent source of minerals, notably Fe and Zn (Pereira et al., Reference Pereira, Carvalho, Dellamora-Ortiz, Cardoso, Carvalho, Viana, Freitas and Rocha2014). To overcome insufficient local production of animal protein and its escalating prices and to increase micronutrient (i.e., mineral and vitamin) intakes by malnourished populations, the development of varieties with high nutritional value and the promotion of cowpea consumption are necessary for the people's health in the region, especially among the poor.
With recent improvements in national economies in Africa, legumes have come under increasing demands and expectations for nutritional value in the diet. Various grain quality-related traits, such as testa and eye colour, testa texture, seed size, cooking time and protein content, affect the market value of cowpea, and the preferred characteristics are often different depending on the region reflecting deep-rooted cultural traditions (Coulibaly and Lowenberg-DeBoer, Reference Coulibaly, Lowenberg-DeBoer, Fatokun, Tarawali, Kormawa and Tamo2002; Faye et al., Reference Faye, Jooste, Lowenberg-DeBoer and Fulton2004). Other factors such as taste, flavour and flatulence may also significantly influence the consumption of cowpea.
Our studies of cowpea breeding and germplasm lines have revealed genetic differences in the protein content of the grain. These differences suggest the possibility of increasing the content through selection; several accessions were identified as potential sources of useful genes for further improvement (Boukar et al., Reference Boukar, Massawe, Muranaka, Franco, Maziya-Dixon, Singh and Fatokun2011, Reference Boukar, Muranaka, Franco, Fatokun, Boukar, Coulibaly, Fatokun, Lopez and Tamo2012). Other studies have examined genetic variance in grain quality-related traits, such as physical characteristics and proximate contents (Nielsen et al., Reference Nielsen, Brandt and Singh1993; Giami et al., Reference Giami, Akusu and Emelike2001; Chinma et al., Reference Chinma, Alemede and Emelife2008; Henshaw, Reference Henshaw2008), AA composition (Tshovhote et al., Reference Tshovhote, Nesamvuni, Raphulu and Gous2003), anti-nutritional factors (Oboh et al., Reference Oboh, Muzquiz, Burbano, Cuadradoa, Pedrosaa, Ayeta and Osagie1998; Giami et al., Reference Giami, Akusu and Emelike2001), flatulence-causing oligosaccharides (Obigbinde and Akinyele, Reference Obigbinde and Akinyele1983; Prinyawiwatkul et al., Reference Prinyawiwatkul, Beuchat, McWatters and Phillips1996; Wang et al., Reference Wang, Lewis, Brennan and Westby1997) and fatty acids (Prinyawiwatkul et al., Reference Prinyawiwatkul, Beuchat, McWatters and Phillips1996). However, the genetic diversity of each trait and interactions among those traits have not been well explored. To facilitate development of improved cowpea varieties that meet nutritional requirements and consumer preferences, we analysed a wide range of cowpea germplasm to (1) compare agronomic traits and the physical and nutritional properties of the grain, (2) identify possible relationships among quality-related traits using selected genotypes covering the variation in physical, nutritional/antinutritional and functional properties and (3) nominate potential resources for the further genetic improvement of cowpea.
Materials and methods
Field trials and sample preparation
We tested a total of 240 genotypes, comprising 214 germplasm accessions, 22 breeding lines and 4 major local varieties (Table S1, available online). Field experiments were carried out at Minjibir experimental farm of the International Institute of Tropical Agriculture, in Minjibir, Kano state, Nigeria (12°09.008′N, 8°39.733′E), during 2011 and 2012. Each genotype was sown in a single 3 m-long row plot with 20 cm distance between plants in rows that are 150 cm apart, on 20 July 2011 and 9 July 2012. At planting, inorganic fertilizer (N:P:K = 15:15:15) was applied at 100 kg/ha. Three seeds were planted per hill, and seedlings were later thinned to one per hill at 3 weeks after planting. Each plot was arranged in an alpha-lattice design with two replications.
