Introduction
Acorus calamus L., sweet flag, has had a long and rich history of ethnobotanical application by many different cultures in many countries (Motley, Reference Motley1994). The plant has good organoleptic properties and is used as a flavouring ingredient in alcoholic beverages. Oleum calami distilled from the rhizomes is used in medicine and in perfumery. In Lithuania, rhizomes of sweet flag (Rhizome Calami) are used for treatment of diarrhoea, dyspepsia, neuralgia and hair loss. Leaves (Herba Calami) are used in baths to relieve arthritis, gout and rheumatism. Crushed leaves exude a distinct tangerine odour and are used in dried foods to prevent infestation by weevils. Moreover, fresh leaves of sweet flag are used in bread making.
Many investigations into sweet flag rhizome essential oil constituents were made in the late 1970s and 1980s, when commercial interest in this species was high. The detection of the mutagenic and carcinogenic effects of β-asarone and other phenylpropene derivatives made the use of the plant less desirable (Goeggelmann and Schimmer, Reference Goeggelmann and Schimmer1983) and limits the use of A. calamus for human purposes. β-Asarone is considered a most important constituent in the rhizome essential oil of A. calamus. Quantitative differences in β-asarone content of the essential oil have been considered to have taxonomic importance within the genus. For example, a higher presence of β-asarone is suggestive of polyploidy in A. calamus (Röst, Reference Röst1979; Keller and Stahl, Reference Keller and Stahl1982; Lander and Schreier, Reference Lander and Schreier1990); β-asarone was not detectable in the North American spontaneous diploid A. calamus var. americanus (Raf.) Wulff (Röst and Bos, Reference Röst and Bos1979; Keller and Stahl, Reference Keller and Stahl1983; Mazza, Reference Mazza1985); and the sterile triploid variety A. calamus var. calamus L. (distributed throughout Europe and temperate Himalayan regions; Röst, Reference Röst1979) accumulates relatively low amounts of β-asarone (3–20%) in its rhizomes and considerable amounts in its leaves (25–45.5%) (Röst, Reference Röst1979; Evstatieva et al., Reference Evstatieva, Todorova, Ognyanov and Kuleva1996; Rode et al., Reference Rode, Mastnak-Culk, Wagner and Pank1996; Venskutonis and Dagilyte, Reference Venskutonis and Dagilyte2003). In the Indian tetraploid A. calamus var. angustatus Bess. β-asarone content varied from 83 to 96% and from 40 to 86% of the total essential oil in rhizomes and leaves, respectively (Röst, Reference Röst1979; Raina et al., Reference Raina, Srivastava and Syamasunder2003).
Raw material containing high percentages of β-asarone has been used as an insecticide and insect repellent for stored-product pest control (Paneru et al., Reference Paneru, Le Patourel and Kennedy1997; Umoetok, Reference Umoetok2000; Kim et al., Reference Kim, Roh, Kim, Lee and Ahn2001; Jiyavorranant et al., Reference Jiyavorranant, Chanbang, Supyen, Sonthichai and Jatisatienr2003). Its antifungal and bactericidal properties suggested further uses of the plant as a natural fungicide (Schmidt and Streloke, Reference Schmidt and Streloke1994; Petrov, Reference Petrov1998; Graham et al., Reference Graham, Quinn, Fabricant and Franswort2000; Mungkornasawakul et al., Reference Mungkornasawakul, Supyen, Jatisatienr and Jatisatienr2002; Park et al., Reference Park, Kim and Ahn2003). Moreover, recent investigations demonstrated anti-proliferative, immuno-suppressive and anti-carcinogenic activity of A. calamus on human carcinoma cells (Vlietinck et al., Reference Vlietinck, De Beuyne, Apers and Pieters1998; Mehotra et al., Reference Mehrotra, Mishra, Maurya, Srimal, Yadav, Pandey and Singh2003).
