Introduction
Cultivated Vitis vinifera subsp. sativa has been domesticated from wild Vitis vinifera subsp. sylvestris (Lacombe et al., Reference Lacombe, Laucou, Di Vacchi, Bordenave, Bourse, Siret, David, Boursiquot, Bronner, Merdinoglu and This2003), distributed from Middle East to European regions (Grassi et al., Reference Grassi, Imazio, Failla, Scienza, Ocete Rubio, Lopez, Sala and Labra2003a), for the development of genes tolerant to biotic and abiotic stresses that it is exposed to in new environments (Liu et al., Reference Liu, Fan, Jiang, Guo, Sun, Zh and Feng2012). Over the past few centuries, historical evidence combined with ampelographic data has frequently been used to characterize grape germplasm and to investigate the origin and relationships among wild and cultivated individuals (Labra et al., Reference Labra, Moriondo, Schneider, Grassi, Failla, Scienza and Sala2002; Barth et al., Reference Barth, Forneck, Verzeletti, Blaich and Schumann2009; Ocete et al., Reference Ocete, Muñoz, López, Pérez, Benito, Cabello and Valle2011). Nowadays, molecular markers are commonly being used to study genetic relationships among Vitis accessions (Grassi et al., Reference Grassi, Labra, Imazio, Spada, Sgorbati, Scienza and Sala2003b; Liu et al., Reference Liu, Fan, Jiang, Guo, Sun, Zh and Feng2012). The EU-PROJECT GENRES CT96 NO81 has identified a universal set of simple sequence repeat (SSR) markers useful for univocally distinguishing each cultivar (This and Dettweiler, Reference This and Dettweiler2003). This marker set has also been used to investigate the evolution of wild grape populations, the gene flow among different areas, and the response of wild accessions to environmental impacts and human activities (De Mattia et al., Reference De Mattia, Imazio, Grassi, Doulaty Baneh, Scienza and Labra2008; Ergül et al., Reference Ergül, Perez-Rivera, Soylemezoglu, Karzan and Arroyo-Garcia2011; Imazio et al., Reference Imazio, Maghradze, De Lorenzis, Bacilieri, Laucou, This, Scienza and Failla2013). Moreover, from the start of grapevine genome analysis (Velasco et al., Reference Velasco, Zharkikh, Troggio, Cartwright and Cestaro2007), several peculiar DNA traits have been identified in the European germplasm (Kuczmog et al., Reference Kuczmog, Galambos, Horváth, Mátai, Kozma, Szegedi and Putnoky2012; Battilana et al., Reference Battilana, Lorenzi, Moreira, Moreno-Sanz, Failla, Emanuelli and Grando2013; Riahi et al., Reference Riahi, Zoghlami, Dereeper, Laucou, Mliki and This2013). Unfortunately until now, only a few DNA investigations have been carried out on the wild germplasm of the Middle East regions (Forneck et al., Reference Forneck, Walker, Schreiber, Blaich and Schumann2003; Doulati-Baneh et al., Reference Doulati-Baneh, Mohammadi, Labra, Nazemieh, De Mattia and Mardi2007; Ergül et al., Reference Ergül, Perez-Rivera, Soylemezoglu, Karzan and Arroyo-Garcia2011). In these regions, wild grapes grow under environmental conditions that are different from those in Europe and plants are exposed to different stresses. Due to these reasons, the Middle East wild germplasm represents an important source of gene resistance traits suitable for improving cultivated grapevines. However, in the last few years, the natural habitats of wild grapevines have been altered by forest exploitation, overgrazing, soil drying, deforestation and other human activities. In Iran, wild grape populations are distributed in riparian wood habitats on river margins and in some regions a few of these populations can still be found near cultivated grape vineyards. The size of the Iranian wild grape populations has clearly decreased (Doulati-Baneh et al., Reference Doulati-Baneh, Mohammadi and Hassani2011) and the germplasm is at a risk of becoming extinct. Therefore, high priority should be given to the characterization and preservation of genetic variability. The present study analysed the genetic constitution of wild grape populations distributed in the Zagros Mountains located in West Azerbaijan and Kurdistan Provinces in the north-west region of Iran (Sabeti, Reference Sabeti1976) to (1) assess the level of genetic diversity; (2) determine the risk of extinction; (3) identify the most suitable conservation strategy.
The Zagros Mountains are considered to be an important site of biodiversity (Djamali et al., Reference Djamali, De Beaulieu, Miller, Andrieu-Ponel, Ponel, Lak, Sadeddin, Akhani and Fazeli2009; Karami et al., Reference Karami, Sefidi and Feghi2014); however, the recent landscape ecology evaluation (Karami et al., Reference Karami, Sefidi and Feghi2014) has shown that human activities (grazing and agriculture) have led to a reduction of natural areas, especially of humid forests where wild grape populations grow. Therefore, accurate characterization and preservation of the existing grapevine germplasm of this area are urgently required to prevent potential genetic erosion.