The rows were hand-weeded during the vegetative growth stage, and insecticide was sprayed once at the vegetative growth, flowering and pod maturing stages. The number of plants per plot at 3 weeks after planting and the date at which 50% of flowers were open in each plot were recorded.
From each plot, 30–60 mature pods were harvested in timely manner before pod shattering occurs, and rinsed with distilled water to remove dust on the pod surface. The cleaned pods were air-dried and threshed by hand with polyethylene gloves in the clean laboratory environment. The remaining pods were harvested two times, when 60% and 95% of pods were matured. At the final harvest, the number of plants per plot and the harvest date were recorded, and fodder was harvested. The fodder was air-dried for 2 weeks in a screen house and weighed.
From each line, approximately 20–60 g of clean threshed grain were shipped to Japan in a sealed plastic bag for chemical analysis. The remaining grain samples were used for the analysis of physical properties at the International Institute of Tropical Agriculture. The grain yield was calculated from the quantities of clean threshed and bulk-harvested grain.
Using the samples collected in both 2011 and 2012, the analysis of the physical properties (testa texture and colour, eye colour, and grain size) and basic chemical properties (crude protein and micronutrient contents) were conducted. The values obtained from all measurements are reported on a dry matter basis.
For further detailed analysis, we selected 20 genotypes to have wide variation in physical properties and basic chemical properties, on the basis of the physical and nutritional characteristics of all 240 genotypes collected in 2011. The grain samples of two replicates collected from the field trial conducted in 2011 were merged per genotype to have enough grain quantity and used for the detailed analysis of physical, nutritional/antinutritional and functional properties, including the following: cooking time; lipid, ash, dietary fibre, AA, oligosaccharide, polyphenol, phytic acid and fatty acid contents; and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and β-amylase activities. All measurements were conducted three times to ensure the sensitivity of the analysis, and average values are reported on a dry matter basis.
Analysis of physical, nutritional/antinutritional and functional properties
Testa texture, eye colour and grain shape were evaluated based on the IBPGR descriptor for cowpea. Testa colour was evaluated against Munsell colour charts and classified as white, cream, light brown, mid-brown, dark brown, red, or black. An image analysis system (Grain Scanner; Satake, Hiroshima, Japan) was used to obtain the average length and width of approximately 100 grains per sample, which were then used to measure the 100-grain weight.
For the analysis of crude protein and micronutrient (Fe, Zn, Mn and Cu) contents, grain samples were ground with a mixer mill (MM200; Retch, Haan, Germany) in a 25 ml Teflon grinding jar with a 15 mm-diameter zirconium oxide grinding ball. Grain N content was determined by NC analyzer (Sumigraph NC-22F; Sumika Chemical Analysis Service Ltd., Tokyo, Japan), and the crude protein content was calculated with an nitrogen-to-protein (N:P) conversion factor of 5.45 (see Results). Micronutrient contents were determined by atomic absorption spectrometry (Z-5010; Hitachi, Tokyo, Japan) of samples digested with 2 N HCl. All contents are reported on the basis of 3 days of oven-drying at 40°C.
For the evaluation of cooking time, 200 unsoaked seeds were boiled in 1500 ml water, and the hardness of ten seeds collected every 10 min was measured by penetrometer (fruit pressure tester 0–1 kg; T.R. Turoni s.r.l., Forlí, Italy) with a 6 mm tip till the average hardness reached 2–4 N. Cooking time was measured three times and average values are reported.
Ash content was determined by a dry ashing method. Crude lipids were extracted in a 2:1 mixture of chloroform:methanol. The lipids were then extracted in ether and saponified, and the composition of fatty acids was measured in a high-performance liquid chromatograph (HPLC) equipped with a Corona CAD (100 pA; ESA Biosciences, Inc., Chelmsford, MA, USA) detector and an Inertsil C8-3 (5 μm, 4.6 mm I.D. × 150 mm, 40°C; GL Sciences, Tokyo, Japan) column. The mobile phase was a 4:3:1 (v/v) mixture of acetonitrile:methanol:water at 1.0 ml/min. The contents of insoluble and soluble dietary fibre were determined using a dietary fibre assay kit (#291-59 701; Wako Pure Chemical Industries, Ltd., Osaka, Japan). β-Amylase activity was determined using a β-amylase assay kit (Betamyl-3, Megazyme International Ireland, Wicklow, Ireland).
