Introduction
Cassava (Manihot esculenta Crantz) is one of the most important staple crops in sub-Saharan Africa, where it is particularly popular with low-income, women-led households (Nweke et al., Reference Nweke, Spencer and Lynam2001). Its popularity is derived from its inherent resilience to abiotic stresses, low input requirements, the diversity of uses to which its starchy roots are amenable and its flexible harvest date which allows it to be ‘banked’ in the soil as a food reserve (Nweke et al., Reference Nweke, Spencer and Lynam2001). In sub-Saharan Africa, cassava roots are primarily used as food in either fresh or processed form. In addition, cassava leaves are an important vegetable and animal feed in some communities (Lutaladio and Ezumah, Reference Lutaladio, Ezumah, Tenry, Oduri and Cavaness1981). Some of the major cassava-producing countries in Africa include: Nigeria; Democratic Republic of Congo (DRC); Ghana; Tanzania; Mozambique; Uganda; Madagascar. To date, the vast majority of cassava varieties from southern, eastern and central (SEC) Africa are not genetically conserved in a formal conservation programme, but they do exist in farmers' fields and National Agricultural Research Systems (NARS) breeding programmes. Cassava germplasm in this region comprises both local farmer varieties (landraces) and cultivars that are derived from formal breeding either by the International Institute of Tropical Agriculture (IITA) or by the NARS. The effect of formal breeding on the genetic diversity of cassava is not known.
The genetic diversity of cassava in SEC Africa has most probably been influenced by early introductions, and also by major pandemics of cassava mosaic disease (CMD) and the current pandemic of cassava brown streak disease (CBSD) (Cours et al., Reference Cours, Fargette, Otim-Nape and Thresh1997; Otim-Nape et al., Reference Otim-Nape, Bua, Thresh, Baguma, Ogwal, Semakula, Acola, Byabakama and Martin1997; Legg et al., Reference Legg, Jeremiah, Obiero, Maruthi, Ndyetabula, Okao-Okuja, Bouwmeester, Bigirimana, Tata-Hangy, Gashaka, Mkamilo, Alicai and Kumar2011). In addition, farmers retain their planting material from year to year and make selections according to their own preferences. Traditionally, farmers also exchange germplasm with friends and relatives in other regions. It is therefore a rational hypothesis that genetic diversity of cassava in the region has been moulded into patterns of diversity.
Genetic diversity of cassava in Africa has been assessed using a range of molecular markers (Fregene et al., Reference Fregene, Bernal, Duque, Dixon and Tohme2000; Fregene et al., 2003; Zacarias et al., Reference Zacarias, Botha, Labuschagne and Benesi2004; Balyejusa Kizito et al., 2005; Benesi, 2005). These assessments have resulted in varying outputs, involving different analysis methods and applications. Other key cassava-producing countries in the region, notably DRC, Madagascar and Rwanda, have not undertaken any significant assessment of cassava diversity. Detailed knowledge on genetic structure and variability of cassava germplasm is critically important for setting effective national and regional conservation priorities, for identifying ‘gaps’ in diversity and/or for defining breeding strategies. The objective of this study was to understand the nature, extent, distribution and hierarchical organization of genetic variation that exists within the NARS breeding programmes of seven countries in SEC Africa: Tanzania; Uganda; Kenya; Rwanda; DRC; Madagascar; Mozambique. Genetic diversity was assessed using simple sequence repeat (SSR) markers that are frequent in eukaryotic genomes (Tautz and Renz, Reference Tautz and Renz1984), show high levels of allelic diversity and are relatively stable and co-dominant (Morgante and Olivieri, Reference Morgante and Olivieri1993; Toro et al., Reference Toro, Fernàndez and Caballero2009).
Materials and methods
Cassava germplasm
The germplasm included in this study consisted of cultivars developed through formal breeding and landraces (farmer varieties). Some of this germplasm was available within the NARS breeding programmes but the majority of the NARS undertook specific collections from farmers' fields to supplement the germplasm base for this study. It provides a reasonable representation of cassava germplasm in each country. Germplasm from each country was grown in a phenotyping trial (Kawuki et al., Reference Kawuki, Ferguson, Labuschagne, Herselman, Orone, Ralimanana, Bidiaka, Lukombo, Kanyange, Gashaka, Mkamilo, Gethi and Obiero2011) from which young fresh leaf samples were collected on dry ice: Tanzania (270 genotypes); Uganda (268); Kenya (234); Rwanda (184); DRC (177); Madagascar (186); Mozambique (82). Of the 1443 plants sampled originally, 1401 cassava genotypes (848 landraces and 553 cultivars) were successfully assayed and used in the analysis. Genotype name, country of origin and classification into cultivars and landraces are given in Supplementary Material 1 (available online only at http://journals.cambridge.org) and summarized in Table 1. Total genomic DNA was extracted using a modified protocol described by Dellaporta et al. (Reference Dellaporta, Wood and Hicks1983). DNA quality was assessed on 0.8% agarose gels, quantified using the NanoDrop ND-1000 and then diluted to 50 ng/μl.
