Introduction
The genus Limnanthes, first described by Robert Brown in 1833, is native to regions of northern California, southern Oregon, and Vancouver Island, British Columbia (Mason, Reference Mason1952). Commonly referred to as meadowfoam, these white flowering plants grow in vernal pools and meadows in valley grasslands along the foothills (Jain et al., Reference Jain, Pierce and Hauptli1977). Limnanthes is a low-growing winter annual that germinates in the early spring in cool, moist environments, maturing rapidly before the climate becomes dry in late spring and early summer (Jolliff et al., Reference Jolliff, Tinsley, Calhoun and Crane1981). The genus includes nine species separated into two sections based on petal position following fertilization. These are the Inflexae (petals enclose the developing fruit): Limnanthes alba, Limnanthes floccosa, Limnanthes gracilis and Limnanthes montana; and the Reflexae (petals reflex as fruit develops): Limnanthes bakeri, Limnanthes douglasii, Limnanthes macounii, Limnanthes striata and Limnanthes vinculans (Mason, Reference Mason1952). Research into the genus increased after Limnanthes was identified as a prospective industrial crop due to the presence of long-chain fatty acids in the seed oil which had larger molecular weights than acids found in common domesticated vegetable oil plants (Gentry and Miller, Reference Gentry and Miller1965). With the use of sperm whale oil banned in 1972, Limnanthes seed oil was proposed as a replacement in lubricants, cosmetics, waxes, detergents and pharmaceuticals (Jain et al., Reference Jain, Pierce and Hauptli1977).
Fatty acids comprise 25% of the seed oil (Miwa and Wolff, Reference Miwa and Wolff1962) with 95% of the oil comprised of four main acids, cis-5-eicosenoic (20:1), cis-5-docosenoic (22:1), cis-13-docosenoic (eruic acid, 22:1) and cis-5-cis-13-docosadienoic (22:2) acids (Smith et al., Reference Smith, Bagby, Miwa, Lohmar and Wolff1960; Bagby et al., Reference Bagby, Smith, Miwa, Lohmar and Wolff1961). The presence of long-chain fatty acids unique to Limnanthes (cis-5-eicosenoic acid and cis-5-docosenoic acid; Smith et al., Reference Smith, Bagby, Miwa, Lohmar and Wolff1960; Bagby et al., Reference Bagby, Smith, Miwa, Lohmar and Wolff1961) and the unique position of unsaturation in the cis-5-cis-13-docosadienoic acid provide stability at high temperatures. Interest in this property fuelled research into the seed oil for use as an industrial lubricant (Jain et al., Reference Jain, Pierce and Hauptli1977). Using standard chemical reactions, Limnanthes oil can be converted to a compound similar to liquid jojoba wax or a solid wax with a high melting point similar to carnauba and candelilla waxes (Miwa and Wolff, Reference Miwa and Wolff1962; Gentry and Miller, Reference Gentry and Miller1965). Limnanthes may have an agricultural advantage over other wax-producing crops as its cultivation is comparatively easy, and it can be double-cropped with rice, an important crop in northern California (Jain et al., Reference Jain, Pierce and Hauptli1977), or it can be maintained in surplus fields with winter grains (Gentry and Miller, Reference Gentry and Miller1965; Jolliff et al., Reference Jolliff, Tinsley, Calhoun and Crane1981).
Traits of agronomic importance, including yield (Jain and Abuelgasim, Reference Jain and Abuelgasim1981), seed retention (Gentry and Miller, Reference Gentry and Miller1965; Higgins et al., Reference Higgins, Calhoun, Willingham, Dinkel, Raisler and White1971), seed oil content (Pierce and Jain, Reference Pierce and Jain1977; Jain and Abuelgasim, Reference Jain and Abuelgasim1981), uniformity in maturity, growth habit and moisture requirements (Gentry and Miller, Reference Gentry and Miller1965), were reported to differ among Limnanthes accessions. Breeding for cultivars exhibiting combinations of desirable agronomic qualities was undertaken from various Limnanthes species (Gentry and Miller, Reference Gentry and Miller1965; Higgins et al., Reference Higgins, Calhoun, Willingham, Dinkel, Raisler and White1971). Research focused on the development of cultivars with high seed oil content that are self-pollinating to avoid the necessity for pollen vector application in production fields (Jolliff et al., Reference Jolliff, Tinsley, Calhoun and Crane1981).
