Introduction
Genetic resources of many crop species have been poorly described (Morris, Reference Morris2009), and much valuable germplasm, particularly in legumes, remains undiscovered (Obiagwu, Reference Obiagwu1997). The cultivation and use of African yam bean (AYB, Sphenostylis stenocarpa A. Rich. Harms, Fabaceae) has only been reported in Africa. This neglected crop is highly valuable for the high protein content of its pulse and tuber. For instance, two amino acids, methionine and lysine, which are limited in most food legumes, are present in metabolizable forms in AYB (Okigbo, Reference Okigbo1973; National Research Council, 1979; Edem et al., Reference Edem, Amugo and Eka1990; Nwokolo, Reference Nwokolo, Nwokolo and Smart1996; Uguru and Madukaife, Reference Uguru and Madukaife2001; Ekpo Reference Ekpo2006).
The International Institute of Tropical Agriculture (IITA) maintains the collection of AYB germplasm for conservation. Accurate characterization of existing genetic diversity in a collection provides an invaluable aid for identifying the most important accessions for conservation and genetic improvement strategies. It is therefore important that the diversity of AYB germplasm, which may host a wealth of undisclosed allelic variants, be mined for useful traits in breeding programmes.
Exploring the available genetic diversity of most crop species through conventional breeding programmes would take decades because of several limitations associated with diversity assessment through phenotypic and biochemical means (Mohammadi and Prassanna, Reference Mohammadi and Prasanna2003; Venkatesha et al., Reference Venkatesha, Ramanjini, Ganapathy, Gowda, Ramachandra, Girish, Channamallikarjuna, Shantala and Gowda2010). The development of DNA markers has to some extent circumvented those limitations. Earlier studies in AYB that used morphological and nutritional descriptors (Potter and Doyle, Reference Potter and Doyle1992; Ajibade et al., Reference Ajibade, Balogun, Afolabi, Ajomale and Fasoyiro2005; Popoola et al., Reference Popoola, Adegbite, Obembe, Adewale and Odu2011; Adewale et al., Reference Adewale, Aremu and Amazue2012a, b) and isozyme and chloroplast DNA data (Potter and Doyle, Reference Potter and Doyle1992, Reference Potter and Doyle1994) have revealed a wide diversity among its accessions. Moyib et al. (Reference Moyib, Gbadegesin, Aina and Odunola2008) performed the first molecular diversity assessment on AYB using random amplified polymorphic DNA on 24 landraces from south-western Nigeria. In underutilized crops such as AYB for which little is known about its genomic diversity, the use of DNA fingerprinting approaches such as amplified fragment length polymorphism (AFLP) that can randomly screen the whole genome would be among the most powerful molecular tools. Indeed, the AFLP approach for diversity analysis has proved very efficient in several plant species (Vos et al., Reference Vos, Hogers, Bleeker, Reijans, Van de Lee, Hornes, Fritjers, Pot, Pelema, Kuiper and Zabeau1995; McKay et al., Reference McKay, Egan, Morris, Scott and Brown1999; Sarutayophat et al., Reference Sarutayophat, Nualsri, Santipracha and Saereeprasert2007; Dolinsk et al., Reference Dolinsk, Kamitani, Machado and Winter2008; Mondini et al., Reference Mondini, Noorani and Pagnotta2009).
In the present study, the genetic diversity of 77 AYB accessions from various geographical origins, and previously characterized at the agro-morphological level (Adewale et al., Reference Adewale, Dumet, Vroh-Bi, Kehinde, Ojo, Adegbite and Franco2012b), was assessed using five AFLP primer combinations. The objective of this study was to provide a framework for the future utilization of AYB genetic resources in breeding programmes.
Materials and methods
DNA extraction
Seeds of 77 AYB accessions previously characterized agro-morphologically by Adewale et al. (Reference Adewale, Dumet, Vroh-Bi, Kehinde, Ojo, Adegbite and Franco2012b) and described by seed coat colour (Adewale, Reference Adewale2011) were collected from the Genetic Resources Centre of the IITA in 2008, and planted in pots in a screen house at IITA, Ibadan. The description of the 77 AYB accessions with matching colour codes (Kornerup and Wanscher, Reference Kornerup and Wanscher1978) are presented in Table S1 (available online). Genomic DNA was extracted from the leaves of these crop species using a modified Dellaporta method (Dellaporta et al., Reference Dellaporta, Wood and Hicks1983).