Table 1 Allelic richness and gene diversity of cassava germplasm used in this study

Tan, Tanzania; Uga, Uganda; Ken, Kenya; Rwa, Rwanda; DRC, Democratic Republic of Congo; Mad, Madagascar; Moz, Mozambique; land, landrace; cult, cultivar.
a Allelic richness was computed using the rarefaction method as described by El Mousadik and Petit (Reference El Mousadik and Petit1996).
b H o is the proportion of heterozygous individuals in a population.
c Nei's unbiased estimator of gene diversity.
Microsatellite genotyping and allele calls
Cassava genotypes were analysed for polymorphism using 26 genomic SSR markers (SSRY5, SSRY9, SSRY12, SSRY19, SSRY21, SSRY38, SSRY51, SSRY52, SSRY59, SSRY63, SSRY64, SSRY69, SSRY100, SSRY102, SSRY110, SSRY135, SSRY147, SSRY148, SSRY151, SSRY155, SSRY161, SSRY169, SSRY171, SSRY181 and SSRY182 (Mba et al., Reference Mba, Stephenson, Edwards, Melzer, Nkumbira, Gullberg, Apel, Gale, Tohme and Fregene2001) and NS911 (A. Zarate, personal communication)). Twenty-four of these SSRs had previously been used in a global diversity assessment of more than 2000 cassava genotypes and found to be highly informative and provided a similar overall population structure to that provided by 30 SSR loci (Hurtado et al., 2008; M. Ferguson, personal communication). These loci were furthermore selected based on single-locus amplification, high degree of polymorphism and reproducibility.
Amplifications with SSR primers were performed in 10 μl reactions containing 50 ng DNA, 1 pmol of each primer, 1 × Taq polymerase buffer, 2 mM MgCl2, 0.2 mM dNTPs and 0.375 U Taq polymerase (New England Biolabs, Inc., Ipswich, MA). The PCR profile was 95°C for 2 min, followed by 30 cycles at 95°C for 30 s, 55–57°C for 1 min and 72°C for 1 min and a final extension at 72°C for 30 min. Different-sized amplification products at different loci and variable fluorescent labels (NED, 6-FAM, PET and VIC; MWG-Biotech) on the forward primer allowed multiplexing of amplicons from the same individual. Seven co-loading sets were optimized and used for the entire genotyping. For each co-loading set, 1–2 μl, depending on amplification efficiency, of the different amplicons were mixed and briefly vortexed. Aliquots of 1 μl of the mixture were added to 9 μl of a master mix containing 1 ml HiDi formamide and 12 μl GeneScan 500 LIZ size standard.
Amplicons were denatured (95°C, 3 min) and subjected to capillary electrophoresis using the ABI 3730 DNA sequencer (Applied Biosystems) and allele calls made using GeneMapper® software version 3.7 (Applied Biosystems® by Life Technologies, Carlsbad, CA). All genotyping was done at the Biosciences eastern and central Africa (BecA) Hub in Nairobi, Kenya. The DNA of sample TMS 30 572 was used as a control. All genotypic data are provided in Table S1 (available online only at http://journals.cambridge.org).
Statistical analysis
Initially, genetic diversity statistics were calculated according to country of origin and according to 14 groups representing country-landrace/cultivar. These statistics included count of alleles per locus, allele frequency per locus, effective number of alleles (A e) (Kimura and Crow, Reference Kimura and Crow1964), observed heterozygosity (H o), expected heterozygosity (Nei's gene diversity; H e) and Shannon's information index (Lewontin, Reference Lewontin1972) calculated using POPGENE v1.32. H e is defined by Nei (Reference Nei1973) as the probability that two alleles chosen at random from a population are different, and was computed using the formula H e= 1 − Σp il2, where p il is the frequency of the ith allele at the lth locus. Allelic richness (number of alleles segregating in the population), compensating for differences in sample size, was calculated by adapting the rarefaction index as suggested by El Mousadik and Petit (Reference El Mousadik and Petit1996). Allele frequency and the number of rare alleles, defined as having a frequency < 0.05, were also computed on a per-country basis using FSTAT version 2.9.3.2 (Goudet, 2001). Quantification of random mating and genetic differentiation was examined using F-statistics (Weir, Reference Weir1990) in PowerMarker version 3.25 (Liu and Muse, Reference Liu and Muse2005).