Materials and methods
Plant material
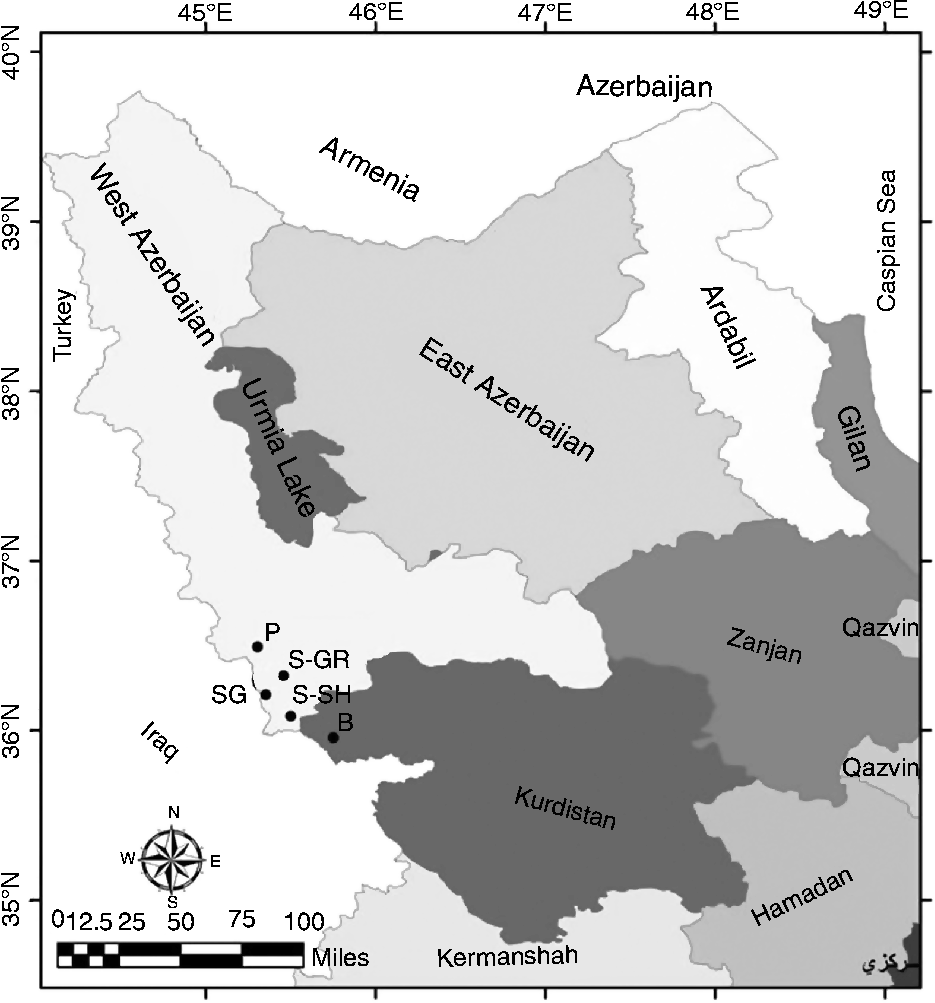
In this study, a total of 65 wild grapevine samples were collected from five populations from the forest and wetland regions of the Zagros Mountains in Kurdistan and West Azerbaijan Provinces, Iran. In Fig. 1, the geographical coordinates and distribution of the analysed populations are shown. Each population is represented by a variable number of wild grape individuals (from 7 to 23) distributed in a limited area of about 500 m2. A distance of at least 40 km separates each population from the other population. The Piranshahr (P) population consisted of 15 individuals (nine males and six females) distributed in the humid area of Piranshahr; the Baneh B population consisted of ten individuals (seven males and three famales) distributed in Baneh in Kurdistan Province. The other three populations were distributed in Sardasht: the Sardasht-Grjal (S-GR) population was the smallest one consisting of five males and two females, the Sardasht-Shalmash (S-SH) population consisted of ten individuals (eight males and two females), and the Sardasht-Ghasmarash (SG) population was the largest one consisting of 23 individuals (12 males and 11 females). Each individual was morphologically characterized based on the International Plant Genetic Resources Institute (IPGRI) descriptors (see Table S1; available online).
