Introduction
More than five million childhood deaths occur due to micronutrient malnutrition every year. Over two billion people worldwide are suffering from deficiency of the key micronutrients such as iron (Fe) and zinc (Zn), afflicting especially woman and children of developing countries (Bouis and Welch, Reference Bouis and Welch2010). Fe deficiency anaemia has major consequences for human health as well as social and economic progress (WHO, 2013). Both Fe and Zn are present in low quantities in most of the staple crops. Biofortification of Fe and Zn and availability in plant foods could be an economical solution to this problem (Bouis and Welch, Reference Bouis and Welch2010). In developing countries, it has been suggested that biofortification strategies should focus on the staple foods that dominate people's diets (Pfeiffer and McClafferty, Reference Pfeiffer and McClafferty2007).
Wheat is a staple food of more than 40 countries for over 35% of the global population especially in the developing world and alone contributes 28% of the world's edible dry matter and up to 60% of the daily Fe and Zn intakes in several developing countries (Peleg et al., Reference Peleg, Saranga, Yazici and Fahima2008). Development of wheat varieties with improved efficiency of uptake and better translocation of Fe and Zn into the grain will improve crop productivity and help to overcome the Fe and Zn deficiencies. The genotypic variation in Fe and Zn contents in grains of commercial wheat cultivars is relatively low for wheat breeding for high contents and bioavailability of Fe and Zn (Cakmak et al., Reference Cakmak, Torun, Millet, Feldman, Fashima, Korol, Nevo, Braun and Ozkan2004). It has also been reported that the wild relatives of wheat have two or three times higher grain Fe and Zn contents than cultivated wheat. Previous studies have suggested that the wild relatives of wheat could be exploited as potential gene(s) source for wheat biofortification (Calderini and Ortiz-Monasterio, Reference Calderini and Ortiz-Monasterio2003; Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Tiwari et al., Reference Tiwari, Rawat, Neelam, Randhwa, Singh, Chhuneja and Dhaliwal2008, Reference Tiwari, Rawat, Neelam, Kumar, Randhawa and Dhaliwal2010; Rawat et al., Reference Rawat, Tiwari, Singh, Randhwa, Singh, Chhuneja and Dhaliwal2009; Farkas et al., Reference Farkas, Molnar, Dulai, Rapi, Oldal, Cseh, Kruppa and Lang2014).
Due to the loss of micronutrients during processing, it is important to understand the localization of Fe and Zn as an important factor in determining grain nutritional quality. The maximum percentage of each nutrient is to be found within bran (Singh et al., Reference Singh, Vogel-Mikus, Arcon, Vavpetic, Jeromel, Pelicon, Kumar and Tuli2013). In major part of the world, mostly the endosperm part of wheat is consumed after milling and processing. Consumption of whole grain flour could minimize this problem by directing most of the nutrients of the grain into flour. Hence, it is important to understand the pathways that lead to the accumulation of Fe in bran and embryo, and the bottlenecks that prevent its sequestration into the endosperm. Most of the studies on variability for micronutrients in the germplasm of wheat were conducted using whole grains, which may be ambiguous as the ratios of bran and endosperm may vary considerably in hexaploid wheat and its wild relatives. Therefore, during the present investigation, we screened hexaploid wheat cultivars, related wild species and wheat–Aegilops kotschyi substitution lines for useful variability of high Fe and Zn contents in endosperm and bran fractions, which is helpful to select the potential donors for biofortification of endosperm/bran fraction of hexaploid wheat.
Materials and methods
The experimental material comprising 16 accessions of progenitor and non-progenitor species of Aegilops and Triticum species, five cultivars of hexaploid wheat and three wheat–Ae. kotschyi derivative lines were grown at Norman E. Borlaug Crop Research Centre, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, India, in three replications (Table S1, available online). Spikes of all plants were harvested and threshed at physiological maturity. Grains of all the three replications were used for the analysis of Fe and Zn contents. Grains of hexaploid wheat, its progenitor and non-progenitor species and wheat–Ae. kotschyi derivative lines were dehusked by hand to avoid any Fe contamination.
