Introduction
Cowpea (Vigna unguiculata (L.) Walp) is the most economically important indigenous African legume crop and has a wide variety of uses as a nutritious component in the human diet as well as nutritious livestock feed (Langyintuo et al., Reference Langyintuo, Lowenberg-DeBoer, Faye, Lambert, Ibro, Moussa, Kergna, Kushwaha, Musa and Ntoukam2003; Singh et al., Reference Singh, Ajeigbe, Tarawali, Fernandez-Rivera and Abubakar2003). Apart from insect pests and diseases, drought is another major constraint to the production of cowpea. Barker et al. (Reference Barker, Campos, Cooper, Dolan, Edmeades, Habben, Schussler, Wrigh and Zinselmeier2005) gave a more precise definition of agricultural drought as stress occurring when available water in the soil drops below 65% of the total accessible by the crop and atmospheric demand is such that significant degrees of leaf rolling are evident. Because the crop cannot photosynthesize when leaves are rolled, it therefore leads to the reduction in crop productivity. Moreover, drought conditions weaken the plants making them more susceptible to disease infection and insect pests. Several factors and mechanisms operate independently or jointly to enable plant cope with drought stress. According to Mitra (Reference Mitra2001), crop plants use more than one mechanisms at a time to cope with drought (drought escape, drought avoidance and drought tolerance) stress. Plant develop strategies for maintaining turgor by increasing root depth or developing an efficient root system to maximize water uptake and by reducing water loss through reduced epidermal (stomata and lenticular) conductance, reduced absorption of radiation by leaf rolling or folding and reduced evapotranspiration surface Mitra (Reference Mitra2001). The mechanisms of drought tolerance are the maintenance of turgor through osmotic adjustment (accumulation of solutes in the cell), increased cell elasticity and decrease cell size and desiccation tolerance by protoplasmic resistance. Agbicado (Reference Agbicado2009) reported that, closure of stomata to reduce water loss through transpiration and cessation of growth (for type 1 drought avoidance) and osmotic adjustment and continued slow growth (drought tolerance in type 2) as observed by Mai-kodomi (1999) have been suggested as the possible mechanism for drought tolerance in cowpea. Muchero et al. (Reference Muchero, Ehlers and Roberts2008) studied 14 genotypes of cowpea at seedling stage confirmed the existence of genetic variation in response to drought stress. Understanding the inheritance of drought tolerance in seedling characters in cowpea is still limited. Therefore, this study was carried out to investigate the mode of inheritance of some seedling traits associated with drought tolerance in cowpea under drought stress.
Materials and methods
This experiment was conducted in the screen house of the Department of Crop Protection and Environmental Biology, University of Ibadan.
The cowpea genotypes used for this experiment consisted of two crosses and their seven generations (P1, P2, F1, RF1, F2, BC1 and BC2). These crosses were derived from the preliminary screening of the parental genotypes (S3). The two crosses, namely Danilla × IT97K-499-35 and Danilla × TVU 7778 were generations involving two highly drought tolerant (Danilla and IT97K-499-35) and one highly susceptible (TVU7778) parents. The Tvu7778 is high yielding under irrigated conditions and well suited to agronomic conditions of the area under study. The IT97K-499-35 is moderately yielding and early maturing variety. The two drought tolerant and one susceptible parent were used for the development of plant generations to obtain F1, RF1, F2, BC1 and BC2. The plant generations were developed in two phases, in the first season, F1 and RF1 were generated. In the second season, the F1 and RF1 were used to generate F2. Also in the second season, the F1 was crossed back to the two parents to develop BC1 and BC2. The seven populations each of the two crosses were evaluated in the screen house during the dry season of November 2011. The cowpea populations: Pa, Pb, F1, RF1, F2, BC1 and BC2 were subjected to water stress in the screen house using the box screening method as described by Singh et al. (Reference Singh, Mai- Kodomi and Terao1999). Wooden boxes of 130 cm length, 65 cm width and 15 cm depth made of 2.5 cm thick planks were constructed and kept in a rain protected screen house. Each box was lined with polyethylene sheets, filled with 25 kg of soil, composed of 80.0% sand, 11.8% silt and 8.2% clay, with water holding capacity of 21.5%. The boxes were arranged in a randomized complete design with three replicates. Equidistant holes were made in straight rows 10 cm apart with a hill to hill 5 cm distance within rows. Two seed were planted per hole and were thinned to 1 at 2 weeks after planting. Each box contained one row of each of the parental line, one row each of the F1 and RF1, one row each of the BC1 and BC2 and five rows of F2 generations with 12 plants per row, which represent one replication. Three treatments made up of three water regime (0 days water stressed (DWS) – no water stress, 10DWS, 15DWS) were applied after suspension of watering. All boxes were watered until the emergence of first trifoliate leaves after which watering was suspended for 10DWS and 15DWS. Data were collected on plant height, terminal leaflet area and seedling vigor and percentage recovery for each treatment separately. The SV was assessed on a scale of 1 (highly-tolerant) – 9 (highly-susceptible), (S1), while RC (%) was evaluated at 5 days interval for 30d. The SLA (cm2) was determined as the product of the length and the maximum width. ‘Continuous watering for the first 14 d until the emergence of the first trifoliolate leaves after which watering was suspended for the 10DWS and 15DWS water regimes. Rewatering started for 10DWS after 10days of water stressed and for 15DWS after 15 days of water stressed. Watering continued for all the water regimes for the next 5 d, thence, and data were collected for all the traits.’ The data were collected on the individual plant basis.