Contents of AAs were determined using an auto AA analyzer (JLC-500/V; JEOL Ltd., Tokyo, Japan) and by HPLC (LC-20AD; Shimadzu, Kyoto, Japan). Saccharides were extracted from aqueous slurry of sample mixed for 30 min in 80% ethanol at room temperature. Extracts were analysed by HPLC (Lachrom Elite HPLC system; Hitachi) equipped with a refractive index detector and a Carbosep CHO682 column (Transgenomic Inc., Omaha, NE, USA) at 80°C using an H2O mobile phase. Polyphenol content was determined by the Folin–Ciocalteu method using a 50% acetone extract and evaluated as the equivalent weight of gallic acid. Concentration of phytic acid was measured using the Wabe method as described by Vaintraub and Lapteva (Reference Vaintraub and Lapteva1988). For the evaluation of anti-oxidative activity, DPPH radical scavenging activity was measured, and an IC50 (half maximal (50%) inhibitory concentration) value was calculated.
Statistical analyses
With the data obtained from all 240 genotypes in both years, a linear mixed model was used to estimate variance due to year, genotype and year-by-genotype interaction, assuming all effects as random. Phenotypic and genotypic correlations were computed to determine the relationship among days to 50% flowering and harvest, grain and biomass yields, crude protein and micronutrient contents, and grain size parameters. Coefficients of genetic correlation were estimated following Holland (Reference Holland2006). The null hypothesis (H 0:ρ = 0) was tested using a confidence interval built from the estimated coefficient and its standard error.
Linear regression was used to evaluate the relationships among grain physical, nutritional/anti-nutritional and functional properties.
Results
Variation in nutritional contents and relationship with agronomic traits and grain physical characteristics in 240 genotypes
The 240 genotypes showed variations in the crude protein and mineral contents in their grain. The crude protein content ranged from 16.6 to 24.8% with a mean of 20.5% in 2011, and from 17.1 to 23.9% with a mean of 20.3% in 2012 (Table 1). The Fe, Mn and Cu contents showed wider variance, and the highest values were approximately double the lowest. The Zn contents showed less variance than the other micronutrients. The variance due to genotype was highly significant (P< 0.01) for crude protein, Fe, Zn and Cu contents; the differences among genotypes explained 17–49% of the total observed variance. The interaction between year and genotype was significant only for crude protein and Fe. In addition, genetic factors explained 21–87% (P< 0.01) of the differences in days to 50% flowering and harvest, biomass and grain yields, and grain size factors, but year had no significant contribution (Table S2, available online).
Table 1 Average, maximum and minimum values of nutrient contents among all 240 genotypes tested in 2011 and 2012
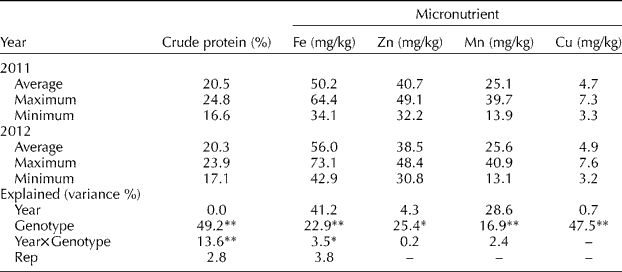
* P< 0.05; ** P< 0.01.
Biomass and grain yield had significant positive genotypic (r= 0.57) and phenotypic (r= 0.49) correlations (Table 2). Days to 50% flowering and harvest also had significant positive genotypic (r= 0.44) and phenotypic (r= 0.45) correlations. Overall, genotypic correlations were higher than the corresponding phenotypic correlations, indicating a greater contribution of genetic factors to the expression of these traits than environmental factors.
Table 2 Phenotypic (upper diagonal) and genotypic (lower diagonal) correlations among agronomic traits, and physical and nutritional properties

* P< 0.05.
a Dflow = days to 50% flowering.
b Dharv = days to harvest.
c Byield = biomass yield.
d Gyield = grain yield.
e CP = crude protein.
f Gweight = 100-grain weight.