Sample preparation and analysis of Fe and Zn
The grains were rinsed with N/10 hydrochloric acid for 1 min to remove any dust particle from seed surface and dried in a hot air oven at 80°C followed by continuous measurement of weight till they retained the same weight on second day of drying. Wheat grains after removing embryos with a scalpel were immersed in distilled water for 12 h at room temperature. After creating an incision at the crease, bran and endosperm were separated manually with the help of forceps and needle. The bran was then washed with deionized water continuously till it became completely free from the endosperm (Antoine et al., Reference Antoine, Peyron, Lullien-Pellerin, Abecassis and Rouau2004). The bran and endosperm fractions were oven-dried at 60°C till constant weight. The dried samples (0.5 g of seeds, 0.25 g of bran and 0.25 g of endosperm) were digested with 5.0 ml nitric acid (65%, w/w) and 2.0 ml hydrogen peroxide (30%, w/w) in a closed vessel in microwave digestion system (Multiwave ECO; Anton Paar GmbH, Austria) with closed door. The digested samples were transferred into a 50 ml graduated falcon tube and diluted up to 25 ml volume with deionized water. Blank was also diluted in the same way as test samples. The samples were analysed by Atomic Absorption Spectrophotometer (Agilent Technologies, USA). The concentration of Fe and Zn was expressed in mg/kg (ppm) on the basis of dry weight.
Statistical analysis
The data on Fe and Zn concentrations of grains and grain fractions of hexaploid wheat, its progenitor and non-progenitor species and wheat–Ae. kotschyi derivatives were subjected to statistical analysis. It included computation of mean performance, analysis of variance and correlation coefficient. Statistical analysis for present investigation was carried out with the help of Statistical Analysis Software (SAS) version 9.2 (SAS Institute, Carry, NC, USA, 2009).
In situ hybridization
The genomic DNA samples of Aegilops longissima (SlSl) and Aegilops umbellulata (UU) were used to prepare genomic probes for utilization in genomic in situ hybridization (GISH) experiments. Clones pAs1 and pHvG38 were used in sequential fluorescence in situ hybridization (FISH). Clone pAs1 contains a 1 kb fragment isolated from Aegilops tauschii that permits identification of the D-genome chromosomes (Rayburn and Gill, Reference Rayburn and Gill1987). The barley clone pHvG38 contains a 900 bp GAA-satellite sequence (Pedersen et al., Reference Pedersen, Rasmussen and Linde-Laursen1996), which has multiple FISH sites on the B-genome and some minor sites on A and D genome chromosomes of wheat as well as dispersed sites in S and U genome chromosomes. As a whole, the FISH pattern of this repeat is distinguishable among hexaploid wheat chromosomes. Using pHvG38 and pAs1 clones, all 21 chromosomes of hexaploid wheat could be identified (Pedersen and Langridge, Reference Pedersen and Langridge1997).
Three of the selected wheat–Ae. kotschyi derivatives namely 117-18-17-9, 63-2-13-1 and 77-33-2-5 were subjected to GISH analysis for identification of alien chromosome(s). Actively growing root tips were collected from germinating seeds and pre-treated in ice-cold tap water for 24 h to accumulate metaphases and fixed in Carnoy's fixative (3:1 ethanol and glacial acetic acid). For the preparation of slides, root tips were stained in 1% acetocarmine and squashed in 45% acetic acid. Genomic probes (0.2–0.6 kb) were prepared by shearing of genomic DNA of Ae. longissima (SlSl genome) and Ae. umbellulata (UU genome) and labelled with fluorescein-12-dUTP (2′-deoxy-uridine-5′-triphosphate) (green) and tetramethyl-rhodamine-5-dUTP (red), respectively (Roche Applied Science, Indianapolis, IN, USA) using the nick translation mix following manufacturer's direction. The labelled probes were purified using the QIAquick Nucleotide Removal Kit (Qiagen, Valencia, CA, USA). In order to prevent the hybridization of labelled genomic probes with wheat chromosomes, unlabelled sheared genomic DNA of Chinese Spring wheat (100–600 bp) was used as blocking DNA in a ratio of 1.0 ng labelled probe:120 ng of blocking DNA. Hybridization conditions, post-hybridization washes and imaging were as described by Zhang et al. (Reference Zhang, Friebe, Lukaszewski and Gill2001). For identification of individual wheat chromosomes, the same slides were probed with pAs1 (red) and pHvG38 (green) repetitive DNA clones after GISH. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole. Slides were analysed with an epifluorescence Zeiss Axioimager M1 microscope (Labexchange – Die Laborgerateborse GmbH, Germany).