Statistical analysis
Analysis of variance was used to determine differences among generations for each trait in each condition separately. The mean values were estimated from the emergent seedling for each generation separately.
The individual scaling tests (A, B and C) as described by Mather (Reference Mather1949) and Hayman and Mather (Reference Hayman and Mather1955) were employed to test their fitness to the additive-dominance model. In the case of the inadequacy and significance of scaling tests, six-parameter and joint scaling test genetic model described by Hayman (Reference Hayman1958) were used to estimate various genetic components. The parameters estimated were mean (m), additive variance (d), variance due to dominance (h), additive x additive epistatic variance (i), additive x dominant epistatic variance (j) and dominant x dominant epistatic variance (l). Estimates were based on population means, from the complete model, and were calculated through the weighted least squares method. The variance associated with each parameter was obtained by using the properties of the variance, under the assumption that the population means are independent. After evaluating the null hypothesis significance for each of these parameters (t-test), those that were not different from zero were eliminated and then the additive-dominance simplified genetic model was utilized. Parameters m, d and h were again estimated by the weighted least squares method and evaluated as to their significance.
The variance estimates attributed to environment, total genetic, additive and dominance deviation effects were obtained from the phenotypic variances for populations P1, P2, F1, F2, BC1 and BC2. These estimates allowed the determination of heritability in the broad and narrow sense and minimum number of genes that control each character, by using Burton's (Reference Burton1951) expression.
Broad sense and narrow sense heritability were estimated by Warner (Reference Warner1552) and Allard (Reference Allard1960) formula.
where V = variance of P1, P2, F1, F2, BC1 and BC2 generations.
The components of F2 variance were obtained by the following formula (Farshadfar et al., Reference Farshadfar, Mahjouri and Aghase2008)
where D is the additive genetic variance; H, the dominance variance; Minimum number of effective factors was estimated as D2, 8VA; where D, (P1-P2) and VA is additive variance. Expected genetic gain was estimated as k.ðp.h2, where k is selection differential; ðp, phenotypic standard deviation and h2, (F1-P1)/D; E, the environmental variance.
Results
Analysis of variance showed significant differences among generations for all the traits across the water regimes, which indicated the presence of genetic variability for all the traits (online Supplementary Table S2). The mean and standard error of the segregating generations of the two crosses in respect of the four traits is presented in Table 1. It was observed that moisture stressed affect in varying degrees the mean expression of all the traits of the parents and the segregating generations of the two crosses.
Table 1. Means and standard error of seedling height (SH), seedling terminal leaflet area (STLA), seedling vigour (SV) and recovery capacity (RC) of two cowpea crosses under three water regimes (0DWS, 10DWS and 15DWS) in the screen house

N, population size; 0DWS, no water stress; 10DWS, 10 d water stressed; 15DWS, 15 d water stressed.
