Introduction
Genus Abelmoschus has quite a number of species which are native to Africa and Indian subcontinents. Southeast Asia is considered as the centre of diversity for okra where cultivated and wild species are overlapping (Charrier, Reference Charrier1984). Out of these wild species, Abelmoschus angulosus occurs in high altitudes in India, Sri Lanka and Indo-China regions (Singh et al., Reference Singh, Mathura, Kalloo, Satpathy and Pandey2007).
Okra Yellow Vein Mosaic Virus (OYVMV) disease which is transmitted by whitefly (Bemisia tabaci Gen.) is the most devastating disease in okra cultivation in the world as well as in Sri Lanka. Infection of OYVMV in the field is common and yield losses range from 50 to 100% depending on the stage of crop growth at which infection occurs (Sastry and Singh, Reference Sastry and Singh1974). This virus belongs to the family Geminiviridae and the disease is caused by a complex consisting of the monopartite begomovirus and small satellite DNA β component (Jose and Usha, Reference Jose and Usha2003). Mode of resistance for begomoviruses has been shown to be able to trigger gene silencing mechanism in response to virus infection (MacDiarmid, Reference MacDiarmid2005).
At present there is no definite and effective control measures against this virus disease. Control of virus is difficult and disease control is mainly based on the use of insecticides to reduce vector populations though with limited success (García-Cano et al., Reference García-Cano, Resende, Boiteux, Giordano, Fernández-Muñoz and Moriones2008). Therefore, as for other plant viruses the use of host plant resistance is the most desirable alternative.
None of the recommended varieties in Sri Lanka shows consistent resistance to this virus disease (Senavirathne et al., Reference Senavirathne, Wasala, Senanayake, Weerasekara, Wickramasinghe and Deepal2016). Therefore, utilization of genetic resources to enhance the resistance in cultivated okra varieties has been identified as one of the solutions to overcome this problem.
Crop-wild relatives which include the progenitors of crops as well as other related species are one of the categories of germplasm containing undeniably beneficial alleles to withstand biotic and abiotic challenges. Singh et al. (Reference Singh, Mathura, Kalloo, Satpathy and Pandey2007) studied on resistant genes for biotic stresses in wild taxa of okra (Abelmoschus species) under natural field evaluation procedures and observed some of the Abelmoschus species have resistance reaction to OYVMV disease. Samarajeewa and Rathnayake (Reference Samarajeewa and Rathnayake2004) evaluated three wild species of okra which are available in Sri Lanka for OYVMV resistance using different transmission methods and observed that A. angulosus has resistance reaction to OYVMV consistently.
Various types of inheritance patterns for genetic resistance to OYVMV disease have been reported in varieties of A. esculentus and wild species. Some scientists have reported that the resistance is controlled by two dominant complementary genes (Thakur Reference Thakur1976; Sharma and Dhillon Reference Sharma and Dhillon1983; Sharma and Sharma Reference Sharma and Sharma1984; Seth et al., Reference Seth, Chattopadhyay, Dutta, Hazra and Singh2017) but others have reported that a single dominant gene (Jambhale and Nerkar, Reference Jambhale and Nerkar1981; Senjam et al., Reference Senjam, Senapati, Chattopadhyay and Datta2018) or two recessive genes (Singh et al., Reference Singh, Joshi, Khanna and Gupta1962) are responsible for the resistance. The current study was conducted to investigate the genetics of wild relative of okra A. angulosus which is available in Sri Lanka against the OYVMV disease to explore the possibility of utilizing A. angulosus as a resistant source of OYVMV disease in future okra breeding programmes.