Results
The Indian bread wheat cultivars possess very low level of Fe and Zn contents and limited variation in whole grain and grain fractions (bran and endosperm) compared with the wild relatives and Chinese Spring wheat. The Fe concentration in the bread wheat cultivars (PBW 343, UP 2338, UP 2382 and WL 711) varied from 28.98 to 32.98 mg/kg in whole grain, 67.10 to 129.40 mg/kg in bran, 6.93 to 10.80 mg/kg in endosperm and 1.186 to 1.499 μg/seed in a single seed (Table 1 and Table S2 (available online)), while the Zn concentration in these cultivars varied from 27.00 to 31.52 mg/kg in whole grain, 58.31 to 120.70 mg/kg in bran, 4.46 to 9.23 mg/kg in endosperm and 0.964 to 1.503 μg/seed in a single seed (Table 1 and Table S2 (available online)). However, in the case of wild relatives of wheat, a very high variation in Fe and Zn concentrations was found in whole grain and grain fractions. In various accessions of Aegilops and wild Triticum species, Fe concentration varied from 30.63 to 103.83 mg/kg in whole grain, 69.30 to 159.30 mg/kg in bran, 0.73 to 14.48 mg/kg in endosperm and 0.334 to 1.810 μg/seed in a single seed; and Zn concentration varied from 31.58 to 111.85 mg/kg in whole grain, 48.99 to 131.10 mg/kg in bran, 1.60 to 25.56 mg/kg in endosperm and 0.337 to 2.075 μg/seed in a single seed (Table 2 and Table S2 (available online)). Among the accessions of wild relatives of wheat, the highest Fe and Zn content in whole grains was recorded in Ae. longissima acc. 3507 (SS). In bran fraction, the highest Fe content was recorded in Triticum dicoccoides acc. TA117 (AABB) and Zn content in Ae. kotschyi acc. 3502 (UkSk). In endosperm fraction, the highest Fe content was recorded in Triticum monococcum acc. W463 (AmAm) and the highest Zn content in Aegilops speltoides acc. 3804 (SS).
Table 1 Fe and Zn contents in whole grain, bran and endosperm in hexaploid wheat cultivars

LSD, least significant difference.
Table 2 Fe and Zn contents in whole grain, bran and endosperm in different accessions of wild Triticum and Aegilops species

LSD, least significant difference.
In wheat–Ae. kotschyi substitution lines, the Fe concentration in whole grain and grain fractions varied from 32.01 to 37.55 mg/kg in whole grain, 99.47 to 136.45 mg/kg in bran, 12.80 to 14.12 mg/kg in endosperm and 1.234 to 1.484 μg/seed in a single seed. The Zn concentration in these substitution lines varied from 30.01 to 36.36 mg/kg in whole grain, 50.34 to 160.40 mg/kg in bran, 8.33 to 14.79 mg/kg in endosperm and 1.102 to 1.437 μg/seed in a single seed (Table 3 and Table S2 (available online)). Among wheat–Ae. kotschyi substitution lines, the highest Fe and Zn content was reported in whole grain and grain fractions of the substitution line 63-2-13-1. In wheat–Ae. kotschyi substitution line, namely 63-2-13-1, the Fe content is higher up to 12.17% in whole grain, 5.45% in bran, 30.74% in endosperm, and the Zn content is higher up to 15.35% in whole grain, 32.89% in bran and 36.94% in endosperm compared with hexaploid wheat cultivars having highest Fe and Zn contents (Table 3). In the analysis of variance for Fe and Zn contents over replicated chemical analysis, highly significant differences were found among wild accessions and cultivars (Table 4). The correlation coefficients between Fe and Zn contents in whole grain and grain fractions of selected wheat cultivars, its progenitor and non-progenitor species and wheat–Ae. kotschyi substitution lines for all possible combinations under the study are shown in Fig. 1. Significantly high positive correlation (r= 0.74) between Fe and Zn content was recorded in whole grain of bread wheat cultivars, its progenitor and non-progenitor species (Fig. 1(a)). A moderate correlation was observed for Zn contents in whole grain and endosperm (r= 0.46; Fig. 1(b)) and whole grain and bran (r= 0.54; Fig. 1(c)). Similarly, a moderate correlation (r= 0.44) was recorded for Fe and Zn in bran (Fig. 1(d)).