AFLP reactions were performed according to the method of Vroh-Bi et al. (Reference Vroh-Bi, Anagbogu, Nnadi and Tenkouano2010), using EcoRI and MseI primer combinations as listed in Table 1. In brief, 500 ng of genomic DNA were digested with EcoRI and MseI (Promega, Madison, WI, USA). DNA digestion, ligation of adaptors, pre-selective and selective amplifications were performed according to standard AFLP procedures (Vos et al., Reference Vos, Hogers, Bleeker, Reijans, Van de Lee, Hornes, Fritjers, Pot, Pelema, Kuiper and Zabeau1995), with a 1:20 dilution factor of pre-amplified products and 4 μl of the dilution used to perform the selective amplification in a final volume of 10 μl as described by Vroh-Bi et al. (Reference Vroh-Bi, Anagbogu, Nnadi and Tenkouano2010). The final PCR products were diluted with 5 μl of 40% formamide (denaturing loading buffer), denatured at 94°C for 2 min, resolved in 6% denaturing polyacrylamide gel in a Bio-Rad sequencing gel rig. The gels were run for 2 h (after a pre-run of 30 min) and visualized by the AFLP silver-staining kit (Promega, Madison, WI, USA) following the manufacturer's protocols.
Data acquisition and analysis
AFLP bands were scored for presence (1) or absence (0) in each accession. The genetic distance between each pair of the accessions was calculated using the Jaccard coefficient. A neighbour-joining (NJ) dendrogram (Saitou and Nei, Reference Saitou and Nei1987) was generated from the distance matrix using 1000 bootstrap repetitions to estimate the robustness of the classification. All calculations were done using the software DARwin version 5.5 (Perrier and Jacquemoud-Collet, Reference Perrier and Jacquemoud-Collet2006).
The percentage of polymorphism of each primer combination across all loci of each accession was calculated, following the method of Martos et al. (Reference Martos, Royo, Rharrabti and Garcia del Moral2005), as the proportion of polymorphic bands over the total number of bands, and recorded as the polymorphic efficiency of each marker. Efficiency denotes the percentage of polymorphic bands produced by a primer combination in a given accession relative to the total number of bands generated in that accession.
The marker index (MI) was calculated for each pair of the primer combinations as the product of the polymorphic information content, the total number of bands and the number of polymorphic bands. An analysis of molecular variance (AMOVA) was performed to study the differences between the NJ clusters. AMOVA pairwise comparisons between groups and estimation of molecular diversity (expected heterozygosity (H e)) within groups were conducted using Arlequin 3.1 (Excoffier et al., Reference Excoffier, Laval and Schneider2005).
Results
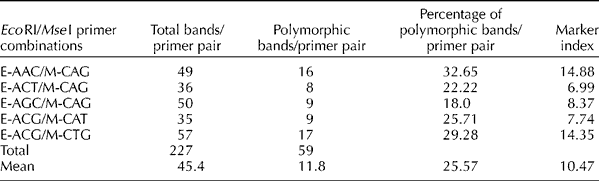
In the present study, five AFLP primer combinations were tested on the 77 AYB accessions. The performance of each pair of the primer combinations is presented in Table 1. The five primer combinations generated 227 AFLP bands. The number of AFLP bands per primer pair varied from 35 to 57 (mean 45.4), and 59 (26%) of the total bands were found to be polymorphic. The mean number of polymorphic bands per primer pair was 11.8. The primer combinations E-ACT/M-CAG and E-ACG/M-CTG generated the least (8) and highest (17) number of polymorphic bands, respectively. The MI per primer pair varied from 7.0 to 14.9, with the lowest from E-ACT/M-CAG and the highest from E-AAC/M-CAG. The mean MI for the five primer combinations was 10.5 (Table 1).