A non-model-based approach, principal component analysis (PCA), was applied to all genotypes according to country of origin, and landraces and cultivars separately, according to country of origin, to elucidate genetic relationships. For this analysis, any clone that had more than two missing SSRs (~10%) was omitted, leaving 1004 clones when landraces and cultivars were considered together, 616 when only landraces were analysed and 388 when cultivars were considered alone. The remaining missing data were replaced with the average value, and analysis conducted using Prcomp function in R (R Development Core Team, 2010).
Genetic relationships among landraces from different countries, cultivars from different countries, and landraces and cultivars from different countries were also calculated using the simple-matching Euclidean distance (DE) followed by cluster analysis using the weighted neighbour-joining algorithm in PowerMarker version 3.25 (Liu and Muse, Reference Liu and Muse2005). Relationships among the groups were visualized as a dendrogram using PowerMarker version 3.25 (Liu and Muse, Reference Liu and Muse2005).
In addition, a model-based approach was also applied to determine genetic structure. The number of populations (K) was determined with the software STRUCTURE using the admixture model (Pritchard et al., Reference Pritchard, Stephens and Donnelly2000). This Bayesian-based model assumes that each individual inherited some portion of its ancestry from each one of the K populations. Initially, the length of the burn-in period and Markov Chain Monte Carlo (MCMC) replications were set at 10,000 for K= 1–17 (number of putative populations in the model); this was repeated ten times. The ΔK statistic, which is based on the rate of change in the log probability of the data between successive K-values, was then used to detect the true number of K populations in the dataset (Evanno et al., Reference Evanno, Regnaut and Goudet2005). The determined population K was rerun at a burn-in period and MCMC replications of 100,000 to verify its consistency. A membership coefficient of Q>0.9 was used throughout.
Results
Allelic richness and gene diversity
Of the 1443 cassava cultivars and landraces genotyped for 26 SSR loci, 42 had more than 10% missing data and were excluded from this analysis. All genotypic data are provided in Table S1 (available online only at http://journals.cambridge.org). A total of 192 alleles were detected, giving an average of 7.38 alleles per locus. All loci were polymorphic in all countries. Generally, rare alleles ( < 5% frequency) were more prevalent in the studied germplasm than alleles of higher frequency (see Table S2 for actual allele frequencies and Table S3 for a summary, available online only at http://journals.cambridge.org). Alleles with frequencies >0.3 were few. When only rare alleles ( < 5% frequency) were analysed (Table S3, available online only at http://journals.cambridge.org), it was generally observed that they were more frequent in germplasm from the DRC, although landraces from Tanzania had the largest proportion of rare alleles (35.2%). The results further indicated that no rare alleles were observed in cultivars from Mozambique and that two loci (SSRY19 and SSRY110) were associated with higher numbers of rare alleles.
Gene diversity and allelic richness statistics among cassava germplasm from the seven countries and country-landrace/cultivar are presented in Table 1. Landraces harboured 186 alleles, whereas cultivars had 169. Allelic richness was very similar in pooled cultivars (88.37), as it was in pooled landraces (86.48) as was the mean A e (cultivars (3.131) and landraces (3.146)), Nei's (Reference Nei1973) gene diversity (H e) (cultivars (0.623) and landraces (0.633)) and Shannon's information index (cultivars (1.272) and landraces (1.261)). The overall mean H o was 0.57.
Landraces from all countries had similar levels of diversity in terms of allelic richness, mean A e, H e and Shannon's information index, with the DRC having marginally more diversity for all parameters, followed by Tanzania. The lowest diversity for all parameters was observed in Ugandan landraces. In terms of the cultivars, cassava from the DRC once again had the highest values for allelic richness, A e, H e and Shannon's information index, followed by those from Kenya. The lowest values were observed in the Tanzanian and Mozambican cultivars.
Genetic variance distribution and genetic differentiation
Wright's F-statistics on all 1401 genotypes grouped into 14 populations (countries-landraces/cultivars) indicated a very slight deficit of heterozygotes within populations with F IS= 0.0084. The overall F IT value (0.1113) also indicated a slight deficit of heterozygotes in the total population. The F ST value between all 14 populations (countries-landraces/cultivars) was 0.1038, indicating moderate differentiation. When seven landrace populations were considered, representing the seven countries, F IS was slightly negative ( − 0.0053), basically indicating random mating, F ST was 0.1040 and F IT was 0.0992, indicating moderate differentiation among populations. When seven cultivars were considered, representing the seven countries, F IS was slightly higher, but still very low (0.0298), F ST was 0.0845 and F IT was 0.1118, indicating some deviation from random mating and moderate differentiation among populations. The first two principal components (PCs) of PCA of 1004 genotypes accounted for 52 and 7% of the variation, respectively (Fig. 1(a)). This revealed a tendency for the germplasm from Uganda and DRC to be somewhat differentiated from the germplasm from other countries.