Materials and methods
Wild-relative species of okra, A. angulosus was crossed with recommended okra variety MI7 (Abelmoschus esculentus), which is highly susceptible to OYVMV at plant house of Plant Genetic Resources Centre, Gannoruwa, Sri Lanka. The variety MI 7 was used as the female parent. Resulting F1 individuals were selfed to obtain seeds of F2 population. A large number of crosses were performed to obtain adequate quantity of seeds. In the next season about 80 F2 individuals along with F1 individuals and two parents were established in the research field at the Field Crops Research and Development Institute (FCRDI), Mahailuppallama, Sri Lanka for the disease evaluation. In this particular location whitefly population and virus incidences are very high during the dry season and late wet season. Plant spacing was maintained at 60 × 90 cm2 (DOA, 1990). All the cultural practices were performed according to the recommendations of the Department of Agriculture, Sri Lanka (DOA, 1990). OYVMV disease pressure was increased by cultivating highly susceptible variety MI7 around the field and between F2 individuals as spreader rows. Natural field infection was allowed.
Seeds were collected from selfed individuals of F2 population. In the following season two parents, F1 and about 60 F2:3 progenies were grown in the field at FCRDI, Mahailuppallama, Sri Lanka. Late planting was performed to increase disease infestation. Plant spacing was maintained at 90 × 60 cm2 and each F2:3 progeny consisted with 20 plants. To increase the disease pressure, MI7, the susceptible parent, was grown as a spreader row after every sixth progeny line and around the field.
Disease assessment
Disease incidences of A. angulosus, MI 7, F1, F2 and F2:3 populations were recorded in 35, 45, 55 and 70 days after sowing. Percent disease incidence (PDI) was calculated according to the formula given by Sankara and Acharyya (Reference Sankara and Acharyya2012). Disease severity was recorded based on the procedure given by Ali et al. (Reference Ali, Khan, Habib, Rasheed and Iftikhar2005) with slight modifications. Virus symptom-free progenies were tested by polymerase chain reaction using virus specific primer to confirm the disease infection. Table 1 summarizes the disease symptoms and respective severity scale used in the current study. Disease severity index (DSI) was calculated based on the formula given by McKinney (Reference McKinney1923). PDI and DSI were calculated based on the disease severity scale. Data were transformed using square root transformation to stabilize the variance of the population and normal distribution of each population was confirmed by the Kolmogorov–Smirnov test (Steel and Torrie, Reference Steel and Torrie1980). Areas under disease progressive curves (AUDPC) were determined for parents, F1 and segregated generation (F2:3) using disease severity data in different time intervals according to the following formula (Wilcoxon et al., Reference Wilcoxon, Skovmand and Atif1975):
where x i is the disease intensity on date i and t i is the time in days in between date i and date i + 1. Mean AUDPC values were used to characterize the inheritance of disease resistance (Bjarko and Line, Reference Bjarko and Line1988).
Table 1. Description of OYVMV disease symptoms used for scoring

Source: Ali et al. (Reference Ali, Khan, Habib, Rasheed and Iftikhar2005) with slight modification.
R, resistant; HR, highly resistant; MR, moderately resistant; MS, moderately susceptible; S, susceptible; HS, highly susceptible.
Assessment of inheritance
Number of genes controlling disease resistance of A. angulosus was estimated by quantitative and qualitative methods. AUDPC values were used to estimate the effective gene number and mode of inheritance by a quantitative method. Number of genes segregating for OYVMV disease resistance were calculated for F2:3 generation using Wright's formula (Wright, Reference Wright1968) with modification for F2:3 generation analyses using the formula of Cockerham (Reference Cockerham1983) as follows:
where GR is genotypic range, V PS, V PR and ![]() $V_{{\rm F}_{2:3}}$ are variances of susceptible parent, resistant parent and F2:3 population, respectively and ‘n’ is the estimated number of genes. This formula assumes that no linkage exists between the loci involved, the effects of all loci involved are equal, dominance and epistasis are absent and all genes responsible for resistance are in a single parent. Three methods were used to estimate the effective gene number. To calculate the number of genes involved, genotypic range was estimated using two methods; mean difference of disease severity of two parents (method I) and the phenotypic range of the disease severity of segregated population (method II). Mean value of methods I and II were considered as method III (Basnet et al., Reference Basnet, Singh, Herrera-Faessel, Ibrahim, Huerta-Espino and Rudd2013).