There were strong positive genotypic correlations between crude protein and Fe (r= 0.70) and Zn (r= 0.70), and between Fe and Zn (r= 0.68), but there was no correlation among crude protein, Mn and Cu contents (Table 2). Days to harvest showed positive genetic correlations with Mn content (r= 0.42) and grain size (r= 0.53). Biomass and grain yields showed no or weak correlations with crude protein, Fe, Zn and Cu contents, and grain size parameters, although there was a positive correlation between grain yield and Mn content (r= 0.53). There was no relationship of testa colour and texture, eye colour, and grain shape with the contents of crude protein and micronutrients (data not shown).
Genotypes TVu-14875, TVu-12802, TVu-2508 and TVu-7127 showed stable high crude protein, Fe and Zn contents between years (means: crude protein, 22.4–24.1%; Fe, 54.2–63.1 mg/kg; Zn, 42.7–44.0 mg/kg). TVu-456, IT97K-131-1, KVx61-1 and IT93K-372-1-2 showed stably low contents (crude protein, 17.0–18.2%; Fe, 43.0–43.8 mg/kg; Zn, 33.4–36.5 mg/kg). No genotypes had high contents of crude protein and all four micronutrients, but TVu-2723 had high contents of crude protein (23.3%), Fe (61.5 mg/kg) and Mn (33.6 mg/kg), and moderately high contents of Zn (41.8 mg/kg) and Cu (5.0 mg/kg). In contrast, TVu-456 and TVu-14890 had constantly low contents of crude protein (17.5 and 17.8%, respectively), Fe (43.7 and 47.3 mg/kg, respectively), Zn (33.4 and 34.2 mg/kg, respectively), Mn (22.5 and 22.6 mg/kg, respectively) and Cu (3.6 and 4.5 mg/kg, respectively).
Physical, nutritional/anti-nutritional and functional properties of 20 selected genotypes
The difference between the two genotypes with the highest (TVu-12802, 24.4%) and lowest (KVx61-1, 16.6%) crude protein contents was 7.8% (Table 3). The differences between the genotypes with the highest and lowest micronutrient contents were 22.0 mg/kg Fe, 15.1 mg/kg Zn, 18.7 mg/kg Mn and 2.9 mg/kg Cu. These differences accounted for 72–89% of the variation observed among the 240 genotypes. There were positive correlations between crude protein and Fe (r= 0.82) and Zn (r= 0.69) contents in grain, and between Fe and Zn (r= 0.71), as found in all 240 genotypes. By contrast, a positive correlation between 100-grain weight and Mn (r= 0.61) found among the 20 genotypes was not detected among the 240 genotypes.
Table 3 Major grain physical and nutritional properties, and N:P factor in 20 selected genotypes

a Testa colour: WT = white, CR = cream, LBN = light brown, BN = mid-brown, DBN = dark brown, RD = red, and BK = black.
b Eye colour: W = brown splash or gray, T = tan brown, B = blue to black, and S = mottled.
c Gweight = 100-grain weight.
d CT = cooking time.
e CP = crude protein.
In general, the genotypes with a rough testa had a shorter cooking time than those with a smooth texture, but TVu-14691 and TVu-4316, with a smooth texture, had fairly short cooking times (Table 3). Testa texture and colour showed no particular relationship with any proximate contents among these 20 genotypes.
Most of the AAs showed a stable composition ratio among the 20 genotypes, although arginine, Trp and cysteine showed greater variance (Fig. 1 and Table S3 (available online)). No relationship between seed characteristics and AA composition was observed. From the total AA, total AA N and total AA residues, an average N:P factor of 5.45 was calculated for the 20 selected genotypes (Table 3).

Fig. 1 Amino acid (AA) composition (as % of total) of 20 selected genotypes. For each AA, the column shows the average value of all 20 genotypes, and the bar indicates the range of the highest and lowest values measured.