Table 3 Fe and Zn contents in whole grain, bran and endosperm fraction of selected wheat–Aegilops kotschyi substitution lines
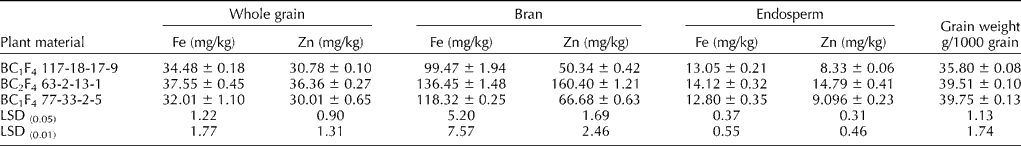
LSD, least significant difference.
Table 4 Analysis of variance for Fe and Zn contents in grain and grain fractions
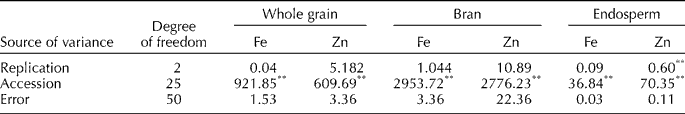
** Significant at 0.01 probability level.

Fig. 1 Correlation between Fe and Zn concentration in whole grain and grain fractions of hexaploid wheat cultivars, its progenitor and non-progenitor species (a) A high positive correlation of Fe and Zn in whole grain, (b) A moderate correlation of Zn in endosperm and whole grain, (c) A moderate correlation of Zn in bran and whole grain, (d) A moderate correlation of Fe and Zn in bran.
To identify the genome of the introgressed Ae. kotschyi chromosome, three of the selected substitution lines, namely 117-18-17-9, 77-33-2-5 and 63-2-13-1, were analysed by GISH. In 117-18-17-9 derivative, a heterologous Uk-wheat (Uk-W) translocation pair was detected after GISH (Fig. 2(c)), and the prophase chromosomes are also showing the same Uk-W translocation in this derivative (Fig. 2(d)). In 63-2-13-1 derivative, the U genome probe strongly hybridized with a pair of chromosomes (Fig. 2(e)) confirmed the presence of a pair of Uk genome of Ae. kotschyi. In the 77-33-2-5 derivative, a pair of Sk genome chromosome was identified using GISH (Fig. 2(a); Tiwari et al., Reference Tiwari, Rawat, Neelam, Kumar, Randhawa and Dhaliwal2010) and a sequential FISH confirmed the presence of a pair of 2Sk chromosomes of Ae. kotschyi in this derivative line (Fig. 2(b)).

Fig. 2 GISH analysis of chromosomes of wheat-Ae. kotschyi substitution lines (a) Mitotic chromosomes of 77-33-2-5 showing the presence of a pair of 2Sk chromosome (green) after GISH (Tiwari et al., Reference Tiwari, Rawat, Neelam, Kumar, Randhawa and Dhaliwal2010), (b) The same chromosomes 77-33-2-5 after the sequential FISH using pAs1 (red) and pHvG38 (green), (c) Mitotic chromosomes of 117-18-17-9 showing the presence of one heterologous Uk-W translocation pair (red) after GISH (d) Prophase chromosomes of 117-18-17-9 showing the presence of heterologous Uk-W translocation pair (red) after GISH (e) Mitotic chromosomes of 63-2-13-1 showing the presence of a pair of Uk chromosome (green) after GISH. The chromosomes were counterstained with DAPI.