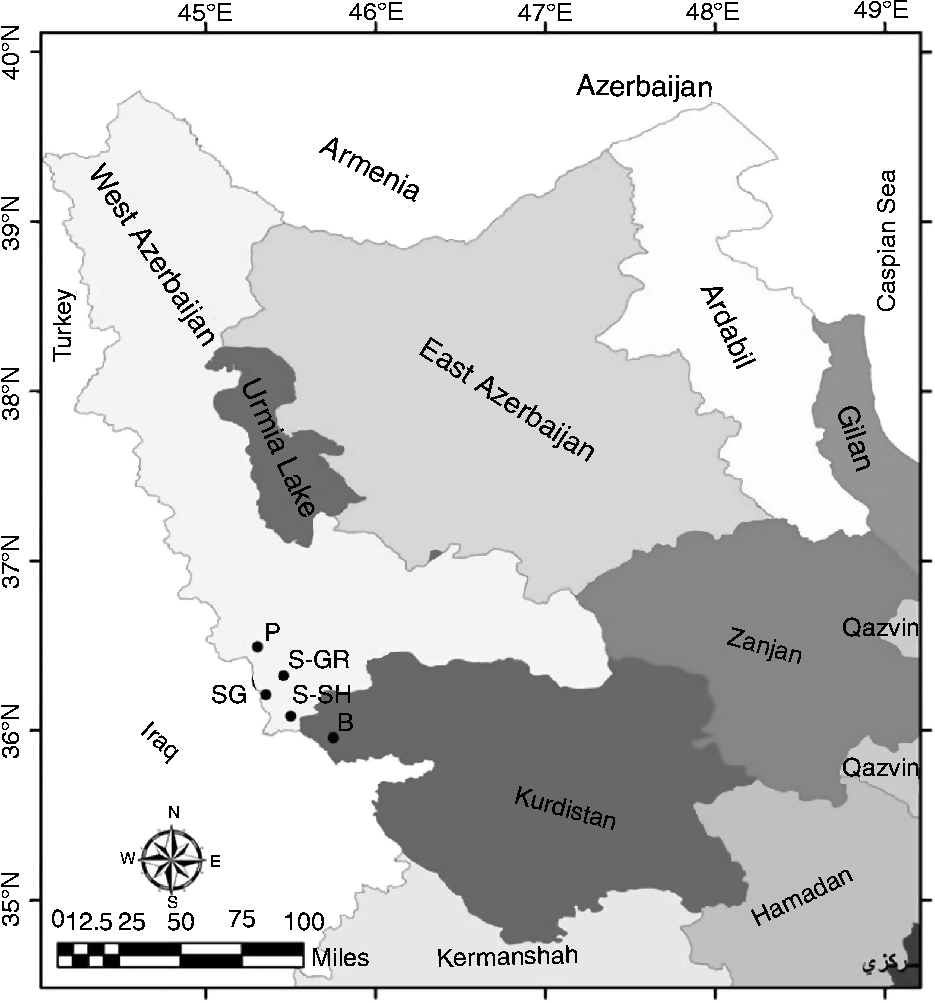
Fig. 1 Geographical coordinates and distribution of the analysed wild grape populations of the north-west region of Iran. P, Piranshahr; B, Baneh; S-SH, Sardasht-Shalmash; S-GR, Sardasht-Grjal; SG, Sardasht-Ghasmarash.
The populations are located at the altitude of 1100–1800 m above sea level and the region was characterized by the typical environmental conditions of wild grapevine habitats: wetlands and forests with a high degree of humidity due to rivers and brooks. Wild grape grows as a liana on different trees such as oak, hawthorn, willow, pear and terebinth.
The plant sampling strategy followed in the study was the same for all the populations and was designed to prevent errors such as collection of clones or individuals of cultivated subspecies (Vitis vinifera subsp. vinifera L.). To avoid sampling of clones, young leaves from individuals spaced at least 30 m apart were collected. The risk of collecting cultivated varieties was very low for the P and B populations, as these were distributed in remote areas far away from vineyards (more than 30 km). In contrast, there were many vineyards in Sardasht. Rasha and Khoshnav (a clone of Rasha) are the main cultivated hermaphrodite varieties followed by Taifi, Angotka, Zardka and Kazav. Although the wild populations analysed in this study were located far from vineyards (almost 5 km), to reduce the risk of sampling of cultivated varieties only dioecious individuals were collected in the SG, S-SH and S-GR populations (Grassi et al., Reference Grassi, Labra, Imazio, Spada, Sgorbati, Scienza and Sala2003b). Moreover, to exclude sampling errors, the SSR profiles of the cultivars (Doulati-Baneh et al., Reference Doulati-Baneh, Mohammadi and Labra2013) were compared with the molecular data obtained in our work related to wild accessions.