Total dietary fibre content ranged widely, from 9.8 to 21.4 g/100 g, and 82–100% was insoluble (Table 4). Three oligosaccharides, stachyose, sucrose and raffinose, were detected, and a weak peak eluted following stachyose was considered to be verbascose (Phillips and Abbey, Reference Phillips and Abbey1989). Since we could not obtain authentic verbascose and little was detected, the content was not quantified. Among the 20 genotypes, sucrose showed wider variance (9.2–39.3 mg/g) than stachyose (24.1–43.8 mg/g) and raffinose (1.7–4.5 mg/g). The oligosaccharide contents were not correlated. Three improved varieties, IT93K-452-1, IT90K-277-2 and IT98K-205-8, showed low contents of stachyose (24.1–28.8 mg/g) and raffinose (2.5–2.9 mg/g), while TVu-12565 showed high contents (43.8 and 4.2 mg/g).
Table 4 Dietary fibre, oligosaccharide, phytic acid and polyphenol contents, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity in 20 selected genotypes

a DPPH IC50: half maximal (50%) inhibitory concentration of DPPH.
N.D., not detected.
KVx61-1 and Aloka, two popular varieties with a sweeter taste, showed exceptionally high-sucrose contents, yet Ife Brown, a popular sweet variety in southeastern Nigeria, showed almost the lowest sucrose content. β-Amylase activity showed wide variation, but no relationship between that and sweetness was suggested (data not shown).
Phytic acid contents ranged from 21.8 to 37.0 mg/g, and polyphenol contents ranged from 0.1 to 48.8 mg/g. DPPH IC50 values ranged widely, from 28.8 to 1403.9 mg/g, a factor of approximately 50, and did not show any relationship with phytic acid or polyphenol contents. Although TVu-14691, with a red testa, had the highest polyphenol content, no relationship between polyphenol content and testa colour was suggested.
Generally, linoleic acid (18:2), linolenic acid (18:3), palmitic acid (16:0), oleic acid (18:1) and stearic acid (18:0) were the major fatty acids (Table S4, available online). There were some variations among genotypes (e.g. 4.0–27.6 mg/g oleic acid). Considering the low lipid content (2.7–5.7%), the variation observed among 20 genotypes makes only a minor contribution to nutritional value, though it may influence the flavour.
Discussion
In sub-Saharan Africa, where the cost of meat can be high, relative to the average income in the region, cowpea, as an affordable source of crude protein, can contribute greatly to nutritional intake. At the same time, the crop is also an important source of cash income for farmers, especially in drier regions, who have limited options for cash crops. Regarding its important role in the region, strategies for the improvement of cowpea must take into consideration its physical, nutritional/anti-nutritional and functional properties, which influence consumers' choices and consumption.
As in our earlier studies (Boukar et al., Reference Boukar, Massawe, Muranaka, Franco, Maziya-Dixon, Singh and Fatokun2011, Reference Boukar, Muranaka, Franco, Fatokun, Boukar, Coulibaly, Fatokun, Lopez and Tamo2012), we found wide genetic variation in crude protein and micronutrient contents in cowpea, which suggests the possible improvement of nutritional value through breeding. The 240 genotypes we analysed showed wide genetic variation in agronomic traits and in physical and nutritional properties, which was adequately represented by the 20 genotypes selected for detailed analysis.
A factor of 6.25 is typically used to calculate the crude protein content from the N content of legumes, although much lower factors, ranging from 5.32 to 6.03, have been suggested (Sosulski and Holt, Reference Sosulski and Holt1980; Fujihara et al., Reference Fujihara, Sasaki and Sugahara2010). From the AA composition of the grain of the 20 genotypes, we calculated an average factor of 5.45. On this basis, the range of crude protein contents in our previous study using 1541 genotypes, 17.5 to 32.5% (Boukar et al., Reference Boukar, Massawe, Muranaka, Franco, Maziya-Dixon, Singh and Fatokun2011), is equivalent to 15.3 to 28.3%, within which our results from the 240 genotypes comfortably fit. A factor of 5.45 for cowpea (or 5.6 for grain legumes in general; Sosulski and Holt, Reference Sosulski and Holt1980) will provide good estimates of crude protein content in cowpea.