In both crosses, F1 and RF1 had highest seedling vigour (2.50 ± 0.00) in the cross Danilla × IT97K-499-35 and least seedling vigour (6.50 ± 1.00) in the cross Danilla × TVU7778. Other hybrids seedling vigour ranged between 3.00 ± 0.00 for BC1 in Danilla × IT97K-499-35 cross to 7.00 ± 2.25 for BC1 in cross Danilla × TVU7778. In both crosses, F1 and RF1 had 100.0% recovery capacity, while other hybrids ranged from 60% for BC2 in cross Danilla × TVU7778 to 100% in cross Danilla × IT97K-499-35 (Table 1).
Complete dominance (Table 1) was observed in the cross Danilla × Tvu7778 for seedling height at 0DWS and 15DWS, while partial dominance was observed in the cross Danilla × IT97-499-35 at both 0DWS and 15DWS. In respect of seedling terminal leaflet area, complete and partial dominance were observed at 0DWS and 15DWS in the cross Danilla × Tvu7778, respectively, while additive gene action was observed at 0DWS and partial dominance at 15DWS in the cross Danilla × IT97-499-35.
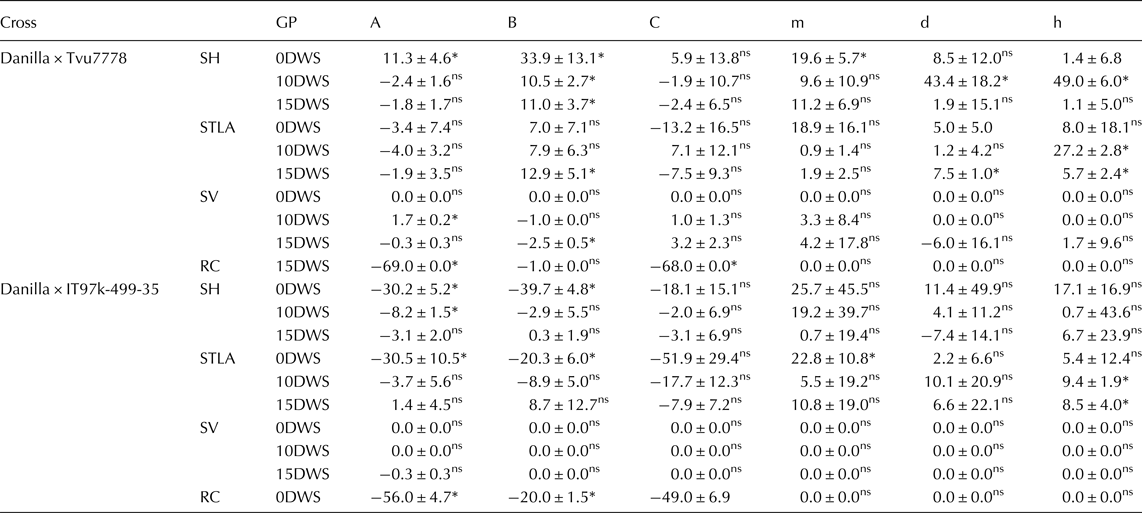
The result of scaling test (A, B and C) (Table 2) indicated adequacy of additive – dominant model for all traits across all the water regimes except recovery capacity at 15DWS in the cross Danilla × IT97K-499-35. A similar result was observed in the cross Danilla × Tvu7778, except the significant of B for seedling height, seedling vigour and seedling leaflet area at 15DWS, which indicated the presence of genetic interactions. Furthermore, in the case of joint scaling test (Table 2), additive-dominant model was adequate in both crosses at 0DWS and 15DWS for all the traits except additive (d) and dominant (h) gene effects in the cross Danilla × Tvu7778 at 15DWS for terminal leaflet area. These results suggested the importance of dominance gene action of these traits except in few cases the presence of genetic interactions.