Fig. 1 (colour online) Principal component (PC) analysis shows some differentiation among cassava: (a) cultivars and landraces pooled, (b) landraces and (c) cultivars from the seven countries: Uganda (red); DRC (dark green); Rwanda (pink); Kenya (blue); Madagascar (black); Mozambique (light green); Tanzania (gold).
Genetic relationships among landraces
The first two PCs accounted for 56 and 10% of the genetic variation in landraces from the seven countries (Fig. 1(b)). The greatest separation appeared to be in the germplasm from Uganda and DRC, to that from Mozambique. DEs among landraces on a country-of-origin basis are provided in Table 2. This shows that landraces from Tanzania are the most closely related to landraces from all other countries except for the DRC which is most closely related to Rwanda, followed by Tanzania. This relationship is not clearly depicted in a dendrogram. Landraces from Tanzania had the lowest mean genetic distance (0.218). Landraces from Mozambique and DRC are overall most distantly related to other countries with mean DEs of 0.306 and 0.294, respectively. Landraces from Mozambique were most distantly related to those from Uganda (0.408), followed by those from Rwanda (0.391). Landraces from the DRC were most distantly related to those from Kenya (0.378) and Mozambique (0.377).
Table 2 Frequency-based Euclidean distance among the 848 landraces from the seven countries within southern, eastern and central Africa
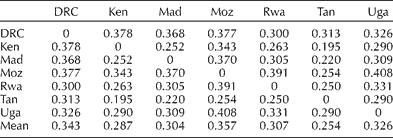
DRC, Democratic Republic of Congo; Ken, Kenya; Mad, Madagascar; Moz, Mozambique; Rwa, Rwanda; Tan, Tanzania; Uga, Uganda.
Genetic relationships among cultivars
Less differentiation was found among cultivars from different countries with the first two PCs accounting for 59 and 6% of the genetic variation (Fig. 1(c)). DE measures among cultivars from different countries are provided in Table 3, and are represented in a dendrogram (Fig. 2). Cultivars from Kenya, Uganda, DRC and Rwanda appear relatively similar, whereas those from Madagascar, Tanzania and Mozambique are more distinct. Madagascar had the highest mean DE (0.330), followed by Mozambique (0.314) and Tanzania (0.313). These were represented by 52, 9 and 21 cultivars each. Cultivars from Madagascar were most similar to those from Tanzania (0.328), and those from Tanzania were most similar to those from Kenya (0.313), followed by Madagascar. Cultivars from Mozambique were most similar to those from Kenya (0.292), followed by Rwanda (0.322).
Table 3 Frequency-based Euclidean distance among the 553 cultivars from the seven countries within southern, eastern and central Africa
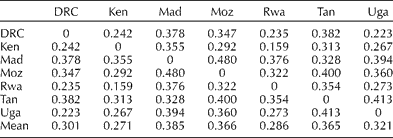
DRC, Democratic Republic of Congo; Ken, Kenya; Mad, Madagascar; Moz, Mozambique; Rwa, Rwanda; Tan, Tanzania; Uga, Uganda.
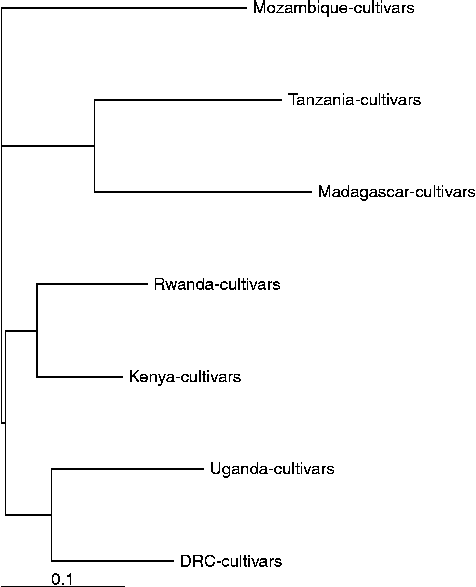
Fig. 2 Genetic relationships among cultivars from different countries in southern, eastern and central Africa based on simple-matching Euclidean distance and the weighted neighbour-joining algorithm using PowerMarker version 3.25.