Discussion
Earlier efforts to promote more diverse diets and to supply extra minerals through supplementation or food fortification are still in practice and are of great importance. However, recent research efforts have shifted towards biofortification of staple food crops that are considered to be one of the best approaches, along with dietary diversification, supplementation and fortification for alleviation of micronutrient malnutrition (Bouis and Welch, Reference Bouis and Welch2010). During the recent period, varieties, landraces and wild species of wheat were explored for their mineral levels and the genotypes having high mineral contents were utilized to transfer gene(s) responsible for high mineral contents in selected species/genotypes/accessions of hexaploid wheat. Metal chelators such as nicotianamine (NA) are important for radial movement of Fe and Zn through the root (Rellan-Alvarez et al., Reference Rellan-Alvarez, Giner-Martinez-Sierra, Orduna, Orera, Rodriguez-Castrillon, Garcia-Alonso, Abadia and Alvarez-Fernandez2010; Deinlein et al., Reference Deinlein, Weber, Schmidt, Rensch, Trampczynska, Hansen, Husted, Schjoerring, Talke, Kramer and Clemens2012) and the Zn transport into the vacuole affects overall Zn transport through the roots into the shoot (Borrill et al., Reference Borrill, Connorton, Balk, Miller, Sanders and Uauy2014). Fe and Zn are loaded into the xylem where Zn can move as a cation or in a complex with organic acids such as citrate (Lu et al., Reference Lu, Tian, Zhang, Yang, Labavitch, Webb, Latimer and Brown2013) and Fe is chelated by citrate (Rellan-Alvarez et al., Reference Rellan-Alvarez, Giner-Martinez-Sierra, Orduna, Orera, Rodriguez-Castrillon, Garcia-Alonso, Abadia and Alvarez-Fernandez2010). Transfer from xylem to phloem can occur in the root or basal part of the shoot or during remobilization from the leaves during grain filling. During grain development, transport of nutrient solution occurs from the vascular strand to the nucellar projection and thereafter to the endosperm. In wheat grains, Fe and Zn are predominantly localized in embryo and bran, whereas endosperm contains much less Fe and Zn (Borg et al., Reference Borg, Brinch-Pedersen, Tauris and Holm2009). The bran and embryo part of the wheat grains removed during milling process, resulting in the loss of 40% of the total grain Fe and Zn (Borrill et al., Reference Borrill, Connorton, Balk, Miller, Sanders and Uauy2014). Hence, particular attention should be given to increase Fe and Zn concentrations in endosperm fraction, as this is predominantly consumed in many developing countries. Furthermore, the low Fe and Zn contents in modern wheat cultivars also emphasize the need of screening and identification of related wild species/accessions of wheat on the basis of whole grain as well as grain fractions to use their effective genetic systems, if any, for wheat biofortification programme.
In addition to a number of previous studies, the present results indicate that the cultivated wheat have limited genetic variation in grain Fe and Zn concentrations. However, wild relatives of hexaploid wheat often possess considerable variation that is in accordance with the earlier reports (Cakmak et al., Reference Cakmak, Torun, Millet, Feldman, Fashima, Korol, Nevo, Braun and Ozkan2004; Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Pfeiffer and McClafferty, Reference Pfeiffer and McClafferty2007; Tiwari et al., Reference Tiwari, Rawat, Neelam, Randhwa, Singh, Chhuneja and Dhaliwal2008, Reference Tiwari, Rawat, Chhuneja, Neelam, Aggrawal, Randhwa, Dhaliwal and Singhm2009). On the basis of whole grain analysis, the wild relatives of wheat, particularly Triticum boeoticum (AA), T. monococcum (AmAm), T. dicoccoides (AABB), Ae. speltoides (SS), Aegilops triuncialis (UtUt), Ae. kotschyi (UkSk), Ae. tauschii (DD), Ae. longissima (SlSl) and Aegilops geniculata (UgMg), were exploited as potential donor to enhance Fe and Zn contents in hexaploid wheat (Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Rawat et al., Reference Rawat, Tiwari, Singh, Randhwa, Singh, Chhuneja and Dhaliwal2009; Tiwari et al., Reference Tiwari, Rawat, Neelam, Kumar, Randhawa and Dhaliwal2010). The results of earlier workers are in agreement with the results of present investigation that there is considerable genetic variation in Fe and Zn concentrations in wild Triticum and Aegilops species/accessions, especially in the U, Sl, S, UkSk and UpSp genomes.