Table 2. Estimated values of A, B, C scaling tests and joint scaling test genetic models for studied traits in two crosses of cowpea under three water regimes (0DWS, 10DWS and 15DWS) in the screen house
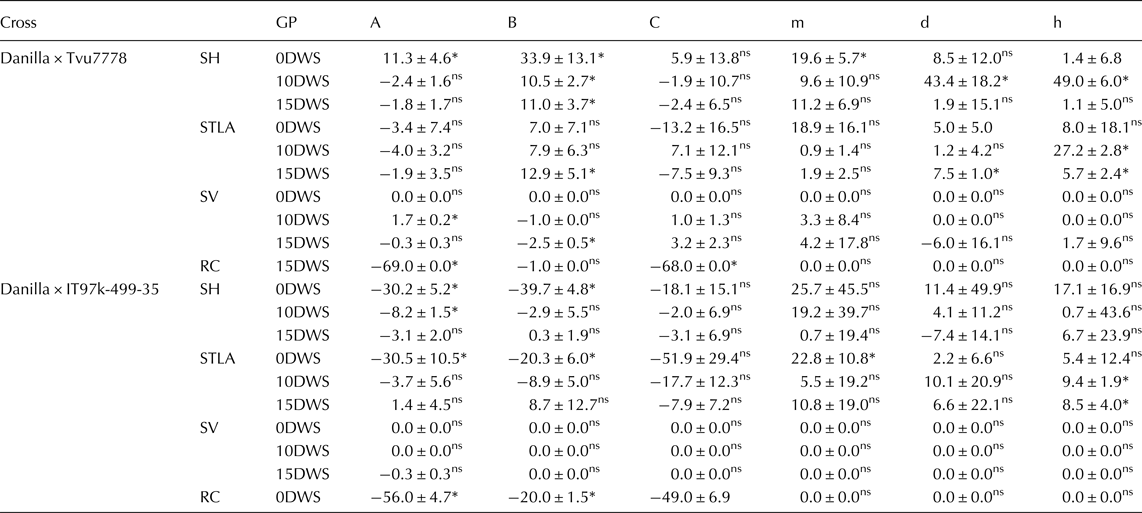
GP, Genetic Parameter; SH, seedling height; STLA, seedling terminal leavelet area; SV, seedling vigour and RC, recovery capacity*significant; ns, not significant.
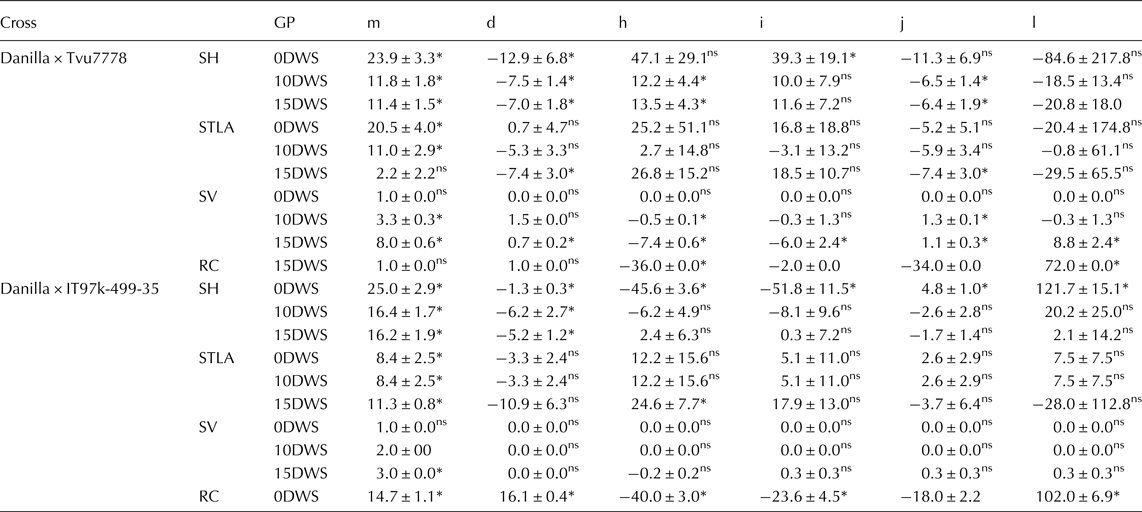
The various gene effects were estimated on the basis of the 6-parameter model (Table 3). Additive gene effect was significantly important for seedling height at all water regimes and recovery capacity at 15DWS in cross Danilla × IT97K-499-35. Similarly, in the cross Danilla × Tvu7778, additive gene effect was also significantly important for seedling leaflet area and seedling vigour at 15DWS. Dominant gene effect was significantly important for seedling height at 0DWS and recovery capacity at 15DWS in the cross Danilla × IT97K-499-35. In the cross Danilla × Tvu7778, dominant gene effect was significant for seedling height and seedling vigour at 10DWS and also for seedling height, seedling vigour and recovery capacity at 15DWS.