Table 1 Percentage of polymorphism of the five amplified fragment length polymorphism EcoRI/MseI primer combinations for the evaluation of diversity in the 77 accessions of African yam bean

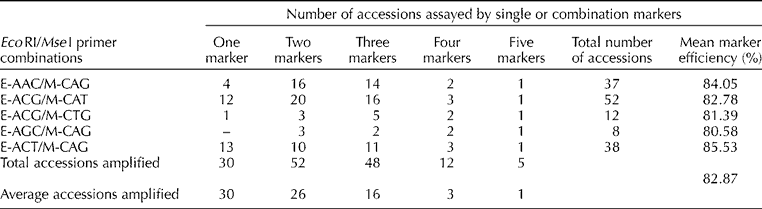
The summary statistics for the 77 AYB accessions amplified by the five AFLP markers with >75% efficiency are presented in Table 2. The most informative primer combinations in increasing order of efficiency were as follows: E-AGC/M-CAG>E-ACG/M-CTG>E-ACG/M-CAT>E-AAC/M-CAG>E-ACT/M-CAG. All AFLP primer combinations except E-AGC/M-CAG singly revealed polymorphism with an efficiency >75% in 30 AYB accessions. The mean percentage of efficiency of the five AFLP markers ranged between 80.6 and 85.5%, with a cumulative mean of 82.9% (Table 2). All the five primer combinations revealed polymorphism with an efficiency >75% in the TSs10 accession.
Table 2 Summary statistics of the five amplified fragment length polymorphism EcoRI/MseI primer combinations with the number of accessions assayed and different efficiencies (%) in detecting polymorphism in the 77 accessions of African yam bean

The genetic distance among the 77 AYB accessions ranged between 0.05 and 0.84 with an overall mean of 0.44 (Fig. 1). The similarity between the pairs of the accessions was calculated as follows: S= 1 − Jaccard distance value. TSs98 and TSs104B were found to be the most similar accessions (S= 0.952). Conversely, TSs11 and TSs94 were found to be the most dissimilar accessions (S= 0.16). Four main clusters (I to IV) were identified. Clusters I to IV comprised 21, 28, 20 and 8 accessions, respectively, with a within-cluster mean genetic distances of 0.45, 0.33, 0.43 and 0.29, respectively (Table 3). Within cluster I, three distinct sub-clusters were identified, with the smallest genetic distance (0.18) between the accessions TSs65 (from Democratic Republic of Congo) and TSs87 (from Nigeria), and the highest value (0.84) between the accessions TSs11 and TSs94.

Fig. 1 Neighbour-joining dendrogram based on Jaccard distance measures showing similarities among the 77 accessions of African yam bean.
Table 3 Characteristics of genetic information in different groups

GD, genetic distance; H e, expected heterozygosity; H t, total heterozygosity.
Cluster II had the highest number of accessions. The mean genetic similarity within this cluster was 0.67. Two sub-clusters were identified (Fig. 1). The genetic distance within cluster II (Table 3) varied between 0.05 (TSs98 and T104B) and 0.69 (TSs16 and TSs77).
Cluster III included 14 accessions from Nigeria, one accession from Bangladesh and five accessions without passport data (Table 3). The mean genetic distance within the cluster was 0.43. The most similar genotypes in the cluster were the accessions TSs10 and TSs117, while TSs119 and TSs23 were found to be the most divergent accessions. All accessions in cluster IV were from Nigeria. Similarity among the eight Nigerian accessions was high (0.71). TSs27 and TSs92 were most closely related.
The highest and lowest genetic distances between the accessions were found in clusters I and II, respectively (Table 3). Cluster IV had the lowest intra-cluster diversity and the lowest number of accessions. The genetic distance between the 60 AYB accessions from Nigeria ranged between 0.15 (TSs81 and TSs112) and 0.84 (TSs11 and TSs94) with a mean of 0.45. Among the remaining 17 accessions, 13 had no passport data. The genetic distances between the four accessions (TSs65, TSs67, TSs76 and TSs77) collected outside Nigeria ranged between 0.30 (TSs76 and TSs77 – both from Ghana) and 0.51 (TSs67 and TSs77) with a mean of 0.41.