The AA compositions among the 20 genotypes show that the composition ratio of each AA was stable, although contents varied among genotypes depending on their total protein contents. These patterns of AA composition are similar to previous results (Tshovhote et al., Reference Tshovhote, Nesamvuni, Raphulu and Gous2003; Fujihara et al., Reference Fujihara, Sasaki and Sugahara2010). As is the case among legumes, the genotypes had high contents of Lys but low contents of sulphurous AAs. These results suggest a greater scope in improving total protein contents than specific AAs.
Among both the 240 genotypes and the 20 selected genotypes, there were strong positive correlations between the contents of crude protein and Fe and of Fe and Zn, as reported in earlier studies with 1541 genotypes (Boukar et al., Reference Boukar, Massawe, Muranaka, Franco, Maziya-Dixon, Singh and Fatokun2011) and 24 elite cowpea lines (Boukar et al., Reference Boukar, Muranaka, Franco, Fatokun, Boukar, Coulibaly, Fatokun, Lopez and Tamo2012). It is also in agreement with the positive correlation between crude protein and Fe contents in 11 populations reported by Moura et al. (Reference Moura, Rocha, Gomes, Filho, Silva and Ribeiro2012). Earlier reports suggested the certain heritability for crude protein content in cowpea (Nielsen et al., Reference Nielsen, Brandt and Singh1993; Tchiagam et al., Reference Tchiagam, Bell, Nassourou, Njintang and Youmbi2011). These facts indicate the possibility of improvement of crude protein, Fe and Zn contents without adverse interactions. However, further investigation of the genetics of these nutritional values and influence of environmental factors are needed to develop effective breeding strategies, since initial attempts in rice for higher Fe contents have indicated a complex mode of inheritance, demonstrating additive and dominant gene and environmental effects (Gregorio, Reference Gregorio2002). Genotypes IT97K-1042-3, IT98K-205-8 and IT98K-503-1, which we had previously nominated as potential genetic resources with high crude protein contents (26.1–29.0%, recalculated with the N:P conversion factor of 5.45) (Boukar et al., Reference Boukar, Muranaka, Franco, Fatokun, Boukar, Coulibaly, Fatokun, Lopez and Tamo2012), again showed consistently high crude protein contents.
Testa texture and colour and seed size are important criteria for consumers (Coulibaly and Lowenberg-DeBoer, Reference Coulibaly, Lowenberg-DeBoer, Fatokun, Tarawali, Kormawa and Tamo2002; Ibro et al., Reference Ibro, Lowenberg-DeBoer and Fulton2005). A rough testa texture and a small seed size are associated with a shorter cooking time (Nielsen et al., Reference Nielsen, Brandt and Singh1993). Our results support this association in general, although some genotypes with a smooth testa texture and a large seed size had a shorter cooking time. As Moura et al. (Reference Moura, Rocha, Gomes, Filho, Silva and Ribeiro2012) suggested that crude protein content and cooking time were not correlated in their analysis with 11 populations, we found no clear correlation in the 20 genotypes. They also reported a negative correlation between crude protein content and grain size, but we found no relationship between crude protein and any grain size parameter in the 240 or 20 genotypes. Certain heritability on shorter cooking time has been reported in cowpea, and the possible improvement of cooking time was suggested (Nielsen et al., Reference Nielsen, Brandt and Singh1993; Mashi, Reference Mashi2006). However, there are several reports suggesting that smooth testa texture that associates with a longer cooking time was dominant to rough testa texture in cowpea (Franckowiak, Reference Franckowiak1973; Drabo, Reference Drabo1981), while small seed size seems partially dominant to large seed size (Drabo et al., Reference Drabo, Redden, Smithson and Aggarwal1984). Since cooking time and grain size are important quality-related traits and influence consumer choice, clear breeding strategy based on the genetics behind and careful selection of parental materials are needed.