Table 3. Estimated values of six-parameters genetic models for studied traits in two crosses of cowpea under three water regimes (0DWS, 10DWS and 15DWS) in the screen house
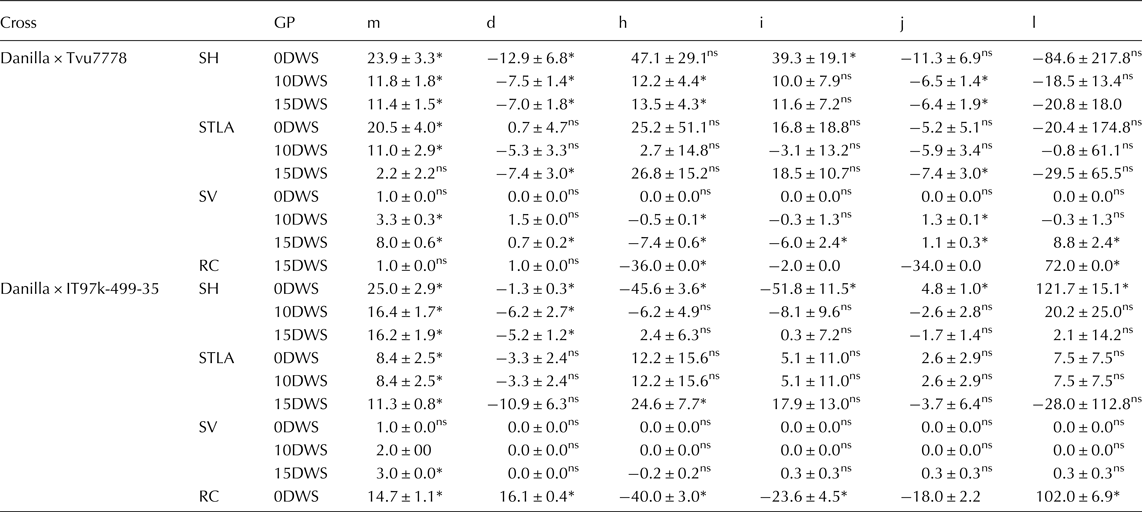
The additive × additive (i) gene interaction was significantly important in the inheritance of seedling height at 0DWS in both crosses and recovery capacity at 15DWS in the cross Danilla × IT97K-499-35, while in the cross Danilla × Tvu7778, additive × additive contributed to seedling vigour and recovery capacity at 15DWS.
The additive × dominance (j) gene interaction was also significant for seedling height at 0DWS and recovery capacity at15DWS in the cross Danilla × IT97K-499-35, while it was significant for seedling height and seedling vigour at 10DWS and for all traits at 15DWS in the cross Danilla × Tvu7778.
The dominance × dominance (l) gene interaction was important for seedling height at 0DWS and recovery capacity at 15DWS in the cross Danilla × IT97K-499-35, while in the cross Danilla × Tvu7778, was only significant for seedling vigour and recovery capacity at 15DWS.
The types of gene action observed among the two crosses depend on the traits combinations (highly tolerant × highly tolerant parents or highly tolerant × highly susceptible parents) and the water regime. However, there was a preponderance of dominance gene action for seedling height, seedling terminal leaflet area and seedling vigour at 15DWS. The partial dominance was pronounced when crosses involved highly drought tolerant and highly drought susceptible parents. The dominance tended towards high drought tolerant parent as observed in the percentage recovery after the resumption of watering. Percentage recovery for hybrids ranged between 50 and –100%. These reports supported the importance of dominance gene action for these traits.