Most of the information concerning the chemistry and bioactivity of A. calamus essential oils relates to the rhizome, and there is little literature concerning the leaf. The use of leaves is more acceptable from the point of view of conservation of plant resources, as in many European countries, populations of A. calamus have been lost or are currently endangered due to habitat loss (Evstatieva et al., Reference Evstatieva, Todorova, Ognyanov and Kuleva1996; Rode et al., Reference Rode, Mastnak-Culk, Wagner and Pank1996; Kozyuk, Reference Kozyuk1997; Lange, Reference Lange1998).
The current work describes the qualitative and quantitative composition of essential oils of A. calamus leaves taken from various plant populations, and an evaluation of the anti-microbial activity of these oils.
Material and methods
Plant material
Natural populations of A. calamus growing in semi-aquatic habitats (marshy meadows near farms, edges of ponds, rivers and lakes) were sampled (Table 1). Whole leaves were gathered during the flowering period (July 2003) from 19 stands and dried at 24°C. Sampling of leaves was randomized. Voucher specimens of each accession were deposited in the herbarium at the Institute of Botany/BILAS, Vilnius, Lithuania.
Table 1 Growing localities and habitats of Acorus calamus populations

Chromatographic analysis
The essential oils were obtained from 20 g of air-dried leaves by hydro-distillation for 1.5 h, using a Clevenger-type apparatus. The samples were analysed by gas chromatography (GC) and gas chromatography–mass spectrometry (GC-MS). Essential oils for analysis were dissolved in hexane (1:15). Analysis by GC-MS was performed using an HP 5890(II) chromatograph interfaced to an HP 5971 mass spectrometer (ionization voltage 70 eV) and equipped with a non-polar capillary column CP-Sil 8 CB (50 m × 0.32 mm i.d., 0.25 μm). The oven temperature was kept at 60°C for 2 min, and then programmed at a rate of 5°C/min up to 160°C, kept for 1 min, followed by an increase to 280°C at a rate of 10°C/min, and held isothermally for 3 min at 250°C. The injector and detector temperature was 250°C. The carrier gas was helium, with a flow rate of 1.0 ml/min. GC analysis was performed using an HP 5890(II) chromatograph equipped with a flame ionization detector (FID) and capillary column HP-FFAP (30 m × 0.25 mm i.d., film thickness 0.25 μm). The GC oven temperature was set at 60°C for 3 min and then programmed to rise from 60 to 240°C at a rate of 4°C/min, held isothermally for 5 min at 240°C. The carrier gas was 0.7 ml/min helium. The injector and detector temperatures were maintained at 200°C and 250°C, respectively.
The relative proportions of the oil constituents were expressed as percentages obtained by GC peak area normalization without applying any correction factors. Qualitative analysis was based on a comparison of relative retention indices (RI) on both columns and mass spectra with corresponding data in Wiley and NBS 54K libraries. RI on the HP-FFAP polar column were calculated relative to n-alkanes (C10–18) and indices on the non-polar column were used to generate Kovats indices. RI means are equivalent to the Kovats indices presented by Adams (Reference Adams2000). The identification of constituents was based on mass spectra and RI values. Two unknown compounds (peaks 54 and 56) with a molecular mass of 222 possessed very similar ionic fragmentation at m/z 81(100), 41(60), 55(57), 69(55), 93(55), 121(47), 136(20), 161(20) (RI = 1515) and 81(100), 93(75), 41(68), 67(65), 55(62), 109(65), 123(48), 136(28), 161(28) (RI = 1530). Peak 74 (RI = 1682) is an unknown compound with molecular mass 218 and ionic fragments at m/z peaks 218(100), 105(64), 91(51), 133(43), 161(39), 119(36), 83(40), 175(27), 203(21), which could be tentatively assigned to a cyclocalorenone isomer, but the RI was not found in the available literature (Adams, Reference Adams2001). A fourth unknown compound (peak 77), eluting from the CP-Sil 8 CB column with RI = 1700, was of molecular mass 222 and produced fragment ions at m/z 84(100), 81(61), 109(50), 83(46), 121(39), 55(43), 41(43), 161(32), 222(11), 67 (38).