The mean H e was 0.28, with a range of 0.22 (cluster IV) to 0.32 (cluster III). The H e values for clusters I and III were higher than the mean value, while those for clusters II and IV were lower. However, within-group diversity in clusters I and III was higher than that in clusters II and IV (Table 3).
A highly significant variation existed among and within the population of the accessions (Table 4). The percentage of variation was 20.3 among the four groups and 79.7 within the groups. The fixation index (F ST) for the molecular variation among the accessions was highly significant (P< 0.0001; Table 4). The four main clusters delineated by the NJ dendrogram (Fig. 1) were significantly different from each other (Table S2, available online). Clusters I and IV were the most divergent, while clusters II and III were nearer neighbours to cluster IV (Table S2, available online).
Table 4 Diversity among and within the population of African yam bean accessions as revealed by analysis of molecular variance

DF, degree of freedom; F ST, fixation index.
Discussion
This study demonstrated the fitness of AFLP markers to assess polymorphism using standard primer combinations in AYB accessions for which genome sequences are not available. According to Du et al. (Reference Du, Zhang and Luo2009) who used the AFLP technique to investigate genetic diversity in Diospyros accessions, the higher MI (10.47) that we obtained herein demonstrated the good performance of AFLP markers in detecting diversity within the AYB accessions.
The five AFLP primer combinations used in this study discriminated among the 77 AYB accessions, although with a varied degree of efficiencies. The variation of marker efficiency in detecting polymorphism has been well documented (Tero et al., Reference Tero, Aspi, Siikamaki, Jakalaniemi and Tuomi2003; Varshney et al., Reference Varshney, Chabane, Hendre, Aggarwal and Graner2007). In the present study, the primer combination E-ACG/M-CAT performed best, as it detected polymorphic bands in 51 (67%) of the 77 AYB accessions studied with an efficiency >75%. However, the primer combination E-ACT/M-CAG that detected polymorphism in 51% of the 77 AYB accessions exhibited 100% polymorphism in accessions TSs9, TSs38, TSs47, TSs57, TSs81, TSs82 and TSs95. This suggests that E-ACT/M-CAG may be an important primer combination for consideration in subsequent AFLP diversity analysis of AYB accessions and possibly related species.
The present study identified TSs98 (sun-burn brown) and TSs104B (greyish white), which differ based on the seed coat colour (Adewale, Reference Adewale2011), as the most similar accessions (S= 0.952). Although these accessions are not duplicates, they are genetically very similar. This study thus reasserts the plasticity of phenotypic descriptors for an intra-specific classification of genotypes, especially when the trait in question has low heritability (Beyene et al., Reference Beyene, Botha and Myburg2005; Siezen et al., Reference Siezen, Starrenburg, Boekhorst, Renckens, Douwe Molenaar and van Hylckama Vlieg2008; Emadzade et al., Reference Emadzade, Lehnebach, Lockhart and Hörandl2010). The correlation between genomic and good phenotypic data in wider and well-structured germplasm can lead to the identification of marker–trait associations for marker-assisted breeding and germplasm management. Such correlation studies between molecular markers and morphological traits would provide information of high value as the size of the current AYB germplasm grows with additional collections. A preliminary study conducted on association mapping between the present AFLP markers and morphological traits, using the mixed-model quantitative trait locus mapping approach of Arbelbide et al. (Reference Arbelbide, Yu and Bernardo2006), has shown a significant (α = 0.01) link of some loci of AFLP markers to days to flowering, seed length and pod length (Adewale, unpublished data). Increased germplasm size and number of molecular markers would provide clearer understanding of the trait–marker association in the crop species. Despite the high similarity within the accessions in cluster IV, a significant variation was observed within the cluster. The wide genetic distance between the genotypes from Nigeria underscores the genetic variability among the Nigerian accessions.
The grouping pattern of the genotypes indicated that the accessions shared many common genetic characteristics. Moreover, the 77 AYB accessions were genetically distinct; no duplication was identified among them. TSs 76 and TSs 77 from Ghana (Adewale et al., Reference Adewale, Dumet, Vroh-Bi, Kehinde, Ojo, Adegbite and Franco2012b), in cluster II were similar (S= 0.69) but different from each other.