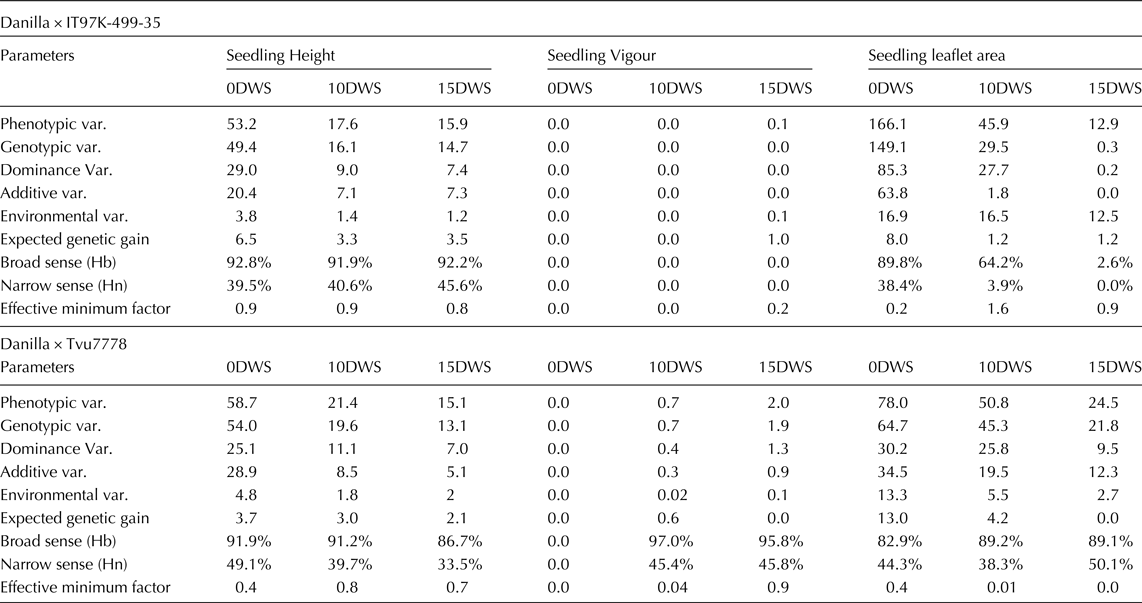
The estimates of phenotypic, genotypic, additive, dominance and environment variances (Table 4) revealed that the dominance (58.7%) and the additive (52.3%) variances were relatively similar and important for the inheritance of seedling height at 0DWS and dominance (50.3%) and additive (49.7%) at 15DWS in the cross Danilla × IT97K-499-35. A similar report was observed in the cross Danilla × Tvu7778, dominance variance ranged between 46.5 and 53.6% and additive between 43.4 and 53.5% across the water regimes in the Inheritance of seedling height. The estimated values for heritability in the broad and narrow senses ranged between 91.2–92.8 and 39.5–45.6% respectively; this represented the proportion at F2 genetic causes and to additive-nature genetic causes. The proportion of environment influence was smaller than the genetic influence on the total variation of the seedling height, hence the possibility of obtaining a satisfactory selection gain. The number of genes estimates suggested a minimum of one gene groups for this trait across the water regimes.
Table 4. Estimates of genetic parameters of two cowpea crosses under three water regimes (0DWS, 10DWS and 15DWS) in the screen house

In respect of seedling vigour, there was no significant dominance and additive variances for the cross Danilla × IT97K-499-35 (two highly tolerant parents) across the water regimes, however, in the cross Danilla × Tvu7778, dominance and additive variances were 68.4% and 31.6% at 15DWS, respectively. Heritability in the broad sense and narrow sense were 95.8% and 45.8%, respectively. The number of genes estimates also suggested a minimum of one gene group for this trait across the water regimes.
The proportion of dominance and additive variances for terminal leaflet area were 57.3% and 32.8% at 0DWS, 93.8% and 6.8% at 15DWS, respectively. The proportions of narrow sense heritability were low at 10DWS and 15DWS due to large environmental variance.
Discussion
There is need to understand the genetic basis of inheritance of drought tolerance in crop plant because drought limits the agricultural production by preventing the crop plants from expressing their full genetic potential. Both agronomical and morphophysiological traits have been reported to show different types of inheritance pattern (monogenic and polygenic) and gene action (additive and non-additive) (Mitra, Reference Mitra2001). Effective screening methods and techniques are pre-requisite for success in selecting the traits that confer adaptation for the gene controlling drought tolerant trait for breeding programs. Lack of efficient screening techniques and incomplete knowledge about the genetic basis of drought resistance are the main constraints for genetic improvement of drought resistance. Exploration of wide genetic variation of relevant characters particularly at seedling stage, consideration of more genes at a time to transfer through breeding under drought and multidisciplinary approach are very important in breeding programme. Some inheritance pattern and nature of gene action have been reported. Root character has been reported to be quantitatively inherited, that is they are controlled by many genes (Ekanayake et al., Reference Ekanayake, O'Toole, Garrity and Masajo1985). Also, long root and high root numbers have been reported to be controlled by dominant alleles and thick root tip by recessive alleles (Armento-Soto et al., Reference Armento-Soto, Chang, Loresto and O'Toole1983). However, further investigation is still needed to have a better understanding of genetic control of morphological and physiological traits contributing to drought resistance.