Antimicrobial assay
The anti-microbial activity of four essential oils was assessed by determining minimum inhibitory concentrations (MIC), using the broth dilution method. The growth medium for fungi and yeasts was malt extract without agar, and for bacteria, nutrient broth (NB, Oxoid). The MIC test was carried out according to Eloff (Reference Eloff1998), using a tissue culture test plate (96 wells). The oils were diluted in dimethyl sulphoxide at eight concentrations (20.0, 10.0, 5.0, 2.5, 1.25, 0.625, 0.313 and 0.156 μg/l). Essential oil of appropriate concentration was added to autoclaved growth medium, which was then inoculated with spores, conidia or cells. Microorganism growth in medium alone, and with the addition of 0.01 ml/ml medium dimethyl sulphoxide represented control treatments. Oil samples were tested in triplicate.
Essential oil activity was evaluated against following microorganism strains: Aspergillus niger Tiegh (DPk0464 and DPk0152), A. flavus Link (DP0314 and DP0407), A. fumigatus Fresen. (DP0124 and DP0431), Aureobasidium pullulans var. pullulans (de Bary) G. Arnaud (DP0308 and DP0042), Fusarium avenaceum (Fr.) Sacc. (DP0050, DP0421 and DP0204), Paecilomyces variotii Bainier (DP0215), Penicillium brevicompactum Dierckx (DP0305, DP0308 and DP0407), P. expansum Link (DP0145 and DP0333), P. verrucosum Dierckx (DP0414 and DP0028), Rhizomucor pusillus (Lindt) Schipper (DP0058 and DP0060), Rhizopus stolonifer (Ehrenb.) Vuill. var stolonifer (D0210 and DP0211), Candida glabrata (H. W. Anderson) S. A. Mey & Yarrow (DPm0204 and DPm0288), Candida albicans (C. P. Robin) Berkhout (DPm0143 and DPm0314) and Rhodotorula rubra (Schimon) F. C. Harrison (DPm0454, DPm0423 and DPm0403), Bacillus subtilis (DPb0116 and DPb0409), Mycobacterium ssp. (DPb0017 and DPb0023) and Pseudomonas aeruginosa (DPb0405, DPb0408 and DPb0111). All strains were isolated from food or an indoor environment and stored in the culture collection of the Institute of Botany (Lithuania). Spore or cell suspensions were prepared in sterile saline (0.85% w/v sodium chloride solution and adjusted to a concentration of 106 spores/ml). The inoculum was added to all wells and the plates were incubated at 32°C for 24 h (bacteria), at 30°C for 48 h (yeast) and at 25°C for 7 days (fungi). Chloramphenicol, amphotericin B and nistatin (Merck) were used as positive growth inhibition control for bacteria, yeast and fungi, respectively. The MIC was defined as the lowest concentration of essential oil that inhibited visible microorganism growth or any other visible changes, such as staining of the growth medium, which indicates a fungal reaction against the inhibitory compounds.
Statistical analysis
The percentage composition of essential oils was used to determine the relationship among different accessions using a hierarchical cluster analysis (SPSS 9.0 software computer package). Only those volatile constituents with percentage content equal or higher than 1% were included in the cluster analysis. The cluster analysis was constructed on the basis of agglomerative grouping and an average linkage between the groups, employing a method based on squared Euclidean distances (Norusis, Reference Norusis1989).
Results
Essential oil composition
The yield of essential oil isolated from dried leaves was between 0.21 and 0.64% (v/w), with a mean of 0.36%. In the 19 oil samples, 84 distinct constituents were identified, accounting for 86.3–98.8% of the total essential oil content (see Table 2 where the components are listed in order of their RI).
Table 2 The ranges of percentage composition of leaf essential oils of Acorus calamus growing in wild populations in Lithuania
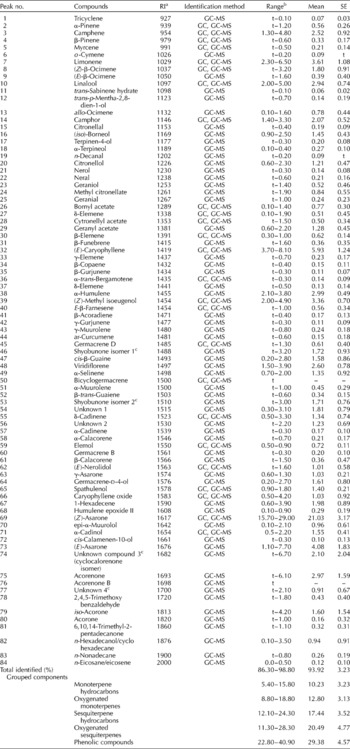
a Relative retention indices on the unpolar CP-Sil8CB column.
b t = trace ( < 0.05%).
c Tentative identification.