Conservation efforts for the populations of Limnanthes were undertaken due to the infringement of urban development and livestock grazing (Dole and Sun, Reference Dole and Sun1992; California Native Plant Society (CNPS), 2005). Four Limnanthes species are listed as endangered: L. douglasii ssp. sulphurea and L. gracilis ssp. parishii by the state of California; and L. floccosa ssp. californica and L. vinculans by both state and the federal governments (CNPS, 2005). Some species of Limnanthes have limited ranges, e.g. L. macounii, which was once believed to be extinct but has since been found in Victoria, British Columbia (Higgins et al., Reference Higgins, Calhoun, Willingham, Dinkel, Raisler and White1971). To preserve wild populations, a germplasm collection of the genus was established in 1972 at the University of California, Davis, California, to maintain genetically diverse resources for breeding (Jain et al., Reference Jain, Pierce and Hauptli1977). However, the seed in that collection is no longer viable.
The utility of a germplasm collection relies on its ability to represent the genetic diversity present in the corresponding natural populations. Until now, the diversity of the USDA Limnanthes collection has only been assessed using morphological traits. For confirmation of morphological diversity data as well as for taking preliminary steps in marker-assisted breeding programs for crop improvement, it is also necessary to document genetic diversity at the molecular level. Many types of molecular markers are currently available. Simple sequence repeat (SSR) markers were chosen for this work. Due to the wide distribution in the genome and high levels of polymorphism exhibited by SSR markers, they have been successfully employed to determine genetic diversity between accessions in other germplasm collections (Godwin et al., Reference Godwin, Mace, Nurzuhairawaty and Henry2001; McLauchlan et al., Reference McLauchlan, Henry, Isaac and Edwards2001; Baranger et al., Reference Baranger, Aubert, Arnau, Laine, Denoit, Potier, Weinachter, Lejeune-Henaut, Lallemand and Burstin2004; Dillon et al., Reference Dillon, Lawrence and Henry2005). In 2004, Kishore et al. identified SSR markers in Limnanthes and used them to screen intra- and interspecific variation in 14 accessions, including 4 inbred lines, 2 open pollinated breeding selections and 8 open pollinated wild species. The objective of the current study was to describe genetic diversity at the molecular level in the Limnanthes germplasm collection preserved by the United States Department of Agriculture, Agriculture Research Service, National Plant Germplasm System (USDA-ARS-NPGS).
Materials and methods
Plant material and DNA extraction
Genomic DNA was extracted from leaf tissue of 62 Limnanthes germplasm accessions maintained as open-pollinated populations isolated by caging at the National Arid Land Plants Genetic Research Unit (NALPGRU), Parlier, California. The 62 accessions belonged to 7 species: L. alba, L. douglasii, L. bakeri, L. floccosa, L. gracilis, L. montana and L. striata. Accessions designated ‘a’ or ‘b’ of the same identification number were collected from the same population but exhibited morphological differences in leaf and stem colour (‘b’ – smaller leaves and purple stems; Table 1). Genomic DNA extractions were performed on a composite sample of 10 plants of each accession using the cetyltrimethylammonium bromide protocol from Lodhi et al. (Reference Lodhi, Ye, Weeden and Reisch1994). A 24:1 solution of chloroform to isoamyl alcohol was used instead of 24:1 chloroform to octanol solution in the organic extraction step. DNA concentrations were determined using a Shimadzu UV-1601 spectrophotometer (Kyoto, Japan).