Tiwari et al. (Reference Tiwari, Rawat, Chhuneja, Neelam, Aggrawal, Randhwa, Dhaliwal and Singhm2009) reported the maximum Fe content in Ae. kotschyi acc. 3573 (90.96 mg/kg) and Zn content in T. dicoccoides acc. 4641 (66.51 mg/kg), and Chhuneja et al. (Reference Chhuneja, Dhaliwal, Bains and Singh2006) reported the maximum Fe content in Ae. tauschii acc. 14 102 (109.40 mg/kg) and Zn content in Ae. tauschii acc. 14 102 (90.4 mg/kg) in whole grain. However, in the present investigation, Ae. longissima acc. 3507 was found to accumulate higher Fe (103.83 mg/kg) and Zn (111.85 mg/kg) in whole grain. T. dicoccoides acc. TA117 (159.10 mg/kg) and Ae. kotschyi acc. 3502 (131.10 mg/kg) were recorded with higher Fe and Zn, respectively, in bran and T. monococcum acc. W463 (14.48 mg/kg) and Ae. speltoides acc. 3804 (25.56 mg/kg) were recorded with higher Fe and Zn, respectively, in endosperm. These results indicate that T. monococcum acc. W463 and Ae. speltoides acc. 3804 may have an effective genetic system responsible for high Fe and Zn in endosperm. Mineral accumulation in whole grain and grain fractions is determined primarily by the amount absorbed through roots from soil during vegetative growth and later on by the proportion of that amount remobilized to grain fractions from vegetative tissues (e.g. stem and leaves) during the grain filling period. The ancestral wild wheat allele encodes a NAC transcription factor (NAM-B1) that accelerates senescence and increases nutrient remobilization from leaves to developing grains, whereas modern wheat varieties carry a non-functional NAM-B1 allele (Uauy et al., Reference Uauy, Distelfeld, Fahima, Blechl and Dubcovsky2006). Distelfeld et al. (Reference Distelfeld, Cakmak, Ozturk, Yazici, Budak, Saranga and Fahima2007) reported that the introgression of the high grain protein content (Gpc-B1) locus from wild tetraploid wheat (Triticum turgidum ssp. dicoccoides) to cultivated wheat (Triticum durum) resulted in higher concentrations of Fe, Zn and protein in its grain. Although previous workers have selected species/accessions of S, UtCt, UkSk, D, Sl and UgMg genomes for transfer of gene(s) into cultivated wheat for high Fe and Zn contents on the basis of information generated through whole grain analysis. However, during present investigation, the analysis of Fe and Zn based on whole grain and grain fractions (bran and endosperm) indicating that T. monococcum acc. W463 (AmAm), Ae. speltoides acc. 3804 (SS) and Ae. longissima acc. 3507 (SlSl) might be used in future to introgress gene(s) in hexaploid wheat for enhancement of both Fe and Zn particularly in endosperm fraction. In view of the above results, it may be concluded that an alien introgression for biofortification programme of wheat, the accessions of wild species for Fe and Zn should be selected on the basis of whole grain and grain fraction (bran and endosperm) analysis instead of only whole grain analysis in order to increase these micronutrients in edible portions.
The progenitor and non-progenitor species of wheat have the ability to uptake very high amount of Fe and Zn by the roots (Cakmak et al., Reference Cakmak, Torun, Millet, Feldman, Fashima, Korol, Nevo, Braun and Ozkan2004; Chhuneja et al., Reference Chhuneja, Dhaliwal, Bains and Singh2006; Pfeiffer and McClafferty, Reference Pfeiffer and McClafferty2007; Tiwari et al., Reference Tiwari, Rawat, Neelam, Randhwa, Singh, Chhuneja and Dhaliwal2008, Reference Tiwari, Rawat, Chhuneja, Neelam, Aggrawal, Randhwa, Dhaliwal and Singhm2009). The wheat–Ae. kotschyi substitution line with chromosome 7Uk also have the ability to uptake high amount of Fe and Zn compared with the commercial cultivars of hexaploid wheat (Priyanka et al., unpublished). We have