Unknown compound 1: M+–207, m/z 81(100), 41(60), 55(57), 69(55), 93(55), 121(47), 136(20), 161(20).
Unknown compound 2: M+–207, m/z 81(100), 93(75), 41(68), 67(65), 55(62), 109(65), 123(48), 136(28), 161(28).
Unknown compound 3: M+–218, m/z 218(100), 105(67), 91(65), 41(50), 133(50), 161(43), 147(39), 119(39), 77(32), 67(30), 175(29), 203(21).
Unknown compound 4: M+–222, m/z 84(100), 81(61), 109(50), 83(46), 121(39).
Phenolic compounds predominated among the essential oils, and contributed from 22.8 to 40.9% of the oil content. The most abundant (15.7–29.0%) single compound was (Z)-asarone (synonym β-asarone). Other isomers of this compound were also represented, such as (E)-asarone (1.1–7.7%) and γ-asarone (0.6–1.3%). (Z)-Methyl isoeugenol (2.0–4.9%) was a major constituent of the leaf oil fraction. The oxygenated compounds were represented by sesquiterpenes (11.3–28.3%), and to a lesser extent (8.8–18.8%) by oxygenated monoterpenes. The major sesquiterpenes were two isomers of shyobunone, whose content varied markedly (trace to 6.6%). Two further peaks (numbers 75 and 76) were identified as acorenones; these were absent from two of the oils, but constituted up to 6.1% of others. The sesquiterpene α-cadinol accounted for between 0.5 and 2.2% of the oil content. Three unidentified compounds (numbers 54, 56 and 77) had mass spectra characteristic of oxygenated sesquiterpenes, and were present in variable concentrations. The major monoterpenes identified were linalool (2.0–5.0%), camphor (1.4–3.3%), citronellol (0.6–2.3%) and isoborneol (0.9–2.5%).
Monoterpene and sesquiterpene hydrocarbons accounted for 22.4–37.4% of the total essential oil content. The most abundant monoterpene constituents were camphene (1.3–4.8%), limonene (2.3–6.5%), and (Z)- and (E)-β-ocimene (1.0–3.8%). The sesquiterpene hydrocarbons were represented by (E)-caryophyllene (3.7–8.1%), α-humulene (2.1–4.2%) and germacrene D (trace to 1.3%). The content of the aroma compound cis-β-farnesene ranged from trace to 1.5%. Asaronaldehyde (2,4,5-trimethoxybenzaldehyde), identified by Mazza (Reference Mazza1985) as the major oxidation product of asarone, was present in minor quantities (trace to 1.8%). Caryophyllene oxide (0.5–4.2%) and humulene epoxide II (0.1–0.9%) were also detected.
A hierarchical cluster analysis, based on the major constituents, generated four major clusters (Fig. 1). All oils shared (Z)-asarone as a main constituent. The first cluster, taking in nine oils, has high concentrations of (E)-caryophyllene (4.1–9.8%), (Z)-metyl isoeugenol (3.0–4.9%), limonene (3.3–4.6%) and α-humulene (2.4–4.2%). The second cluster (eight oils) contained (E)-caryophyllene (4.5–6.9%) and (Z)-metyl isoeugenol (2.9–3.2%) and was recognizably distinct with respect to content of (Z)-asarone (17.6–29.0%) and the acorenones (0–6.1%). Two oils formed separate clusters. The former (oil no. 12) contained high concentrations of (E)-asarone (7.2%), caryophyllene oxide (4.2%), acorenone (4.2%) and acorone (4.2%). The latter (oil no. 14) was distinguished by its low (Z)-asarone (15.7%) content, whereas other principal components such as limonene (6.5%), linalool (5.0%) and camphene (4.8%) were present at higher concentrations.