Table 1 Limnanthes germplasm accessions evaluated for simple sequence repeat marker diversity

Inventory of the USDA-ARS-NALPGRU-Parlier Limnanthes collection can be accessed at http://www.ars-grin.gov
PCR amplification and PCR product visualization
Fifteen primer pairs developed by Kishore et al. from SSRs of the species L. alba (2004) were used to screen the Limnanthes DNA pools for polymorphism (Table 2). These primers were sub-selected based on high levels of polymorphism observed across 17 taxa in the Limnanthaceae (Kishore et al., Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004). Touchdown PCR was performed according to Kishore et al. (Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004) in 20 μL reactions containing 1 × PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.03% BSA (bovine serum albumin), 15 pmol of forward and reverse primers (Operon Technologies, Alameda, CA), 0.75 U of Taq polymerase (Invitrogen, Carlsbad, CA) and 10 ng of genomic DNA. Reactions without DNA were run as negative controls. PCRs were carried out in an Eppendorf Mastercycler Gradient thermocycler using an initial denaturation step of 94°C for 3 min, followed by 1 cycle of 94°C for 30 s, 68°C for 30 s and 72°C for one min. Subsequent cycles were performed with the annealing temperature decreasing by 1°C to a base temperature between 53 and 63°C depending on the optimum annealing temperature for the primer pair used. Reactions were continued for 30 cycles: (94°C for 30 s, the optimum annealing temperature for the respective primer pair for 30 s, and 72°C for 1 min), followed by a final elongation step of 72°C for 20 min.
Table 2 Primer, annealing temperature, G/C content, expected and observed size ranges and the number of amplicons across all Limnanthes accessions analyzed

a Primers used to screen Limnanthes cultivars by Kishore et al. (Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004).
PCR products were diluted 1:2 with 2 × Novex, TBE-Urea Sample Buffer (Invitrogen). The samples and 40 ng/μL solutions of 10 and 50 bp standards (Invitrogen) were denatured at 95°C for 5 min. The samples and standards were immediately placed on ice to prevent strand re-annealing for a minimum of 5 min prior to gel loading. The gel was loaded with 4 μL of each standard and 5.5 μL of each sample. Samples were then run on a 6% (w/v) polyacrylamide sequencing gel to determine amplicon sizes. Electrophoresis was performed at 1800 V, 90 W and 200 mA, between 2.5 and 3.5 h depending on expected amplicon sizes. The gels were stained using 0.2% silver nitrate solution following the Promega Silver Sequence DNA Sequencing System protocol (Promega, Madison, WI).
The gels were visually scored based on the presence or absence of the same size amplicons among the Limnanthes samples for each primer set, regardless of the band intensity.
Cluster analysis
A binary data matrix representing presence and absence of bands in each pooled DNA sample was assembled. The dataset contained 62 accessions and 140 simple-sequence length polymorphisms (SSLPs), each of them treated as a separate character in the analysis. Approximately, 23% of the matrix contained missing data. A series of detailed simulation studies has shown that phylogenetic analysis methods are robust to missing data and, in most cases, can yield accurate results with much higher levels of missing data than that seen here, although resolution may suffer (Wiens, Reference Wiens2006). Treebased analyses of genetic diversity among samples were performed using PAUP*4.0 (Swofford, Reference Swofford2002). A parsimony bootstrap tree was generated using 10,000 replicates and the TBR-M search strategy of Debry and Olmstead (Reference Debry and Olmstead2000). A neighbour-joining bootstrap tree was also generated in order to confirm the parsimony results.
Principal coordinate analysis of the binary matrix was performed with NTSYS (Exeter Software) using Jaccard distances. In order to determine whether any statistically significant clusters of principal coordinates existed, we used PCOMC (Reeves and Richards, Reference Reeves and Richards2007; http://lamar.colostate.edu/~reevesp/PCOMC/PCOMC.html). PCOMC uses a nonparametric density clustering procedure as an objective means to identify natural discontinuities in multidimensional principal coordinate data. All principal coordinate axes were considered simultaneously.