Except for accessions in cluster IV, the grouping pattern of the accessions in the other clusters did not reflect the geographic origin of the genetic materials, meaning that there was no clear-cut geographical origin in the genetic structure of the AYB accessions studied. It is noteworthy to state that AYB is an autogamous species with an outcrossing rate of about 10%. The non-geographical delineation of the accessions analysed in this study may be due to the small size of collections from other AYB-growing regions. However, the grouping pattern of the Nigerian accessions was exceptional. Nigeria has been identified as one of the centres of diversity of AYB (National Research Council, 1979; Potter and Doyle, Reference Potter and Doyle1992). Additionally, testing more AFLP marker combinations could increase the resolution of the genetic structure of AYB. The accessions from the same origin were loosely grouped in clusters I, II and III. Grouping within some sub-clusters within each of the larger groups was with respect to the same geographical origin (e.g. TSs76 and TSs77 from Ghana, which grouped in cluster II, and eight Nigerian accessions in cluster IV). The clustering pattern that we found shares some resemblance with that reported by Asare et al., (Reference Asare, Gowda, Galyuon, Aboagye, Takrama and Timko2010) for 141 Ghanaian cowpea accessions. The accessions grouped into five main clusters without reference to their geographical origin. As the collection of AYB germplasm continues and the number of accessions and geographical coverage increases, further studies with more accessions per geographical origin and more DNA markers would be warranted to gather more insights into geographical locations and diversity of AYB.
The genetic diversity within the AYB accessions studied herein is wide compared with that of other legumes such as cowpea. The genetic base of cowpea has been consistently reported to be low (Pasquet, Reference Pasquet2000; Coulibaly et al., Reference Coulibaly, Pasquet, Papa and Gepts2002; Ba et al., Reference Ba, Pasquet and Gepts2004). The highest genetic distance reported by the study of Asare et al., (Reference Asare, Gowda, Galyuon, Aboagye, Takrama and Timko2010) for 141 cowpea accessions was 0.68, whereas 0.84 was reported for the 77 AYB accessions studied herein. Self-pollination in cowpea has been suggested as the major reason for the narrow genetic base of the crop. AYB, similar to other legumes, exhibits self-pollination. However, Adewale (Reference Adewale2011) documented an outcrossing rate of 10% in this crop species. The mean F ST of 0.21 reported in this study indicates that large differentiation exists among the accessions within each cluster. The observed diversity among the accessions within the same cluster could be due to the fact that AYB exhibits inbreeding. Thus, populations tend to retain their distinctness and higher levels of diversity among the accessions when compared with allogamous species. The results from the present study concur with those of Moyib et al., (Reference Moyib, Gbadegesin, Aina and Odunola2008) who recorded wide genetic diversity among 24 AYB landraces from south-western Nigeria. The level of genetic diversity found in this study indicates that the AYB germplasm studied can be exploited for genetic improvement.
The present results agreed with the initial morphological characterization of the same accessions (Adewale et al., Reference Adewale, Dumet, Vroh-Bi, Kehinde, Ojo, Adegbite and Franco2012b); however, there were obvious differences in the structure of both classifications, such as those observed in other crop species (Venkatesha et al., Reference Venkatesha, Ramanjini, Ganapathy, Gowda, Ramachandra, Girish, Channamallikarjuna, Shantala and Gowda2010). The agro-morphological characterization and the present grouping pattern of the 77 AYB accessions into four genetic groups based on DNA profiles provides a basis for parental selection and the creation of heterozygous breeding populations. Unravelling useful trait–marker associations in such heterozygous AYB populations is very important, as it can facilitate breeding programmes in this underutilized legume. Indeed, combining phenotypic evaluations and molecular analysis can provide a platform for the selection of parents for genetic improvement programmes and germplasm conservation. Such programmes can use some of the approaches devised in this study in relation to marker generation and analysis in larger germplasm collections with more molecular markers.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262114000707
Acknowledgements
The authors thank Oyatomi Niyi, Faloye Benjamin and Adebowale of the Genetic Resources Centre of the IITA, Ibadan for their assistance during the first author's doctoral programme. The authors sincerely appreciate the reviewers for their constructive criticism that has greatly enhanced the quality of this article.