observed a large variation between Fe and Zn contents in whole grain as well as grain fractions in different accessions of same species, which indicate genotype-specific variation as also observed in the case of rice (Sperotto, Reference Sperotto2013). It may be due to that the genotypes with different Fe and Zn use efficiencies can show different remobilization patterns (Impa et al., Reference Impa, Morete, Ismail, Schulin and Johnson-Beebout2013). The loading and remobilization mechanism of Fe and Zn from vegetative tissues to grain fractions is crucial to the enrichment of Fe and Zn in hexaploid wheat; however, the exact mechanism is still not very much clear. However, an inverse relationship between grain yield and grain micronutrient concentration has been demonstrated (McDonald et al., Reference McDonald, Genc and Graham2008; Cakmak et al., Reference Cakmak, Kalayci, Kaya, Torun, Aydin, Wang, Arisoy, Erdem, Yazici, Gokmen, Ozturk and Horst2010a, Reference Cakmak, Pfeiffer and McClaffertyb), and it is desirable to combine the ability to accumulate Zn into the grain with a high-yield potential. To obtain reliable information of level of genetic variation in grain nutrient concentration, McDonald et al. (Reference McDonald, Genc and Graham2008) proposed that any possible dilution and concentration effects associated with differences in yield potential need to be taken into account. Comparing the grain yield (expressed as 1000-grain weight values in Tables 1–3) with the micronutrient content, the parental wheat line UP 2338 and WL 711 showed higher yield than wheat–Ae. kotschyi substitution lines. Therefore, wheat–Ae. kotschyi substitution lines developed and identified in this study may not be very suitable for commercial exploitation due to undesirable linkage drag. However, for precise transfer of defined chromosomal regions containing gene(s) controlling high grain Fe and Zn contents is in progress using radiation hybrid approach and induction of homoeologous chromosome pairing.
On the basis of GISH results of the selected wheat–Ae. kotschyi substitution lines, one pair of chromosome of Uk or Sk genome of Ae. kotschyi was introgressed in wheat. In wheat–Ae. kotschyi substitution lines with chromosome 2Sk, 7Uk and Uk-W translocation, Fe and Zn contents in whole grain as well as grain fractions were considerably higher than the recipient hexaploid wheat cv. WL 711 and UP 2338 but less than the donor Ae. kotschyi. The wheat–Ae. kotschyi substitution line with chromosome 7Uk had higher Fe and Zn contents in whole grain and endosperm than that having 2Sk chromosome and Uk-W translocation. These results indicate that the gene(s) present on 7Uk chromosome of Ae. kotschyi are highly effective for uptake, translocation and mobilization of Fe and Zn contents in endosperm of grains. It was also reported earlier that substitution of wheat chromosomes with chromosome 2Sk and 7Uk of Ae. kotschyi improved Fe and Zn concentrations in wheat grains (Tiwari et al., Reference Tiwari, Rawat, Neelam, Kumar, Randhawa and Dhaliwal2010) and the major quantitative trait loci for grain Fe concentration are located on linkage group 2A, 6B and 7A (Tiwari et al., Reference Tiwari, Rawat, Chhuneja, Neelam, Aggrawal, Randhwa, Dhaliwal and Singhm2009). However, the overall concentration of Fe and Zn in the endosperm of wheat–Ae. kotschyi substitution lines was higher than both of the parental species (donor and recipient). In wheat–Ae. kotschyi substitution lines, the Fe and Zn concentrations in whole grain and grain fractions (bran and endosperm) indicating that homoeologous groups 2Sk and 7Uk chromosomes carry the gene(s) responsible for high Fe and Zn in whole grain and storage in endosperm fraction of the grain.