Results
Amplification and polymorphism
SSR-based PCR was used to identify SSLPs between the 62 accessions of the NPGS Limnanthes germplasm collection. Statistics were calculated for SSLPs and haplotypes instead of alleles and genotypes due to the amplification of multiple loci with the SSR markers used in this study. A total of 140 SSLPs were identified using 15 SSR markers, ranging from 144 to 437 bp in size. Eleven of the fifteen SSR primer pairs amplified products within 10 bp of the expected size ranges reported by Kishore et al. (Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004). The remaining four primers ampli fied products within 50 bp of the expected ranges (Table 2).
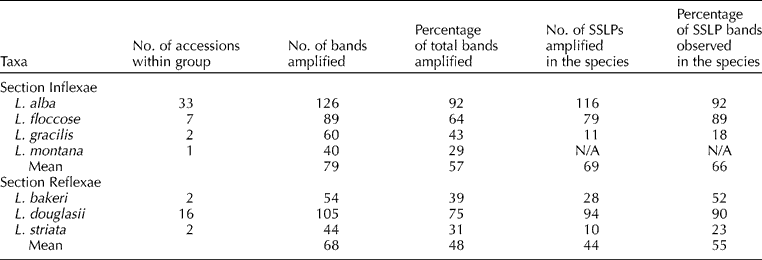
The number of scorable SSLPs observed with the primers ranged from 2 to 24 per marker with an average of 9.5 SSLPs per marker. The total number of bands amplified for the different species was 126 for L. alba, 54 for L. bakeri, 105 for L. douglasii, 89 for L. floccosa, 60 for L. gracilis, 40 for L. montana and 44 for L. striata. The mean number of bands for accessions in the Section Inflexae (L. alba, L. floccosa, L. gracilis and L. montana) was 79, and a mean of 68 bands amplified among the accessions in the Section Reflexae (L. bakeri, L. douglasii and L. striata) (Table 3). The percentage of the total bands amplified that were polymorphic within each species was 92% in L. alba, 52% between the two L. bakeri accessions, 90% in L. douglasii, 89% in L. floccosa, 18% in L. gracilis, and 23% between the two L. striata accessions. Only one accession from L. montana was available and therefore no statistics were calculated. The mean polymorphism observed in the Inflexae accessions was 66%, greater than the mean observed in the Reflexae at 55% (Table 3).
Table 3 Simple sequence repeat amplification in the analyzed Limnanthes germplasm collection. Number of accessions, number of amplified bands, percentage of total bands, and the percentage of bands polymorphic within each species
SSLP, simple-sequence length polymorphism.
Dendrograms and principal coordinate analysis
A maximum parsimony tree based on the SSLP data is shown in Fig. 1. The accessions split into two principal groups with little resolution within groups. The first group (Group I) included all of the accessions of L. alba, L. floccosa, L. gracilis and L. montana. This group corresponds to Section Inflexae. The second group (Group II) included all of the accessions of L. douglasii, L. bakeri and L. striata, equivalent to Section Reflexae given current sampling. The split between Group I and Group II was confirmed by neighbour-joining bootstrap analysis (results not shown). The separation of the 62 accessions into the two groups was also supported by PCO-MC analysis. Two statistically significant clusters (P < 0.05) found using PCO-MC (Fig. 2) matched exactly with Groups I and II from the dendrogram analyses.

Fig. 1 Maximum parsimony tree based on simple-sequence length polymorphisms identified for accessions in the USDA-NPGS Limnanthes germplasm collection. Numbers at internodes are bootstrap support values. Branches with < 50% bootstrap support have been collapsed. Tree is displayed as unrooted to emphasize the central branch, which splits the accessions into two groups also found to be significantly distinct using principal coordinate analysis. Group I (Inflexae) is on the left; Group II (Reflexae) is on the right.