Fig. 1 Dendrogram obtained by hierarchical cluster analysis of the percentage composition of essential oils from leaves of Acorus calamus; clustering method based on squared Euclidean distances.
Antimicrobial activity
The antimicrobial activity of the essential oils in the leaf of A. calamus is reported in Table 3. The inhibitory concentrations against bacteria were between 0.156 and 0.625 μg/ml, against yeasts, between 0.313 and 1.250 μg/ml, and against fungi, between 2.500 and 20.000 μg/ml. The individual oils varied in their antimicrobial activity. Oil no. 12 had the lowest MIC values (0.156–0.313 μg/ml) for bacteria, followed by oil no. 13 and no. 1. For comparison, the average MIC value chloramphenicol was 0.14 μg/ml. Among the microorganisms tested, Mycobacterium ssp. and B. subtilis (MIC values of 0.313 and 0.156–0.313 μg/ml, respectively) were the most susceptible to A. calamus essential oils. Antibiotic activity against the fungi tested was low. Aspergillus fumigatus, A. flavus and A. niger were most resistant, while Fusarium avenacium and Rhizomucor pusillus were more susceptible to A. calamus leaf essential oils.
Table 3 Minimal inhibitory concentrations (μg/ml) of leaf essential oils of Acorus calamus

The numbers of oils corresponded.
Discussion
Comparisons between the oil compositions measured in the present study and those presented in the literature is difficult, as the leaf oil of A. calamus has not attracted much scientific attention. Some of the compounds we have detected have not been reported elsewhere (Evstatieva et al., Reference Evstatieva, Todorova, Ognyanov and Kuleva1996; Venskutonis and Dagilyte, Reference Venskutonis and Dagilyte2003), and the content of (Z)-asarone, acorenone, (Z)-methyl isoeugenol and shyobunones are not comparable. Some of these differences may arise from inconsistenncy in the plant material analysed, because the content of (Z)-asarone and some other compounds tends to increase from the base to the tip of leaves (Röst and Bos, Reference Röst and Bos1979). Populations accumulating relatively low quantities of the toxic (Z)-asarone may be suitable as a source in pharmaceuticals. Iguchi et al. (Reference Iguchi, Nishiyama, Koyama, Yamamura and Hirata1968) and Yamamura et al. (Reference Yamamura, Iguchi, Nishiyama, Niwa and Koyama1971) isolated shyobunones from rhizome oils and noted that shyobunone-like sesquiterpenoids were responsible for the characteristic odour of the plant, whereas Choi (Reference Choi2004) identified cis-β-farnesene as the key aroma compound. These odour compounds were present in low, although variable concentrations in leaves which had less smell than the rhizomes.
The antimicrobial activity of this plant essential oil underlines the value of natural resources. The MIC values of the essential oils were lower than those of the standard antibiotics chloramphenicol, amphotericin B and nistatin, possibly because of their poor solubility in the liquid media, which enables the antibiotic to more easily interact with its molecular target. On the other hand, since essential oils comprise a mixture of different compounds, it may be possible to exploit the existence of synergistic effects. The differences in antimicrobial activity of the oils is tested on microorganisms. So why now say that there are differences? Some of these differences analysed may be due to the variation in relative content of the various components. Essential oil no. 12, which displayed good antimicrobial activity, was distinguished from the others by its high relative contents of (E)-asarone, acorernone and acorone, although oils with a high content of (Z)-asarone were not associated with high antimicrobial activity.
Further investigations of antimicrobial activity are planned using a wider selection of oil samples (such as rhizome oils), and a wider range of microorganisms, especially clinical isolates of fungi and dermatophytes, in order to determine which component(s) have the most potent antimicrobial activity and to establish any synergistic effects between components.
Acknowledgements
The authors thank the Lithuanian State Science and Studies Foundation for financial support in the form of a research grant (T-89/05).