$V_{{\rm F}_{2:3}}$ are variances of susceptible parent, resistant parent and F2:3 population, respectively and ‘n’ is the estimated number of genes. This formula assumes that no linkage exists between the loci involved, the effects of all loci involved are equal, dominance and epistasis are absent and all genes responsible for resistance are in a single parent. Three methods were used to estimate the effective gene number. To calculate the number of genes involved, genotypic range was estimated using two methods; mean difference of disease severity of two parents (method I) and the phenotypic range of the disease severity of segregated population (method II). Mean value of methods I and II were considered as method III (Basnet et al., Reference Basnet, Singh, Herrera-Faessel, Ibrahim, Huerta-Espino and Rudd2013).
Inheritance study for resistance through a qualitative approach was performed by grouping plants of the F2 population into resistant, segregating and susceptible classes at 60 days after sowing based on the F2:3 disease response. F3 progenies derived from F2 individuals were classified according to the disease response. A plant was considered resistance if its disease severity rating was <20%, whereas, a plant was considered as susceptible if the rating is above 60% and plants having severity rating in between these two groups were classified as intermediate (Ali et al., Reference Ali, Khan, Habib, Rasheed and Iftikhar2005). The χ 2 test was performed to determine the goodness of fit of observed segregation ratio for OYVMV disease reaction with different theoretical ratios to identify the number of genes involved. The model was not rejected if the calculated χ 2 value was lower than the tabulated value at the 0.05 probability level.
Results
Poor seed setting was observed in a cross between wild relative of okra A. angulosus and recommended variety MI 7 (A. esculentus). Many of the F1 seeds were deformed in shape. Those seeds were unable to give rise to F1 seedlings. Further, >90% seed abortion was observed at seed formation on F1 plants hence difficult to obtained F2 seed. By increasing the number of crosses F2 seeds were obtained and successful 80 F2 individual plants were established in the field along with A. angulosus, MI 7 and F1 plants for disease screening. However, due to unfavourable weather condition for whitefly population and disease infection none of the plants in the populations were infected with OYVMV disease. In this particular season highly susceptible variety MI 7 also showed zero infestation. F2 population grew well and selfed seeds from all the F2 individuals were harvested separately. In the following season, F2:3 progenies from each individual F2 plant were established in the field and disease screening was performed. In this season high disease pressure was observed. Spreader rows of susceptible parent showed very high disease incidences indicating that the disease pressure was uniformly present across the experimental plots.
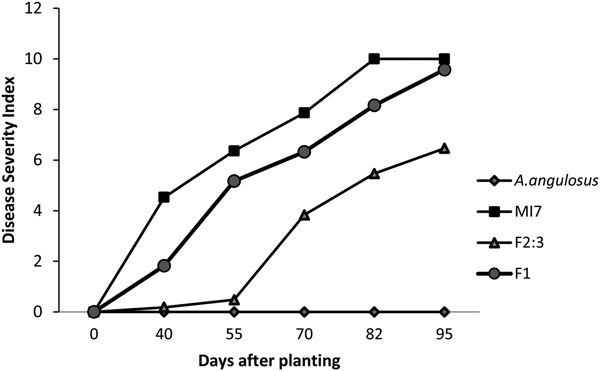
Two weeks after planting, disease symptoms were observed in MI 7, F1 and in F2:3 populations. Disease progress curves for susceptible parent MI 7, resistant parent A. angulosus, F1 and F2:3 populations are plotted in Fig. 1. Susceptible parent showed progressive disease severity while resistant parent showed zero disease symptoms. Disease severity of F1 generation recorded uniform susceptible reaction. Disease severity of F2:3 progenies showed between and within progeny segregation for the disease symptoms. It was low at the beginning and became progressive later which stay below the susceptible parent. Three disease-free F2:3 progenies were identified and absence of disease was confirmed using molecular techniques (data not shown). Identification of three disease-free F2:3 progenies was a strong indication to show that the study was successful though limited number of F2 plants and F2:3 progenies utilized for the disease screening in the field.

