Introduction
Lying between the Antarctic Peninsula and South America, the Drake Passage is the shortest path to the Antarctic coasts. However, this passage is crossed by an intense oceanic and atmospheric circulation that has configured for Antarctica a continuous biogeographic barrier during the last ~23 million years (Myr) (Clarke et al., Reference Clarke, Barnes and Hodgson2005; Barnes et al., Reference Barnes, Hodgson, Convey, Allen and Clarke2006). Despite this fact, and the harsh polar conditions, two vascular plants are established in Antarctica: Deschampsia antarctica Desv. (Poaceae) and Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) (Convey, Reference Convey1996). Antarctic species could be divided into Recent Holocene (~11,000 years) immigrants, or ancient inhabitants with a Mid-Early Cenozoic origin or even Late Cretaceous origin (Convey and Stevens, Reference Convey and Stevens2007). Nevertheless, the latter would imply that these species have survived periodically in highly isolated glacial refugia, at least since Late Neogene, ~2.5 Myr BP (Barnes et al., Reference Barnes, Hodgson, Convey, Allen and Clarke2006). In this sense, ancient (biogeographic) and recent (demographic) processes have been proposed to explain the current presence of vascular plants in Antarctica (Mosyakin et al., Reference Mosyakin, Bezusko and Mosyakin2007; Parnikoza et al., Reference Parnikoza, Kozeretska and Kunakh2011). Indeed, there is evidence of glacial refugia for terrestrial biota (Stevens et al., Reference Stevens, Greenslade, Hogg and Sunnucks2006; Convey and Stevens, Reference Convey and Stevens2007; Convey et al., Reference Convey, Gibson, Hillenbrand, Hodgson, Pugh, Smellie and Stevens2008), but for Antarctic vascular plants, current information, besides being scarce, seems not to be consistent with this hypothesis (Mosyakin et al., Reference Mosyakin, Bezusko and Mosyakin2007). On the other hand, the presence of both species in cores from Mid-Holocene peats (~6000 years BP) is the only concrete evidence of their historical presence in Antarctica (Birkenmajer et al., Reference Birkenmajer, Ochyra, Olsson and Stuchlik1985).
Current genetic information of Antarctic vascular plants has been obtained almost exclusively from D. antarctica (Chwedorzewska and Bednarek, Reference Chwedorzewska and Bednarek2008, Reference Chwedorzewska and Bednarek2011; van de Wouw et al., Reference van de Wouw, van Dijk and Huiskes2008; Volkov et al., Reference Volkov, Kozeretska, Kyryachenko, Andreev, Maidanyuk, Parnikoza and Kunakh2010; but see Gianoli et al., Reference Gianoli, Inostroza, Zúñiga-Feest, Reyes-Díaz, Cavieres, Bravo and Corcuera2004); despite this, both species have been reported as being highly similar when compared with non-Antarctic conspecifics (Gianoli et al., Reference Gianoli, Inostroza, Zúñiga-Feest, Reyes-Díaz, Cavieres, Bravo and Corcuera2004) and even congeneric populations (Chwedorzewska et al., Reference Chwedorzewska, Bednarek and Puchalski2004). This would support the Holocene origin of Antarctic vascular plant populations because if they had remained in glacial refugia, high differentiation of these species would have been expected (Convey et al., Reference Convey, Gibson, Hillenbrand, Hodgson, Pugh, Smellie and Stevens2008). Moreover, for D. antarctica, a recent stepping-stone process of dispersion into Antarctica (north to south) has been proposed, with a decreasing latitudinal gradient of its genetic diversity among sub-Antarctic and Antarctic populations being observed as moving further south (van de Wouw et al., Reference van de Wouw, van Dijk and Huiskes2008). For C. quitensis, a perennial herb which is distributed from Mexico to Antarctica (Smith, Reference Smith, Huiskes, Gieskes, Rozema, Schorno, van der Vies and Wolff2003), the actual genetic information was not captured on the population scale (Gianoli et al., Reference Gianoli, Inostroza, Zúñiga-Feest, Reyes-Díaz, Cavieres, Bravo and Corcuera2004). Therefore, we present here the first estimation of genetic diversity for Antarctic populations of C. quitensis, and its pattern of spatial distribution across the most plausible path for Antarctic colonization.
Experimental
Samples from 15 individuals per site, separated by at least 1 m, were collected in three populations of C. quitensis across the Drake Passage (Fig. 1). In the fourth site (Antarctic Peninsula), only seven individuals were sampled due to the small size of the population. Total DNA from these samples was obtained by the cetrimonium bromide (CTAB) protocol (Doyle and Doyle, Reference Doyle and Doyle1987). Amplified fragment length polymorphism (AFLP) was applied using the original protocol (Vos et al., Reference Vos, Hoger, Bleeker, Reijans, Van de Lee, Hornes, Frijters, Pot, Peleman, Kuiper and Zabeau1995) and the manufacturer's recommendations for the capillary sequencer analysis of DNA fragments (PE Applied Biosystems, 1996). DNA fragments were amplified twice by the PCR using complementary primers for the EcoRI and MseI adaptors, plus one and three nucleotides as selective factors. After validating the results for one sample of each population, 16 combinations of primer pairs were analysed (Genographer 1.1.0; Montana State University, 1998), obtaining a total of 331 loci from two combinations of selective primers (M+CA/E+ACA and M+CA/E+ACT). The analysis of molecular variance (AMOVA) was carried out using GENALEX-6 software (Peakall and Smouse, Reference Peakall and Smouse2006). Also, the mean number of alleles per locus (A) and mean expected heterozygosity (H e) as diversity estimators (Frankham et al., Reference Frankham, Briscoe and Ballou2002) were obtained.