Phytic acid is the major inhibitor of Fe and Zn absorption from cowpea and is more tolerant of heat-cooking than other anti-nutritional factors (Akpapunum and Achinewhu, Reference Akpapunum and Achinewhu1985; Ogun et al., Reference Ogun, Markakis and Chenoweth1989; Abizari et al., Reference Abizari, Moretti, Schuth, Zimmermann, Armar-Klemesu and Brouwer2012). In addition, polyphenolic compounds can interact with crude protein and reduce its digestibility, and alter AA availability and functional properties (Lin et al., Reference Lin, Humbert and Sosulski1974). Conversely, polyphenols and phytic acid may benefit human health as antioxidants (Singh, Reference Singh2012). Polyphenol and phytic acid contents showed no relationship with testa colour and no correlation with DPPH IC50 value. Oboh (Reference Oboh2006) reported that the high phytate content of brown cowpea varieties did not contribute to free radical scavenging ability. Although genotypes with red and black testa colour had a high DPPH radical scavenging activity, these results indicate the contribution of other factors to anti-oxidative activity. Further investigation of the relationships among the traits is required for their better understanding. We found relatively wide variance in phytic acid and polyphenol contents in the grain among the 20 genotypes, among which TVu-12802 and TVu-467 had low ratios of phytic acid to Fe (0.36–0.42) and Zn (0.49–0.58) contents. TVu-12802 also had a low level of polyphenols and high levels of crude protein, Zn, Mn and Cu, while TVu-14691 had high levels of phytic acid and polyphenols and low levels of crude protein and micronutrients.
The presence of flatulence-causing oligosaccharides continues to pose problems with regard to complete nutritional utilization of cowpea, and even to its consumption. We observed wide genetic variation in both stachyose and raffinose, as reported previously (Obigbinde and Akinyele, Reference Obigbinde and Akinyele1983). Interestingly, the raffinose contents of the 20 genotypes were much lower than reported previously (Obigbinde and Akinyele, Reference Obigbinde and Akinyele1983), although the stachyose contents were similar to those reported previously (Obigbinde and Akinyele, Reference Obigbinde and Akinyele1983; Prinyawiwatkul et al., Reference Prinyawiwatkul, Beuchat, McWatters and Phillips1996). IT93K-452-1 had the lowest stachyose content (55% of the highest), and IT93K-503-1 had the lowest raffinose content (38% of the highest). Alkali treatment using potash (sodium sesquicarbonate and sodium bicarbonate), as used in Africa to reduce the cooking time and alter the texture of the grain, can decrease stachyose contents by 61% and raffinose contents by 69% (Onyenekwe et al., Reference Onyenekwe, Njoku and Ameh2000). The results indicate the potential to breed improved varieties with less flatulence-causing effects, and to contribute to enhanced consumption in combination with suitable cooking methods. Among the 20 selected genotypes, three early-maturing improved breeding lines, IT93K-452-1, IT90K-277-2 and IT98K-205-8, can be recommended for breeding for a lower content of flatulence-causing oligosaccharides.
This study has identified genetic diversity in various grain quality-related traits within cowpea germplasm and breeding lines and major local varieties, and relationships among them. Strong correlations among crude protein, Fe and Zn contents suggest the possibility of improving the concentrations of these nutrients simultaneously. Low associations among physical, nutritional/anti-nutritional and functional properties suggest the possibility of introgressing favorable traits from the genetic resources. Several potential genotypes with a variety of quality-related traits that can be matched to consumer preferences and that enhance the nutritional value of the grain were identified and could therefore be used in crossing to generate segregating populations. The fundamental information and genetic resources that we have collected will help to reveal the reasons underlying the correlation between explanatory variables and grain quality-related traits. They will stimulate the development of varieties with excellent agronomic characteristics and favorable grain quality-related traits, helping to ameliorate the poverty and nutrient deficiencies common among developing communities.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S147926211500009X
Acknowledgements
This work was conducted as part of the international collaborative research project ‘Strategic approach to develop value-added cowpea varieties with higher food and nutrition quality (EDITS-Cowpea)’ funded by the Japan International Research Center for Agricultural Sciences (JIRCAS).