In Sardasht, three female varieties, Mam Braima, Bol Mazu and Sarghola, are also distributed in a few vineyards. To differentiate the female wild grapevine varieties from the cultivated ones, we evaluated the berry characteristics. All the collected female wild grapevine varieties had small, round and black berries with sour taste, while the fruits of the three cultivars were big and sweet with yellow (Mam Braima and Bol Mazu) or red (Sarghola) colour.
DNA extraction and SSR analysis
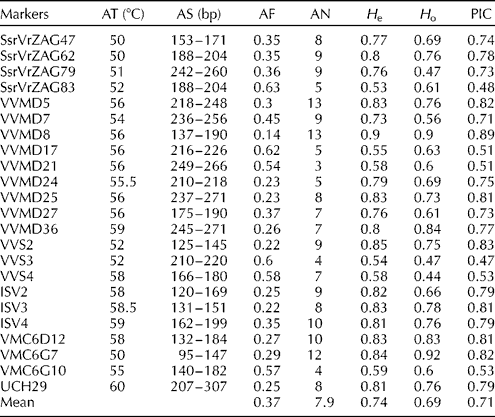
For each sample, two to three young leaves were used for genomic DNA extraction according to the procedure described by Lodhi et al. (Reference Lodhi, Ye, Weeden and Reisch1994). Molecular characterization was carried out using 23 SSR markers for each sample (Table 1). The SSR loci and annealing temperatures used for polymerase chain reaction (PCR) analysis are listed in Table 1. Primer sequences were obtained from Thomas and Scott (Reference Thomas and Scott1993); Bowers and Meredith (Reference Bowers and Meredith1996); Bowers et al. (Reference Bowers, Boursiquot, This, Chu, Johansson and Meredith1999); Sefc et al. (Reference Sefc, Regner, Turetschek, Glöessl and Steinkellenr1999); Lefort et al. (Reference Lefort, Pelsy, Schehrer and Merdinoglu2003). PCRs were carried out in a final volume of 10 μl, containing 15 ng of each primer, 1 U of Taq DNA polymerase (Sinagen Company, Teheran, Iran), 100 μM of each of the four dNTPs, 1 × PCR buffer (10 mM Tris–HCl, 50 mM KCl and 1.5 mM MgCl2), 2.5 mM of MgCl2, 50 ng of template DNA and distilled deionized water. Amplification reactions were carried out using the following cycling profile: initial denaturation at 94°C for 4 min, followed by 35 cycles of 94°C for 1 min, 58–68°C for 1 min (see Table 1), and 72°C for 2 min, and a final extension step at 72°C for 7 min. The PCR products were separated on 6% (w/v) polyacrylamide gels and visualized by silver staining.
Table 1 Genetic parameters of SSR markers across the analysed wild grape genotypes
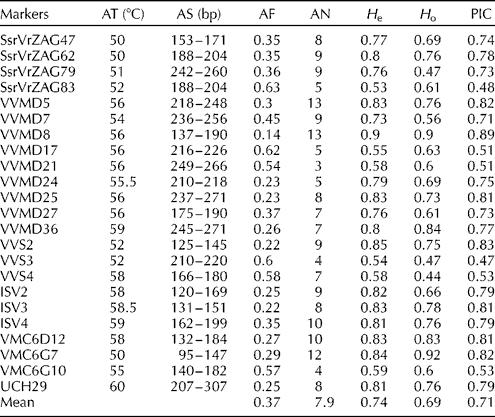
AT, annealing temperatures; AS, allele sizes; AF, allele frequency; AN, allele number; H e, expected heterozygosity; H o, observed heterozygosity; PIC, polymorphic information content.
The allele sizes of the SSR bands were determined using internal size markers and by comparison with a standard set of microsatellite reference alleles (This et al., Reference This, Jung, Boccacci, Borrego, Botta, Costantini, Crespan, Dangl, Eisenheld, Ferreira-Monterio, Grando, Ibáñez, Lacombe, Laucou, Magalhães, Meredith, Milani, Peterlunger, Regner, Zulini and Maul2004). For each SSR locus, allele number, allele frequency, expected heterozygosity (H e), observed heterozygosity (H o) and polymorphic information content (PIC) were calculated. Gene diversity, often referred to as expected heterozygosity, is the probability that two randomly chosen alleles at a locus within a set of genotypes will be different (Nei, Reference Nei1987). The PIC values were estimated as described by Botstein et al. (Reference Botstein, White, Skolnick and Davis1980). These genetic parameters were estimated using PowerMarker version 3.20 (Liu, Reference Liu2002). For each population, genetic parameters, such as observed average allele number (N a), allelic richness (A [14]), H o, H e, coefficient of inbreeding (F is) and private allele (P a), were calculated using the program FSTAT (Goudet, Reference Goudet2001).