Fig. 1 Spatial distribution of Colobanthus quitensis populations analysed in this study: Punta Arenas: 53°51′S–70°57′W; Arctowski: 62°09′S–50°28′W; Hannah Point: 62°39′S–60°36′W; Antarctic Peninsula: 64°53′S–62°54′W. Geographic (km) and genetic (Nei-D) distances are provided for each between-site link. Significant genetic differentiation between the sites is indicated by an asterisk at the end of the link's note. *P< 0.05 (Tukey's HSD) for the respective pairwise comparison.
Discussion
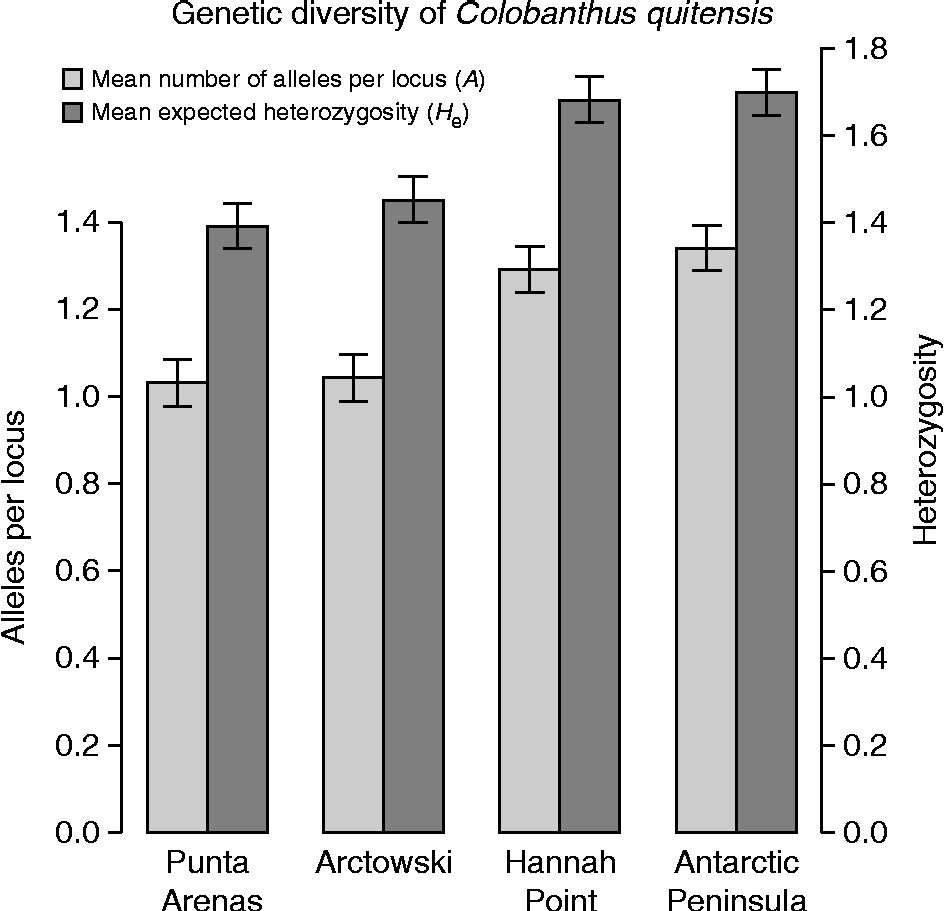
Although low levels of genetic diversity were found among the four populations (H e(all)± 1 SE = 0.155 ± 0.01; A (all)± 1 SE = 1.175 ± 0.03), southern sites had significantly higher levels of allelic richness as well as higher expected heterozygosity (Hannah Point and Antarctic Peninsula; Fig. 2). Interestingly, significant pairwise genetic differentiation, as measured by Tukey's honestly significant difference (HSD) test for unequal N (Zar, Reference Zar1999), was found between the populations, except in the pair Hannah Point–Arctowski. This suggests allelic differences despite the low overall mean number of alleles per locus (A (all)), even between the Antarctic pearlwort populations and their neighbouring South Shetland populations. Surprisingly, the South American populations showed the lower levels of both genetic diversity estimators (Fig. 2). The AMOVA confirmed a significant genetic population structure (AMOVAINTER: df = 3; SS = 582.6; MS = 37.5; F= 182.9; P= 0.001), with 71% of genetic variability being explained by populations and the remaining 29% explained by individuals within populations.