Genetic relationships among cultivars and landraces
The relationship between cultivars and landraces from different countries is best revealed through STRUCTURE analysis. The results from STRUCTURE analysis indicated that the 1401 individuals genotyped largely belonged to three subpopulations, i.e. K= 3 (assignment of individuals to the three populations is presented in Fig. 3). ΔK values were K= 2 (24.982), K= 3 (33.601) and K= 4 (8.636). Cultivars from DRC, Mozambique, Kenya and Rwanda had over 70% of their individuals assigned to subpopulation 1. Nearly 40% of Ugandan cultivars were assigned to this subpopulation as well. Landraces from Madagascar, Tanzania, Mozambique and Kenya had over 70% of their individuals assigned to subpopulation 2. The majority of cultivars from Tanzania and Madagascar were also included in this group. A relatively similar number of individuals from the landraces from Rwanda were assigned between subpopulations 1 and 2. Over 80% of Ugandan landraces were assigned to subpopulation 3, together with 56.9% of Ugandan cultivars, while the landraces from the DRC were assigned between subpopulations 1 and 3.

Fig. 3 (colour online) Assignment of population structure using the admixture model of the 1401 individuals to three subpopulations (subpopulation 1 = red; subpopulation 2 = green; subpopulation 3 = blue): 1 = Ugandan cultivars; 2 = Ugandan landraces; 3 = DRC cultivars; 4 = DRC landraces; 5 = Malagasy cultivars; 6 = Malagasy landraces; 7 = Tanzanian cultivars; 8 = Tanzanian landraces; 9 = Mozambican cultivars; 10 = Mozambican landraces; 11 = Kenyan cultivars; 12 = Kenyan landraces; 13 = Rwandan cultivars; 14 = Rwandan landraces.
Discussion
A major objective of this research was to study the extent of genetic diversity in cassava landraces and cultivars immediately available within the cassava breeding programmes of Tanzania, Uganda, Kenya, Rwanda, DRC, Madagascar and Mozambique, and to ascertain any patterns of variation. It was hypothesized that the independent introductions of cassava into the East African region (Jones, Reference Jones1959), contrasting farmers' selection needs across the region, disease pandemics and germplasm movement, could have contributed to shape cassava into distinct groups or subpopulations. For this purpose, selectively neutral microsatellites that are suited for understanding population structure or history were used (Toro et al., Reference Toro, Fernàndez and Caballero2009). It is important to note that genetic models used for analysis are based on several assumptions, including random mating and sexual reproduction (Weir, Reference Weir1990). Data presented in this research were generated from representative samples of landraces (farmer varieties) and cultivars (derived from formal breeding). Cassava is a clonally propagated, outcrossing crop that is propagated through stem cuttings. Flowering is variable and the number of pods and seeds limited. Silva et al. (Reference Silva, da Bandel and Martins2003) performed a quantitative analysis of mating system parameters of ethno varieties of cassava using progeny arrays assayed for eight allozyme markers. They found the multilocus outcrossing rate (t m) to be 0.915 ± 0.04, with a range from 0.69 to 1.00, revealing high levels of outcrossing with variable levels of selfing.
Cassava was one of the first crops to arrive in Africa from the east coast of Brazil. After several years of debate on its origin (Allem, Reference Allem1994), molecular markers have provided strong evidence that cassava was probably domesticated from a single wild Manihot species (M. esculenta spp. flabellifolia Pohl) and that the crop originated from the southern Amazon basin (Olsen, Reference Olsen2004). It was from this region that cassava was exported to other parts of the world. The crop was first introduced to West Africa some time during the 1700 s (Jones, Reference Jones1959; Carter et al., Reference Carter, Fresco, Jones and Fairbairn1992). Thereafter, it was quickly adopted and rapidly spread within the West African region. Cassava was subsequently introduced to the continent via the East African coastline in the 1750 s, after the French introduced cassava from Brazil to Mauritius (Jones, Reference Jones1959). Thereafter, the crop was introduced to Madagascar in the early 19th century and to mainland Africa where it spread to various countries in the eastern, central and southern African region (Jones, Reference Jones1959; Langlands, Reference Langlands1966). Cassava also reached Lake Tanganyika in Tanzania from West Africa via Congolese farmers and farmer-to-farmer spread (Carter et al., Reference Carter, Fresco, Jones and Fairbairn1992). Further spread was reinforced by administrators in the region who encouraged farmers to grow cassava and increased cultivation in the late 19th and 20th centuries. The natural spread of the crop was facilitated by its vegetative propagation, hardiness and viability of cuttings (Masumba, 2006). The introduction of cassava into East and West Africa thus represents a ‘genetic bottleneck’ whereby Africa received a portion of the genetic diversity present in the crop's centre of origin. In addition, cassava in Africa was isolated from the great diversity available in Brazil and from the majority of its wild relatives.