Intrapopulation genetic diversity was also determined in terms of A (El Mousadik and Petit, Reference El Mousadik and Petit1996) using a fixed sample size of seven (14 gene copies), corresponding to the number of individuals of the smaller population (S-GR). The F is was calculated for polymorphic loci both to test the deviation from the Hardy–Weinberg equilibrium and to estimate the genetic differentiation among the populations (Weir and Cockerham, Reference Weir and Cockerham1984). The significance of deviations from the Hardy–Weinberg equilibrium, as evidenced by the deviation of F is from zero, was tested by randomization using the FSTAT software (Goudet, Reference Goudet2001). All the calculations were done using POPGENE version 3.2 (Yeh et al., Reference Yeh, Yang and Boyle1999) and FSTAT (Goudet, Reference Goudet2001).
Genetic distance was calculated by determining microsatellite allele frequencies using Nei's index (Nei, Reference Nei1987). The resulting genetic distance matrices were used to construct a neighbour-joining (NJ) dendrogram. The dendrogram was constructed using the MEGA4 software (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007). A hierarchical analysis of molecular variance (AMOVA) was carried out to separate total molecular variance into within-population and between-population variance using PowerMarker version 3.20 (Liu, Reference Liu2002).
To exclude hybrids between local cultivated varieties and the analysed wild individuals, we used the Bayesian clustering method of Pritchard et al. (Reference Pritchard, Stephens and Donnelly2000), implemented in the program STRUCTURE 2.1. In this case, the program was run using the ‘admixture model’, and all STRUCTURE analyses included no previous population information and correlated allele frequencies and were carried out using runs of one million interactions, following a burn-in period of 200,000 interactions. Clustering analyses were carried out in quadruplicate to ensure the convergence of parameters. The program New Hybrids (Anderson and Thompson, Reference Anderson and Thompson2002) was also used to carry out a more detailed analysis of admixture proportions and hybrid ancestry, by inferring the posterior probability assignment of each sampled individual to six genotype frequency classes: pure wild (W), pure cultivated (C), F1, F2, wild backcross and cultivated backcross. Tests were carried out for all the 23 loci with uniform priors and with a run of 500,000 iterations after a burn-in period of 50,000 iterations. This approach reduces the influence of low-frequency alleles, preventing sampling and genotyping errors in closely related populations.
Results
DNA of good quality was observed in all the analysed samples. The amplification of the 23 SSR loci yielded 182 different alleles, ranging from three (VVMD21) to 13 (VVMD5 and VVMD8) per locus, with an average of 7.9 alleles/locus (Table 1 and Table S2, available online). The mean PIC value was consistent (0.71); the highest value was obtained for VVMD8 (0.89) and the lowest value for VVS3 (0.47).
The H e values ranged from 0.53 (SsrVrZAG83) to 0.90 (VVMD8), while the H o values ranged from 0.44 (VVS4) to 0.97 (VMC6G7).
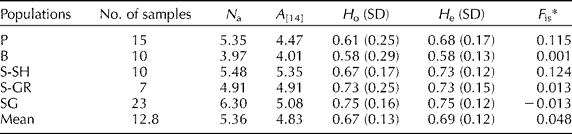
The consistent polymorphisms detected among the used SSR markers allowed characterizing the genetic structure of the analysed populations. The results given in Table 2 reveal consistent A in all the analysed populations ranging from 4.01 for the B population to 5.35 for the S-SH population. The mean values of H o and H e were 0.67 and 0.69, respectively. A modest heterozygosity deficiency was detected only in the P and S-SH populations; the other populations had quite similar H o and H e. In accordance with the detected heterozygosity deficiency, the F is values of the P (0.115) and S-SH (0.124) populations indicated slight inbreeding. In the B, S-GR and SG populations, the F is values were close to 0, implying a randomly mating population.
Table 2 Genetic characteristics of the analysed populations evaluated based on the polymorphisms of the 23 simple sequence repeat loci
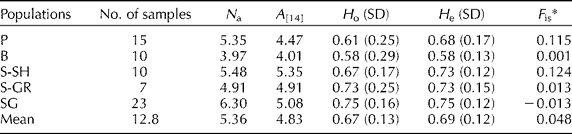
P, Piranshahr; B, Baneh; S-SH; Sardasht-Shalmash; S-GR, Sardasht-Grjal; SG, Sardasht-Ghasmarash; N a, average number of alleles; A, allelic richness; H o, observed heterozygosity; H e, expected heterozygosity; SD, standard deviation; F is, coefficient of inbreeding.
* Significant deviation of the F is value from zero was tested with 1000 randomizations (P< 0.05).
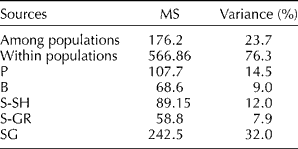
The AMOVA (Table 3) revealed that 76.3% of the total molecular variation was within the populations and only 23.7% between the populations. The minimum within-population variation was recorded in the S-GR population, while the SG population exhibited the highest molecular variation (32%).