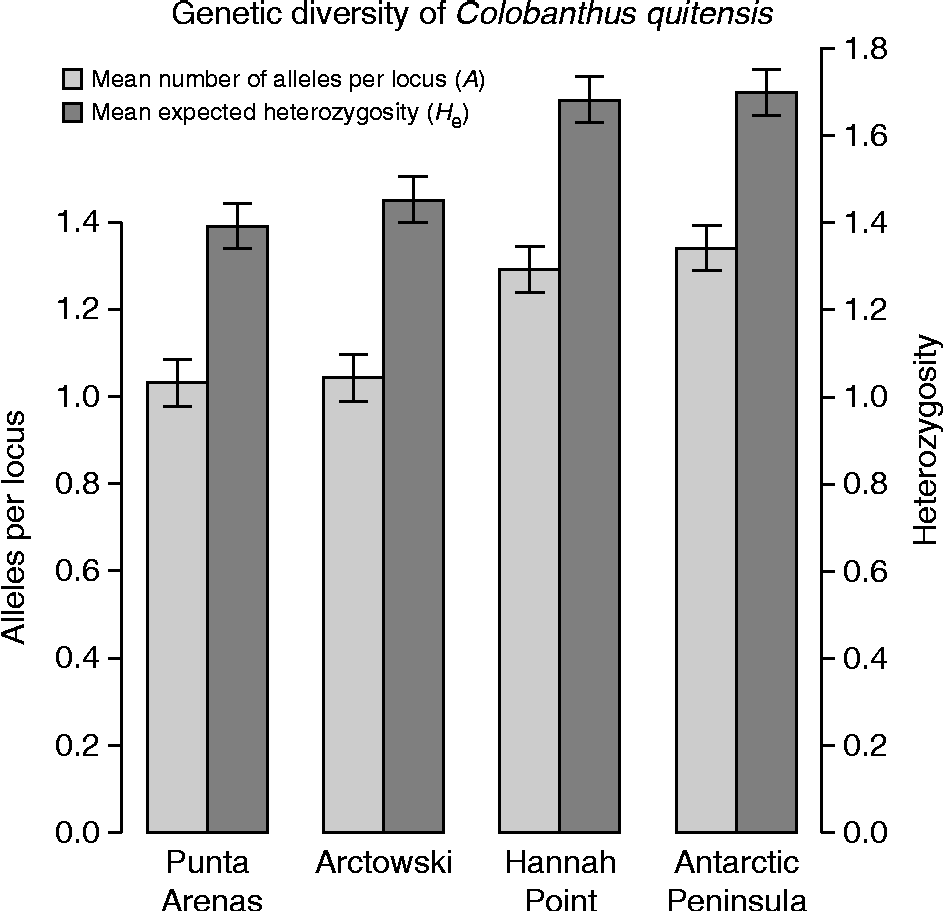
Fig. 2 Genetic diversity of Colobanthus quitensis populations expressed as the mean number of alleles per locus (A) and mean expected heterozygosity (H e). Sites are organized from left to right as they appear in the north-to-south latitudinal gradient. Error bars show ± 1 SE.
A north-to-south increase of genetic diversity suggested that, opposite to D. antarctica, a recent stepping-stone process is not such a plausible hypothesis for C. quitensis to explain its presence in Antarctica. Certainly, the observed genetic diversity distribution (higher at the southern sites) could have been generated recently (i.e. during the Holocene) by random long-distance dispersal events (Muñoz et al., Reference Muñoz, Felicísimo, Cabezas, Burgaz and Martínez2004; Parnikoza et al., Reference Parnikoza, Dykyy, Ivanets, Kozeretska, Kunakh, Rozhok, Ochyra and Convey2012), but the idea of glacial shelters remains somehow latent. And this is not just for the higher diversity levels found in the Antarctic Peninsula; instead, the low values observed in Punta Arenas (South America) seem counterintuitive thinking in the total range of distribution of C. quitensis. The apparent high potential for connectivity, and thus for genetic variability of any South American pearlwort population compared with those from Antarctica, was the motive for the inclusion of just one South American point in our gradient. We nonetheless recognize that this is not an extensive genetic study, and it is clear that additional South American and Antarctic populations are needed to unravel the connectivity history of these species in the Southern Hemisphere. However, this is the first AFLP approximation to the genetic variability of C. quitensis and its distribution among Antarctic populations and, for now, insights of a new pattern of genetic diversity distribution seem to arise for Antarctic vascular plants. Detailed and extended research should be conducted in this group of species, which, albeit small, encloses a true enigma among Antarctic biota.
Acknowledgements
This work was funded by the INACH T-14-08 project of the Chilean Antarctic Institute (INACH). We thank INACH, the Chilean Navy and the Arctowski Polish Antarctic Station for their logistical support. We also thank K.J. Chwedorzewska and one anonymous reviewer for their valuable comments. The corresponding author thanks Arturo Restrepo for his opportune assistance.