Fig. 1. Disease progress curves for parents, F1 and F2:3 populations of MI 7 × A. angulosus.
Quantitative analysis of inheritance
Mean AUDPC values for parents F1 and F2:3 generations are summarized in Table 2. These values were used to estimate the number of genes involved in OYVMV disease resistance in A. angulosus. According to Wright's formula modified for F3 population, effective number of genes segregated for OYVMV disease was estimated as 2 to 3 from method 1 and method II, respectively. Method III estimated effective gene number as two (Table 2).
Table 2. Mean AUDPC for each population and quantitative estimates of effective gene number for OYVMV disease in A. angulosus × MI-7 based on modified Wright's formula for F2:3 population using DSI

a Method I: genotypic range was estimated using difference of the parental means; method II: genotypic range was estimated using phenotypic range of the segregated population; method III: genotypic range was estimated using mean value of models I and II.
Qualitative analysis of inheritance for disease resistance
DSI value for susceptible parent MI 7 ranged from 80 to 100% while resistant parent, A. angulosus showed zero value. Disease severity of F1 generation grouped with the susceptible parent indicating that the susceptible reaction is dominant. Based on the disease severity at 60 days after sowing the F2:3 progeny lines were grouped into three distinct groups namely completely susceptible (homozygous for susceptibility), segregated for disease reaction (Seg.) and completely resistant (homozygous for resistant). Number of progenies in each group is summarized in Table 3. Range of DSI values in F2:3 population was 0–90%. F2 segregation ratio could be predicted using number of susceptible homozygous, segregated for disease reaction and completely resistant homozygous F2:3 progenies. The χ 2 test for predicted F2 segregation ratio based on disease severity of F3 progeny proved the goodness of fit to the expected ratio of 9 homozygous susceptible:6 segregated heterozygous:1 homozygous resistant with 95% confidence limit (χ 2 = 0.1757 at P ≤ 0.05). It explains the involvement of two recessive genes for inheritance of OYVMV disease resistance in A. angulosus (Table 3). This is in close agreement with the quantitative analysis which estimated the involvement of two to three effective genes in controlling resistance to OYVMV in wild relative of okra A. angulosus.
Table 3. Segregation of parents, F1 and F2:3 progenies for disease resistance/susceptibility and χ 2 test for populations

*Significant at 0.05 probability level.
Hom S, homozygous susceptible; Seg, segregating populations; Hom R, homozygous resistant.
Discussion
In the presence of substantial disease pressure A. angulosus showed highly resistant reaction to OYVMV disease while MI 7 showed highly susceptible reaction. High sterility in the wide hybrid between A. angulosus and MI 7 hinders the proper genetic analysis of OYVMV disease in wild species of okra A. angulosus using large segregated population. Samarajeewa (Reference Samarajeewa2003) studied about the crossability of this wild species with A. esculentus (cultivated okra) and observed that it was compatible with A. esculentus and can produce few viable seeds when A. angulosus was used as the male parent and 100% sterility was observed in the reciprocal cross. Patel et al. (Reference Patel, Malik, Negi, John, Yadav, Chaudhari and Bhat2013) identified that delayed pollen growth along with other structural abnormalities resulted poor seed setting in wide crosses of Abelmoschus species. However, results of the current study indicated that the genetic analysis of disease resistance of wild relatives which has reproduction barriers to develop sufficient amount of seeds for inheritance studies can be achieved through adequate population size for F2 and giving more emphasis to F2:3 generation.