Cowpea has been considered to be one of the most drought tolerant crops in semi-arid Africa (Ehlers and Hall, Reference Ehlers and Hall1997), significant genetic variability still exists among cowpea genotypes for drought tolerance (Mai-Kodomi et al., Reference Mai-Kodomi, Singh, Myers, Yopp, Gibson and Terao1999; Watanabe et al., Reference Watanabe, Hakoyama, Terao, Singh, Singh, Mohan Raj, Dashiell and Jackai1997). Muchero et al. (Reference Muchero, Ehlers and Roberts2008) also confirmed the existence of genetic variation in response to drought stress, while studying 14 genotypes of cowpea at seedling stage. In the present study, the genetic analysis of the two crosses studied indicated that, their parents were genetically different for all the traits studied and their sensitivity to environmental effects as a result of adopted varying water regimes. In both crosses, the F1 and RF1 showed the best performance at terminal water stressed condition, suggesting dominance of the allele controlling drought tolerant trait. Where highly tolerant and highly susceptible parents were involved in the cross, the F1 and RF1 tended towards the highly tolerant parent indicating partial dominance of the highly dominant parent. Complete and partial dominance were observed for seedling height in the cross Danilla × Tvu7778 and Danilla × IT97-499-35, respectively. Additive gene effect was also found to contribute to the inheritance of seedling height at all water regime in the cross Danilla × IT97-499-35. The additive and dominance also contributed to the seedling height at terminal water stressed in the cross Danilla × Tvu7778. For seedling terminal leaflet area, complete and partial dominance were also contributed to the inheritance in either of the crosses. In the cross Danilla × Tvu7778, additive gene effect was also significantly important for seedling leaflet area at terminal water stressed. The dominance, additive × additive and dominance × dominance were all found to contribute to the inheritance of recovery capacity. Incorporating traits that confer adaptation of drought tolerant trait at seedling stage in cowpea will enhance breeding rapidity. The inheritance of the seedling characters for drought tolerance indicated that both dominant and additive gene actions were found to be important with the preponderance of dominant or dominace × dominacegene action. Kumar and Sharma (Reference Kumar and Sharma2005) reported that, among the various growth stages of the wheat crop, the seedling stage is very important with regard to vigour of the plants. Moisture stress at the initial stage affects germinations and other seedling traits. The author suggested that incorporation of the seedling traits viz., germinations capacity, longer root, shoot and coleoptiles length development will benefit the breeding programme that is targeted for drought prone areas and conditions where water availability is limited. However, Wallace et al. (Reference Wallace, Ozbun and Munger1972) stated that, with the exception of few monogenic traits influencing drought resistance character, most drought resistance mechanism are polygenic. Lebreton et al. (Reference Lebreton, Lazic-Jancic, Steed and Quarrie1995) also reported that while most secondary traits associated with drought tolerance are quantitatively inherited, there are two examples of simple inheritance.
The gene interactions for all characters showed the complex nature of both additive × additive and dominance × dominance fixable and non-fixable components of genetic variation, respectively. This report also similar to that of Kumar and Sharma (Reference Kumar and Sharma2005) working on bread wheat showed that, coleoptiles length and shoot length were predominantly governed by additive genetic effects and there is equal importance of both additive and dominance genetic effects for root length and seedling vigour index with the involvement of epistatic effects particularly dominance × dominance interactions for all the traits. So under such situation, bi-parental mating and/or diallel selective mating, which can exploit both components of variation could be useful for developing cowpea populations with desirable drought tolerance trait through breeding.
The expected genetic gain as showed in Table 4 reflected the possible gain from selection as percent increase in the F3 over the F2 mean at 5% selection (K = 2.06) from the F2 plants for seedling height and terminal leaflet area in both crosses. The estimates of both heritability and genetic advance showed that selection in F2 would lead to a substantial improvements in these traits.
With the assumption of no dominance, no linkage and no epistasis, it is possible to estimate the minimum number of effective factors involved in the inheritance of these traits using population variances. These estimates were likely to bias downward due to dominance and epistatic effects.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262117000235.
Acknowledgements
Department of Crop Protection and Environmental Biology, University of Ibadan, Nigeria.
Conflict of Interest
There is no conflict of interest.