Genetic diversity
Moderate to low levels of genetic variation were observed in all countries. Although the number of alleles per locus was relatively high (see Table S2, available online only at http://journals.cambridge.org), the majority of alleles occurred at a very low frequency ( < 5%), and therefore do not contribute much to heterozygosity. This is reflected in low values of Shannon's information index (between 0.95 and 1.25) and A e (between 2.37 and 3.06). Much higher values have been noted in Guyana where landraces grown at one location had the same level of genetic diversity (Shannon's information index = 4.293) as a sample from the core collection at the International Centre for Tropical Agriculture (CIAT) (Shannon's information index = 4.289) (Elias et al., Reference Elias, Panaud and Robert2000). This indicates a genetic bottleneck in both landraces and cultivars in SEC Africa. If a stepwise mutation model is assumed for the SSRs used in this study, then several alleles appear to be missing or at low frequency (see Table S2, available online only at http://journals.cambridge.org), providing further evidence of a genetic bottleneck.
Marginal differences were observed in allelic richness among the seven countries, with the highest number of segregating alleles being registered in the DRC (96) and lowest in Uganda (85). It appears that this difference largely stems from the number of rare alleles. This indicates that cassava from the DRC is slightly more genetically diverse than cassava from the other six surveyed countries. It is possible that, due to its location, the DRC may have received more cassava germplasm from West Africa, as well as from SEC Africa, contributing to the gene pool. New gene combinations are generated through unmanaged sexual reproduction between different cultivars within cassava stands in farmers' fields, but this does not increase SSR diversity.
Allelic richness, A e, H e and Shannon's information index were very similar in landraces and cultivars. Vellvé (Reference Vellvé1993) reported that formal breeding significantly reduced genetic diversity in European agriculture. Other studies have strongly demonstrated increased wheat genetic variability with formal breeding (Maccaferri et al., Reference Maccaferri, Sanguineti, Donini and Tuberosa2003). To date, it appears that there is limited genetic variability in both landraces and cultivars in SEC Africa, and that there is scope for increasing diversity through the introduction of germplasm from the centre of origin and diversity of cassava in Brazil.
Partitioning of variation
A high level of mean H o (H o= 0.57) was expected due to the outbreeding nature of cassava, as was an F IS close to zero, indicating random mating. This is consistent with reports of some degree of inbreeding in cassava which is likely to occur spontaneously in farmers' fields with progeny being selected from volunteer seedlings. In addition, if outcrossing occurs between two clonally propagated plants, it would appear as inbreeding. Farmers tend to grow a number of plants of the same clonally propagated genotype in close proximity; this may tend to overestimate levels of true inbreeding. In addition, it appears that the cassava inflorescence architecture and seed dispersal mechanisms limit long-distance gene flow, but favour mating among plants in close proximity, which would also tend to reduce heterozygosity. Besides inbreeding, high frequencies of null alleles (Brookfield, Reference Brookfield1996) have been reported to cause heterozygosity deficits within populations. The estimate on the frequency of null alleles was computed using the method of Brookfield (Reference Brookfield1996), which assumes that null homozygotes are present in the sample. The estimated frequency of null alleles was low (0.04), suggesting that inbreeding (or outcrossing among identical plants) could account for random mating. Elsewhere, heterozygosity deficits have been reported for both cultivated and wild relatives of cassava (Olsen and Schaal, Reference Olsen and Schaal2001).
Genetic differentiation
In general, if F ST is 0.05–0.15, then differentiation in allele frequencies between populations is considered moderate. Here, F ST among all 14 populations when landraces and cultivars were considered was 0.1038, indicating moderate differentiation. Traditionally, it is customary for farmers to exchange germplasm frequently, and often across substantial distances, including across international borders. This would tend to reduce genetic differentiation among countries.
Slightly greater differentiation was observed, in terms of deviation from random mating, in landraces (F ST= 0.1040), compared with cultivars (F ST= 0.0845). This is likely to reflect the common origin from IITA-Nigeria of many of the cultivars now present in a number of countries, tending to reduce population differentiation. The levels of differentiation observed here are slightly higher than those observed by others. Balyejusa Kizito et al. (2005), though working with fewer cassava genotypes (ranging between 19 and 195), observed no appreciable gene differentiation among cassava genotypes from Uganda (East Africa) and Ghana (West Africa) (F ST= 0.04) and between cassava genotypes from Ghana and Tanzania (East Africa) (F ST= 0.05). Peroni et al. (Reference Peroni, Kageyama and Begossi2007) analysed sweet (76 entries) and bitter (60 entries) cassava and observed higher genetic diversity within populations (higher within sweet cassava), and moderate genetic differentiation (R ST= 0.057) among the two populations. Several other studies using neutral microsatellites observed no appreciable gene differentiation within different cassava populations (Asante and Offei, Reference Asante and Offei2003; Lokko et al., Reference Lokko, Dixon, Offei, Danquah and Fregene2006; Siqueira et al., Reference Siqueira, Queiroz-Silva, Bressan, Borges, Pereira, Pinto and Veasey2009).