Table 3 Analysis of molecular variance among and within the analysed populations
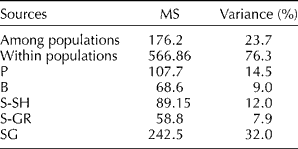
P, Piranshahr; B, Baneh; S-SH; Sardasht-Shalmash; S-GR, Sardasht-Grjal; SG, Sardasht-Ghasmarash; MS, mean square.
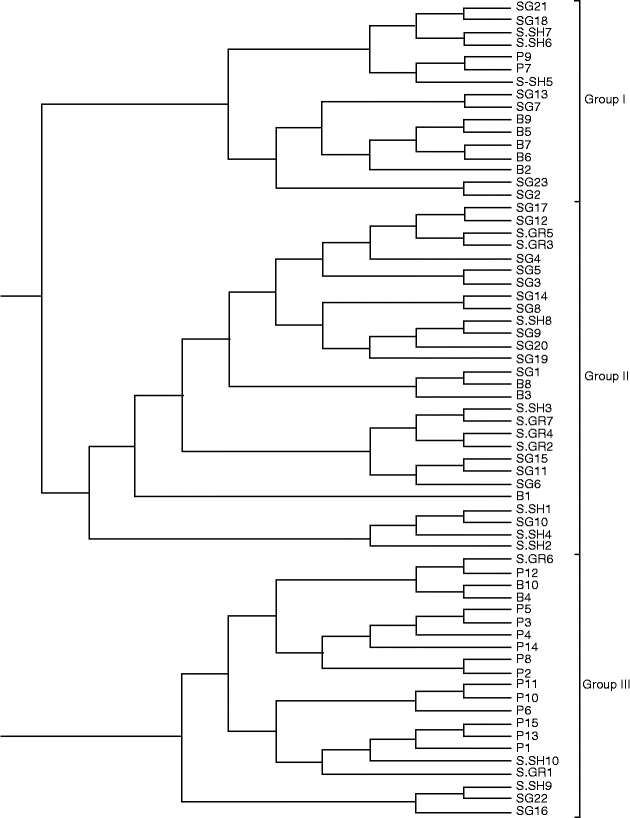
To better evaluate the relationships among different grape accessions, a dendrogram based on NJ algorithm was constructed (Fig. 2). The analysed samples were assigned to three major groups. Group 1 consisted of 16 samples, with most of the samples belonging to the B population. Group 2 consisted of 28 genotypes belonging to the Sardasht populations (SG, S-SH and S-GR). All the samples of the S-GR population clustered in this group with the exclusion of the S-GR2 population, while the samples of the S-GR population exhibited a consistent genetic difference and clustered in all the three branches of the dendrogram. Group 3 consisted of 21 wild genotypes, with most of them belonging to the P population.
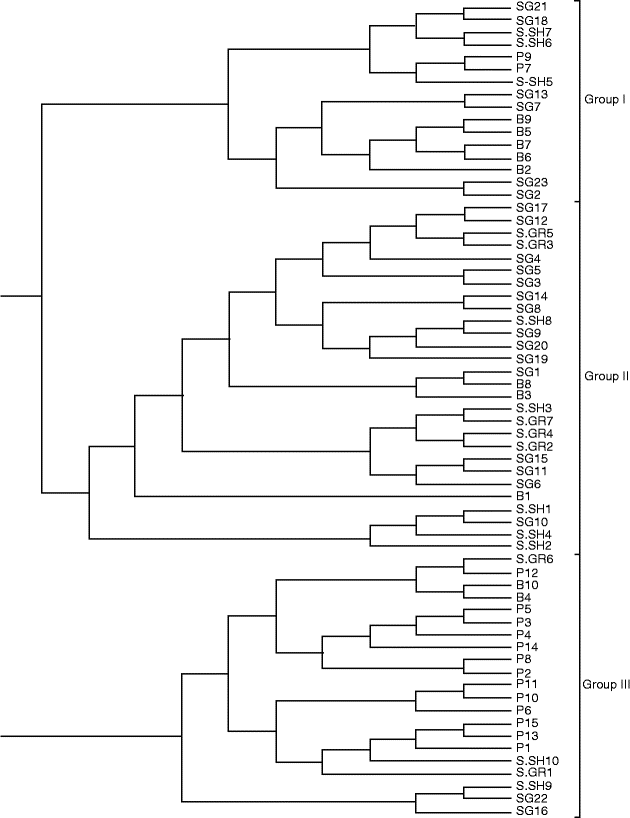
Fig. 2 Dendrogram depicting genetic relationships among the wild grape populations based on shared allele distance and neighbour-joining algorithm. P, Piranshahr; B, Baneh; S-SH, Sardasht-Shalmash; S-GR, Sardasht-Grjal; SG, Sardasht-Ghasmarash.