Fig. 2 Plot of sampled accessions along the first two principal coordinate axes. Using PCO-MC and data from all principal coordinate axes, two statistically significant clusters (P < 0.05) that were consistent with parsimony analysis were identified. A dashed line is used to indicate the discontinuity between significant clusters. Group I (Inflexae) is above the line; Group II (Reflexae) is below the line.
Fingerprinting accessions
Five of the SSR markers (Ls-402, Ls-466, Ls-483, Ls-484 and Ls-278) were able to distinguish 59 (95.2%) of the 62 bulked accession fingerprints from each other. Two additional SSR markers were needed to fully distinguish all accessions. Ls-107 was needed to distinguish PARL 428 L. douglasii rosea from PARL 423 L. douglasii nivea as well as PARL 430b L. alba alba from PARL 431b L. alba alba. Ls-273 was needed to distinguish PI 374802 L. alba from PI 608039 L. alba versicolor.
Discussion
In this study, all of the SSR primer pairs amplified multiple products in the Limnanthes accessions. The amplification of multiple loci in Limnanthes using SSR markers was also observed in a study by Kishore et al. (Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004), in which 1–28 unique bands were amplified for each SSR marker, with 56% of the primers amplifying multiple loci. Here, where samples were bulked, the level of allelic richness of 9.5 alleles per SSR primer set is comparable to the average number of alleles found in the survey of individual genotypes (10.7 per marker) in Kishore et al. (Reference Kishore, Velasco, Shintani, Rowe, Rosato, Adair, Slabaugh and Knapp2004). In other studies of SSRs in higher plants, where specific allelic status was unknown because of polyploidy, bulked samples found comparable levels of allelic richness. For example, mean allelic richness was 8 per marker in cultivated sugarcane cultivars (Cordeiro et al., Reference Cordeiro, Taylor and Henry2000), 12.6 per marker in open-pollinated Zea sp., 6.9 per marker in inbreds in Zea sp. (Matsouka et al., Reference Matsuoka, Mitchell, Kresovich, Goodman and Doebley2002) and 11.8 per marker in Sorghum sp. (Dillon et al., Reference Dillon, Lawrence and Henry2005).
Pooled DNA samples have been used successfully in prior studies of genetic variability in plant collections (Havey, Reference Havey1995; Charters et al., Reference Charters, Robertson and Wilkinson1996; Gilbert et al., Reference Gilbert, Lewis, Wilkinson and Caligari1999; Sham et al., Reference Sham, Bader, Craig, O'Donovan and Owen2002). When accessions are represented using SSR fingerprints from bulked samples, special care is necessary during analysis. SSR fingerprints produced from bulked samples comprise a composite phenotype of the alleles sampled within an accession. Because of this, commonly used genetic distances based on allele frequencies cannot be used. Here, we have chosen to use parsimony as a conservative criterion to classify accession fingerprint data. In addition, we used a non-hierarchical ordination method to cluster fingerprints and employed a quantitative metric to identify significant clusters.
The SSR fingerprint data were sufficient to distinguish all samples from one another. This suggests that all of the Limnanthes accessions in the NPGS collection contain unique alleles and are thus distinct. However, the possibility that this finding is a sampling artefact due to the sample pooling strategy cannot be categorically eliminated. While the maximum parsimony tree showed limited substructure among SSR fingerprint phenotypes, the division of accessions into Group I and Group II is clear and is supported by neighbour-joining as well as by principal coordinate analysis (Fig. 2). The lack of substructure within Group I and II observed in the dendrogram may be due to a variety of factors, including bulking, missing SSLP data, lack of sufficient DNA marker resolution, scoring of non-allelic fragments of the same size as allelic, and gene flow in the wild. The first three issues may be resolved by other approaches that could improve genetic resolution in the data including addition of loci or marker density and the collection of individual genotypes. Avoiding scoring of non-allelic fragments of the same size as allelic could be dealt with by sequencing of Limnanthes amplicons of interest to ensure that the SSLPs amplified with each primer are truly allelic, as seen in rice by Panaud et al. (Reference Panaud, Chen and McCouch1996). The fifth issue, natural gene flow between taxa, would probably require extensive DNA sequencing to detect.