Genetic relationships of landraces from different countries
PCA showed a tendency for landraces from a specific country to group together (Fig. 1(b)) and some loose structure among the countries. DE revealed that landraces from Tanzania have a pivotal position in the region, being most closely related to germplasm from all other countries apart from that from the DRC which were most closely related to landraces from Rwanda, followed by Tanzania (Table 2). Tanzania is centrally located in the region, having borders with Rwanda, Uganda, Kenya and Mozambique. This is likely to reflect traditional practices of exchanging cassava germplasm, even across international borders. The relative distance of Mozambique and DRC from other germplasm in the region could reflect their geographical peripheral location in the region, with DRC probably receiving a relatively greater proportion of germplasm from West Africa. Germplasm from West Africa has been shown on the basis of 1190 single nucleotide polymorphism markers and a small number of genotypes to be somewhat genetically different from that from SEC Africa (Ferguson et al., Reference Ferguson, Hearn, Close, Wanamaker, Moskal, Town, de Young, Marri, Rabbi and de Villiers2012). The close relationship of landraces from the DRC to those of Rwanda indicate substantial exchange between these countries. It is interesting to note that landraces from Madagascar were not more distinct from the rest of the region, due to its relative geographical isolation; however, Madagascar was struck by a severe epidemic of CMD in 1934–1936. This led to the almost total elimination of local varieties (Cours et al., Reference Cours, Fargette, Otim-Nape and Thresh1997). Many of the ‘landraces’ may be relatively recent introductions from mainland Africa. A similar epidemic struck Uganda in the 1990s where the severity of symptoms commonly led to the almost complete elimination of the most vulnerable varieties (Legg et al., Reference Legg, Jeremiah, Obiero, Maruthi, Ndyetabula, Okao-Okuja, Bouwmeester, Bigirimana, Tata-Hangy, Gashaka, Mkamilo, Alicai and Kumar2011). This epidemic spread rapidly to the Great Lakes region and beyond acquiring the status of ‘pandemic’ (Otim-Nape et al., Reference Otim-Nape, Bua, Thresh, Baguma, Ogwal, Semakula, Acola, Byabakama and Martin1997). Many areas of SEC Africa are currently experiencing another pandemic, of CBSD (Legg et al., Reference Legg, Jeremiah, Obiero, Maruthi, Ndyetabula, Okao-Okuja, Bouwmeester, Bigirimana, Tata-Hangy, Gashaka, Mkamilo, Alicai and Kumar2011). This is likely to affect the nature and frequency of particular landraces in the region. The lack of conservation of these landraces in any kind of germplasm repository in the region is of particular concern in the face of disease pandemics.
Genetic relationships of cultivars from different countries
The observed close relationship among cultivars from Kenya, Uganda, Rwanda and DRC from PCA and DE can be explained by the fact that many of these improved varieties grown in these countries have been acquired through the IITA through the ‘International Nurseries’ initiative. This germplasm often has a West African genetic background and/or a Latin American genetic background if CIAT parental lines were used in hybridization schemes at the IITA.
The isolation of cultivars from Madagascar, and their slight proximity to cultivars from Tanzania, may reflect efforts to combat, particularly, CMD in these countries. As a result of the CMD epidemic in Madagascar, a broad diversity of genotypes were screened for resistance to the disease at Alaotra Agricultural Research Station, Madagascar from 1935. Adequate resistance to CMD was found in Javanese varieties and intra-specific crosses (Cours, Reference Cours1951; Cours-Darne, 1968). In addition, inter-specific crosses with ceara rubber (Manihot glaziovii) and other species were conducted to obtain increased resistance. From the 1930s, a similar but unconnected programme at Amani, Tanzania, conducted extensive hybridization between cassava and its wild relatives (Nichols, Reference Nichols1947). Examples of these hybridization schemes included: (1) utilization of Manihot dichotoma Ule, M. glaziovii Muell.-Arg. and Manihot catingae Ule for sources of disease resistance (Hillocks and Jennings, Reference Hillocks and Jennings2003) and (2) Manihot melanobasis Muell.-Arg. and Manihot saxicola Lang. for disease resistance and protein enhancement (Jennings, Reference Jennings1959). These latter two species are now considered to be forms of M. esculenta subsp. flabellifolia. High levels of resistance to CMD were obtained from M. glaziovii× M. esculenta crosses (Jennings, Reference Jennings1994). This cassava improvement programme continued during the 1940s and 1950s, supported by the British Government and under the auspices of the East African Agriculture and Forestry Research Organization. In the 1950s, a number of selections derived from crosses were distributed to research stations across East Africa (Nichols, Reference Nichols1947), and some of this material has recently been retrieved for use in current disease resistance breeding programmes. These initiatives generated new genetic variability in the region, and could explain the relative isolation of Malagasy and Tanzanian cultivars from those from Uganda, Rwanda and DRC. The relative isolation of Mozambique was somewhat surprising; however, this may be due to its peripheral location in the region, and the fact that it was only represented by nine cultivars.