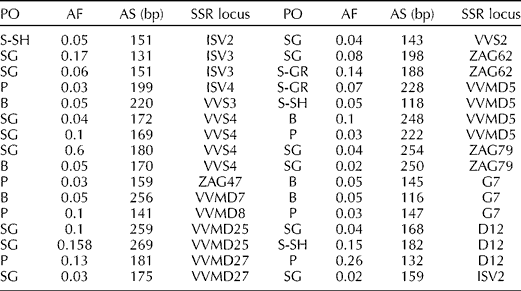
The analysis of rare alleles indicated that 34 (18.68%) of the 182 detected alleles at the 23 loci were private (Table 4). A total of 14 private alleles were observed in the SG population followed by the B and P populations with eight and seven private alleles, respectively.
Table 4 Distribution of rare SSR alleles in the wild grape populations
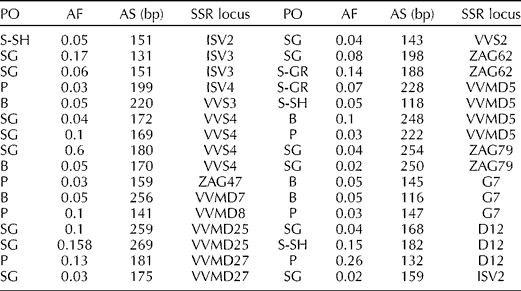
PO, name of the population; AF, allele frequency; AS, allele size; S-SH; Sardasht-Shalmash; SG, Sardasht-Ghasmarash; P, Piranshahr; S-GR, Sardasht-Grjal; B, Baneh.
To exclude the hybrid origin of wild accessions, the obtained SSR profiles of the cultivated and wild varieties were analysed with STRUCTURE 2.1 analysis (Zecca et al., Reference Zecca, De Mattia, Lovicu, Labra, Sala and Grassi2010). The results revealed that the W samples were genetically distinct from the C ones (Fig. S1, available online). At the same time, the analysis carried out using the New Hybrids program confirmed the sharp distinction between the W and C accessions, where each plant was assigned to each genotype frequency class with an average posterior probability >0.95 (Fig. S2, available online).
Discussion
Consistent genetic diversity was detected among the analysed wild grape populations distributed in the Zagros Mountains (Iran). Similar results have been obtained for Anatolia populations (Ergül et al., Reference Ergül, Perez-Rivera, Soylemezoglu, Karzan and Arroyo-Garcia2011), and they indicate that the genetic diversity of wild grape populations in the Middle East regions is higher than that of populations distributed in other areas such as the Mediterranean basin (Grassi et al., Reference Grassi, Imazio, Failla, Scienza, Ocete Rubio, Lopez, Sala and Labra2003a; Lopes et al., Reference Lopes, Mendoca, Rodrigues do Santos, Eiras-Dias and da Câmara Machado2009; Zinelabidine et al., Reference Zinelabidine, Haddioui, Bravo, Arroyo-García and Martínez-Zapater2010). Moreover, no inbreeding depression events were observed in the analysed populations. Previously, studies had demonstrated a clear reduction in H o in most of the European wild grape populations (Grassi et al., Reference Grassi, Imazio, Failla, Scienza, Ocete Rubio, Lopez, Sala and Labra2003a; Di Vecchi-Staraz et al., Reference Di Vecchi-Staraz, Laucou, Bruno, Lacombe, Gerber, Bourse, Boselli and This2009; Lopes et al., Reference Lopes, Mendoca, Rodrigues do Santos, Eiras-Dias and da Câmara Machado2009), probably due to the reduction of population size by human activities. We can conclude that although in the last few years the Zagros Mountains have been subjected to intense deforestation activities (Karami et al., Reference Karami, Sefidi and Feghi2014), the local wild grape germplasm has not yet undergone a genetic erosion. However, most of the analysed populations exhibited an unbalanced sex ratio. The modest number of female individuals in most of the Zagros Mountains populations (i.e. B, SG and S-SH) reduced the seed-dispersal ability of the species. This was also confirmed by our field evaluation during plant sampling activity; a large proportion of individuals were very old (more than 25–30 years old) and a few young plantlets were detected only in the SG population. We conclude that Iranian wild grape populations have a reduced regeneration ability probably due to modest seed productions or/and environmental alterations that lead to an inhospitable habitat for young seedlings (Arnold et al., Reference Arnold, Schnitzler, Douard, Peter and Gillet2005; Ocete et al., Reference Ocete, López, Gallardo and Arnold2008).