Within the USDA collection, the L. alba, L. floccosa and L. douglasii accessions showed the highest level of genetic diversity (92, 89 and 90% of all alleles found experiment-wide, respectively) based on the SSR analysis (Table 3). These taxa have shown the greatest promise for cultivar development (Jolliff et al., Reference Jolliff, Tinsley, Calhoun and Crane1981) and, therefore, have been sampled extensively and are represented by a large number of accessions in the NPGS collection (33, 7 and 16, respectively). The number of accessions for L. bakeri, L. gracilis and L. striata (2 for each species) was low as was the percentage of total allelic diversity observed (52, 18 and 23%, respectively). These findings support the need for increased sampling of wild L. bakeri, L. gracilis, L. striata and L. montana populations (which is represented by only one accession in the NPGS collection). Further study may find that the genetic diversity truly is lower in the L. bakeri, L. gracilis, L. striata and L. montana taxa compared with other Limnanthes species, but at present, assumptions cannot be made due to small sample size. Representatives of L. macounii and L. vinculans should also be included in the collection to cover all taxa of the genus.
In this study, taxa of the Inflexae exhibited more diversity than taxa of the Reflexae, with 66% of the amplified products observed to be polymorphic in the Inflexae compared with 55% in Reflexae (Table 3). This may prove to be important in cultivar development due to the taxa in the Inflexae having superior seed retention qualities than the Reflexae taxa (Gentry and Miller, Reference Gentry and Miller1965; Higgins et al., Reference Higgins, Calhoun, Willingham, Dinkel, Raisler and White1971). For breeding purposes, having a large repertoire of accessions with traits of agronomic value is of importance. The data suggest that accessions of the NPGS germplasm collection within the Inflexae are of diverse genetic backgrounds and therefore may provide a suitable platform for cultivar development.
Conservation of wild relatives of cultivated crops is necessary to prevent the loss of genetic diversity with repeated breeding. In domesticated crops, genetic variation is often subjected to a ‘domestication bottleneck’ when selection for desirable characteristics results in the loss of diversity. Such loss of variation occurred in maize (Matsuoka et al., Reference Matsuoka, Mitchell, Kresovich, Goodman and Doebley2002) and wheat (McLauchlan et al., Reference McLauchlan, Henry, Isaac and Edwards2001), where wild relatives showed greater diversity among SSR markers than did cultivated varieties. Breeding efforts aimed at the development of new cultivars may lead to losses in Limnanthes genetic diversity. Hence, the development of a germplasm collection with high genetic diversity is important. As this study showed, the Limnanthes species most used in cultivar development, L. alba, L. douglasii and L. floccosa, are the species most highly represented in the NPGS germplasm collection and have the greatest amount of intraspecific diversity. In comparison, species L. bakeri, L. gracilis, L. striata and L. montana show little intraspecific variation and should be collected more extensively to ensure the genetic diversity of the genus and the respective species in the collection.
Diverse germplasm collections provide a range of traits for use in breeding programs from increased yield to traits that may be required in the future due to unforeseen abiotic and biotic challenges. The data from the current study showed that the NPGS Limnanthes germplasm collection is genetically diverse and that the accessions within the species likely contain novel alleles. The collection therefore forms an important contribution to the conservation of the Limnanthes gene pool.
Acknowledgements
This work was supported in part by USDA CRIS # 5302-21000-008-00D. We would like to thank Dr Christopher Richards (USDA-ARS-NCGRP, Fort Collins, CO), Dr Jefferey Dole (The University and Jepson Herbaria, UC, Berkeley, CA) and Jeremy Ross (CSU Fresno, CA) for their technical assistance, and the CSU Fresno, College of Science and Mathematics for supporting J. Prince during the preparation of this manuscript.