Genetic relationships of cultivars and landraces from different countries
When the robust Bayesian model-based analysis was done on all the 1401 cassava individuals, they were assigned into three subpopulations. Most cultivars from DRC, Mozambique, Kenya and Rwanda, and nearly 40% of cultivars from Uganda were assigned to the same subpopulation, together with a proportion of landraces from DRC and Rwanda. This subpopulation is likely to be characterized by a higher frequency of alleles from West Africa. This was hypothesized earlier to explain the relatively high genetic distance of landraces from the DRC to other landraces in the region. Cultivars derived from IITA-Nigeria are also more likely to have a West African genetic background with more of its characteristic allelic composition and frequency.
The second subpopulation consisted of cultivars and landraces of more East African origin. This included landraces from Madagascar, Tanzania, Mozambique, Kenya and a proportion from Rwanda. This subpopulation also included cultivars from those two countries, Tanzania and Madagascar, which had early breeding programmes, including inter-specific breeding programmes (Jennings, Reference Jennings1994), and disseminated cassava varieties to neighbouring countries (Nichols, Reference Nichols1947). This indicates a relatively close relationship and introgression between cultivars and landraces from these countries, and less influence of West African-derived germplasm from IITA-Nigeria. This is not necessarily the case in Kenya and Mozambique, where the landraces and cultivars appear more distinct, indicating a lack of introgression between landraces and cultivars, possibly due to the maintenance of integrity of introductions from IITA-Nigeria.
It is interesting to note that STRUCTURE analysis of cultivars and landraces together revealed the relative difference between Ugandan cultivars and landraces. This is likely to reflect the breeding programme in Uganda where IITA-Nigeria varieties are frequently introgressed with local landraces. This, together with its intermediate position between West African-influenced DRC and East Africa, could explain why germplasm from this country is largely allocated to a separate subpopulation.
Conclusions
In conclusion, the results from this research highlight that cassava landraces and improved cultivars available within the NARS of Tanzania, Kenya, Uganda, DRC, Madagascar, Mozambique and Rwanda contain moderate genetic variability resulting from a dominance of a few alleles with the majority of alleles having low frequency. This indicates a genetic bottleneck in the region and the need to increase diversity through the importation of germplasm from South America if substantial genetic gain is to be made in breeding. The majority of variation exists within individuals as opposed to among countries. This reflects the heterozygous nature of cassava. PCA, genetic distance and STRUCTURE analysis revealed subtle and rather complex patterns of genetic diversity of cassava germplasm of SEC Africa. This appears to be shaped by gene flow from West Africa into the landraces from the DRC; introduction and preservation of IITA-improved varieties in the breeding programmes of DRC, Rwanda, Kenya and Mozambique; independent introductions of cassava into East Africa and its subsequent dissemination throughout the region which may have contributed to the pivotal role of Tanzanian landraces in the region. This and the close relationship of cultivars and landraces in Tanzania and Madagascar are likely to reflect early independent breeding programmes in these countries, which involved germplasm selections and intra- and inter-specific crosses with landraces. This is the first large assessment of genetic variation in the SEC African region, and the first study of variation in the NARS of DRC, Madagascar and Rwanda. Information generated from this study has immediate application to justify and guide a regional cassava genetic resource conservation strategy, to identify gaps in cassava diversity in the region in relation to global diversity, to contribute information to a global cassava reference set and to guide parental selection during breeding.
Acknowledgements
This research was supported by funds from the Generation Challenge Program (GCP), Rockefeller Foundation and Biosciences eastern and central Africa Network (BecANet). Martha Hamlin of Cornell University assisted with PCA. All the genotyping work was carried out at the BecA Hub, Nairobi, Kenya. We thank the various NARS partners that were involved in this work.