In the last few years, the Zagros Mountains have been subject to consistent anthropic pressure, deforestation and overgrazing (Noroozi et al., Reference Noroozi, Akhani and Breckle2008) with the consequence being environmental alteration. Deforestation and river management have been indicated as the main threats to wild grape populations (Arnold et al., Reference Arnold, Schnitzler, Douard, Peter and Gillet2005). The effect of habitat modifications was particularly evident in Sardasht region, where grazing, forest fire and agriculture (Haidari et al., Reference Haidari, Bazyar, Hosseini, Hossein Haidari and Shabanian2013) drastically reduced the riparian areas suitable for wild grape growth (Grassi et al., Reference Grassi, Imazio, Failla, Scienza, Ocete Rubio, Lopez, Sala and Labra2003b). The S-GR and S-SH populations are relegated to a small area surrounded by cultivated fields and human activities, especially in Shalmash region. These rapid environmental alterations do not alter the genetic constitution of the existing wild grape individuals, as indicated by our molecular investigation, but induce population decline and prevent the development of new plantlets. In 2005, the UNDP (United Nations Development Programme) approved a ten-year project for biodiversity conservation in the central Zagros Mountains (http://www.undp.org.ir/index.php/component/content/article/77) with the main goal being the preservation of the endangered habitat including the humid forest and wetland areas. This is an essential step for preserving the wild grape and several riparial taxa distributed in the Zagros Mountains.
The SG population is distributed in a large humid area located at the Iraq–Iran border. This area is subject to conspicuous rainfall that guarantees full rivers and humidity. The consistent number of individuals and a proper balance between males and females and the presence of young plantlets indicate that the SG population is not at a risk of becoming extinct. Moreover, due to the war between Iran and Iraq, in the last 8 years, very few people have travelled to this area and thus the anthropic pressure on biodiversity is modest.
The evident effect of population erosion was also observed in the B population. In this area, local communities mainly depend on ranching and a serious forage shortage has occurred in the last few years. Local people began to exploit trees such as oak to feed cattle (Fatahi, Reference Fatahi1994; Moradi et al., Reference Moradi, Oladi, Fallah and Fatehi2010) and deforestation has led to drastic effects on climber species such as wild grape.
The P population is distributed in Prdanan tourist area and it is distributed far from the other populations. Although this is the second largest analysed population, the increase in tourism, overgrazing, seasonal river floods and agricultural activities has led to negative effects on riparian plants including wild grape. To preserve the genetic diversity of this population, some individuals have been propagated and transferred to the germplasm collection of the Kahriz Horticultural Research Station in Ourmia.
The results of our molecular investigation combined with field evaluation suggested that wild grape populations distributed in the Zagros Mountains have consistent genetic richness, but are at a risk becoming extinct due to human activities, especially deforestation, and increase in agricultural activities.
Ex situ conservation approaches are convenient and rapid for preserving genetic traits (Barth et al., Reference Barth, Forneck, Verzeletti, Blaich and Schumann2009). Most of the wild grape accessions had peculiar alleles (see Table S2, available online); these samples should be the first to be preserved in germplasm collections. Moreover, preliminary evaluation carried out by a team of researchers of the Agricultural Research Center of West Azerbaijan provides evidence that characteristics such as powdery mildew and fanleaf virus resistance and salinity tolerance are present in some accessions in the germplasm (H. Doulati-Baneh, pers. commun.). This is in agreement with the results of a recently conducted analysis highlighting the presence of pest resistance traits in the wild grape germplasm (Riaz et al., Reference Riaz, Boursiquot, Dangl, Lacombe, Laucou, Tenscher and Walker2013). We underline that such evaluations are very important for future breeding programmes of table and wine grape.
Plants propagated using ex situ strategies should also be also used for restoration ecology programmes (i.e. replanting and reinforcement). Reintroduction programmes that have been very successful have been implemented using local genotypes; for this reason, we suggest that the wild grape collection be separated into three distinct groups corresponding to the dendrogram groups that reflect the geographical distribution of the populations.
Unfortunately, plants conserved by ex situ strategies do not evolve under natural forces and processes. By contrast, the conservation of genetic resources under in situ conditions ensures that evolutionary dynamic forces continue to influence plant adaptation and survival. Based on the results of the present study, we suggest that the SG population be maintained in its natural habitat through the establishment of a local protected area where the river margins are preserved and tourism activity is regulated to reduce the negative effects of human activities. In situ conservation is a practical way to complement ex situ conservation and meets the need of expanding germplasm collections with new genetic traits coming from the natural evolutionary processes (Pavek et al., Reference Pavek, Lamboy and Garvey2003).
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262114000598