The present results were analysed as described in Taylor (Reference Taylor1990), according to which correlation coefficients (in absolute value) that are < 0.35, usually considered to be significantly low or weak correlations, 0.36 to 0.67 modest or moderate correlations and 0.68 to 1.0 strong or high correlations with r coefficients >0.90 very high correlations. The consistent positive correlation (r= 0.74) of Fe and Zn over a wide range in grain-Fe and grain-Zn concentrations suggests transport to and/or storage of these nutrients in the grain is linked (Fig. 1(a)). These results are in accordance with several earlier reports on positive correlations between grain Fe and Zn concentrations in wheat germplasm based on whole grain analysis (Cakmak et al., Reference Cakmak, Torun, Millet, Feldman, Fashima, Korol, Nevo, Braun and Ozkan2004), which may be due to common transport mechanisms or the genes for Fe and Zn are possibly co-segregating (Cakmak et al., Reference Cakmak, Kalayci, Kaya, Torun, Aydin, Wang, Arisoy, Erdem, Yazici, Gokmen, Ozturk and Horst2010a,b). A moderate correlation was observed for Zn in whole grain and endosperm (r= 0.46; Fig. 1(b)) and whole grain and bran (r= 0.54; Fig. 1(c)). Similarly, a moderate correlation (r= 0.44) was also observed for Fe and Zn in bran (Fig. 1(d)). This might be due to that the Fe and Zn concentrations in different fractions of grain are dependent on the source (i.e. soil and vegetative tissue) to sink (i.e. grain and grain fractions) relationship. A source limitation could either be due to a limited transport capacity or a very high Fe and Zn sequestration capacity in the vacuoles of vascular tissues. Generally, in cereal, grain nutrients are transported into the maternal seed coat region through the phloem and then translocated into the apoplastic region between the maternal seed coat and the aleurone and endosperm through different types of efflux and influx transporters such as zinc/iron-regulated protein (ZIP), natural resistance-associated macrophage proteins and Yellow Stripe Like (YSL) (Tauris et al., Reference Tauris, Borg, Gregersen and Holm2009). Several Fe and Zn loading barriers are present in seed coat. First, several types of transporters and chelating molecules such as YSL, ZIP, NA and 2′-deoxymugineic acid are localized in the outer tissue region of the resting seed (bran) and transport Fe and Zn into the inner endosperm only during germination, a process that clearly indicates low bioavailable Fe content in the endosperm (Walker and Waters, Reference Walker and Waters2011). Second, the presence of very low amounts of ferritin protein moieties in the endosperm tissue of the mature grain (Stein et al., Reference Stein, Ricachenevsky and Fett2009) is a major drawback to bioavailable Fe. The earlier reports indicate that Zn concentration in cereal grains is linked to levels of proteins while the Fe sequestration is determined mainly by phytate and to a lesser extent on the proteins, mainly within the vacuole of the seed coat, peripheral aleurone and embryo (Cakmak et al., Reference Cakmak, Kalayci, Kaya, Torun, Aydin, Wang, Arisoy, Erdem, Yazici, Gokmen, Ozturk and Horst2010a,b) and thus check mobilization into the endosperm (Kutman et al., Reference Kutman, Yildiz, Ozturk and Cakmak2010). Further, the identities of the proteins that bind Fe and Zn in the endosperm are still unknown (Xue et al., Reference Xue, Eagling, He, Zou, McGrath, Shewry and Zhao2014). Thus, it is important to not only consider the total content of Fe and Zn in grain but also the tissue localization and speciation (as chelates, protein particles or other) that affect their bioavailability.
Conclusion
The findings of the present investigation indicate the necessity of Fe and Zn analysis in whole grain and grain fractions before the selection of species/accession for successful Fe and Zn biofortification of wheat cultivars with high-yield potential. Screening of different accessions of T. monococcum, T. dicoccoides, Ae. tauschii, Ae. umbellulata, Ae. longissima, Ae. speltoides, Ae. kotschyi and Aegilops peregrina confirmed that Ae. longissima acc. 3507 for Fe and Zn in whole grain, T. dicoccoides acc. TA117 for Fe in bran, Ae. kotschyi acc. 3502 for Zn in bran, T. monococcum acc. W463 for Fe in endosperm and Ae. speltoides acc. 3804 for Zn in endosperm might be used as the most promising genetic resource for Fe and Zn biofortification in cultivated wheat. Wheat–Ae. kotschyi derivative line, i.e. BC2F4 63-2-13-1, with chromosome 7Uk showed a higher concentration of Fe and Zn in whole grain, endosperm and per seed, which indicates that the introgression of alien chromosome in hexaploid wheat clearly influenced the Fe and Zn concentrations in whole grain and grain fractions. The work on precise transfer of useful gene(s) from 7Uk to reduce linkage drag is in progress using wheat–Ae. kotschyi derivative line, i.e. BC2F4 63-2-13-1.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S147926211500012X
Acknowledgements
The authors thank the support of Department of Biotechnology (DBT), Government of India through a project, ‘Biofortification of wheat for micronutrient through conventional and molecular approaches’. One of the authors Priyanka Mathpal (SRF) gratefully acknowledges the University Grant Commission (UGC) India.