Individuals of F1 generation were grouped into susceptible class in which the susceptible parent was grouped. This reaction of F1 individuals emphasized the fact that the dominance of susceptibility over resistance. Quantitative analysis proved the involvement of two genes in an additive manner. In the analysis of effective gene number, the genotypic range was calculated using two different procedures. In method I genotypic range was estimated by calculating the difference of parent means assuming all genes responsible for the disease resistance are in one parent. Further, assumptions were taken that no linkage, no dominance and no epistasis exists between loci involved. However, the presence of linkage, dominance and epistasis may course underestimation of actual number of segregating genes (Luke et al., Reference Luke, Barnett and Pfahler1975; Bjarko and Line Reference Bjarko and Line1988). Method II genotypic range was considered as the phenotypic range of the segregated population. Here the genes for resistance can be come from either parent and assumed that there were no linkage, epistasis and dominance. However, because of the environmental effects on the phenotypic range, this method may overestimate the actual number of segregating resistant genes. Therefore, average number of genes estimated from method III (average of number of genes from methods I and II) will give the correct number of genes involved for disease resistance (Basnet et al., Reference Basnet, Singh, Herrera-Faessel, Ibrahim, Huerta-Espino and Rudd2013). In the current study two effective genes was determined by the average of methods I and II. Further, the presence of zero SDI value for the disease in resistant parent confirmed that the two responsible genes are in one parent (A. angulosus). Based on both qualitative and quantitative inheritance pattern in the current study, the involvement of two recessive genes in an additive manner to control the OYVMV disease resistance in A. angulosus was ascertained.
Crop-wild relatives are important group of germplasm which can be identified as reservoir of desirable genes to endure adverse effects of climate change. Mining of such genes and introgress it into cultivated species are challenging due to its reproductive barriers. The presence of high sterility in wide hybrids, study of mode of gene action of candidate genes of wild relatives cannot be analysed according to the developed methods for large segregated population sizes. Under such situation the current study successfully identified two recessive gene actions for OYVMV disease using small population size. In such situation screening of disease severity at F2:3 population increases the precision of identification of effective gene number and mode of gene action.
Inheritance studies on OYVMV disease resistance in cultivated and wild species of okra have reported that OYVMV disease resistance is a genetically controlled trait and there are various types of inheritance patterns for resistance. Ali et al. (Reference Ali, Hossain and Sarker2000) studied the inheritance of OYVMV tolerance using inter-varietal crosses and revealed that it is a quantitative trait involving two major gene effects and the tolerance depends on gene dosage with incomplete dominant gene action. Inheritance studies of Seth et al. (Reference Seth, Chattopadhyay, Dutta, Hazra and Singh2017) and Sharma and Dhillon (Reference Sharma and Dhillon1983) concluded that the inheritance of OYVMV resistance in okra is controlled by two dominant complementary genes. Jambhale and Nerkar (Reference Jambhale and Nerkar1981) reported that resistance to OYVMV is controlled by a single dominant gene. Vashisht et al. (Reference Vashisht, Sharma and Dhillon2001) revealed that genetic control of resistance to OYVMV is complex as higher order interactions are involved and reported that the inheritance of resistance to OYVMV is governed by the epistasis gene action. The presence of major genes along with minor genes for resistance to OYVMV implies that the resistance mechanism is complex (Arora et al., Reference Arora, Jindal and Singh2008; Senjam et al., Reference Senjam, Senapati, Chattopadhyay and Datta2018). The current study revealed that two recessive genes are involved in an additive manner in OYVMV resistance in A. angulosus.
Identification of inheritance pattern in wild okra species A. angulosus is important to tag those responsible genes with the aim of introgression into the cultivated type; A. esculentus by wide hybridization. As two major recessive genes are involved with OYVMV resistance in A. angulosus, proper backcross breeding programme along with molecular marker aided selection would make the necessary introgression of these recessive genes into cultivated species.
Acknowledgements
This work was financially supported by the Grant no. 12-148 of National Research Council, Sri Lanka.