Introduction
Salinity afflicts crop production on more than 800 million hectares worldwide, either because of salinity which affects 397 million hectares or due to the associated sodicity prevailing on 434 million hectares. This forms nearly 7% of the world's land (Munns, Reference Munns, Ashraf, Ozturk and Athar2009) with estimated annual losses currently being over USD 12 billion due to salinity (Shabala and Bose, Reference Shabala, Bose and Volkov2012). The crop plants are more prone to salinity as it decreases yield of agricultural crops since most plants are salt-sensitive glycophytes. Effects of salinity on plants comprise of: (1) osmotic stress, (2) disruption of membrane ion transport, (3) direct toxicity of cytoplasmic sodium and chloride at high concentrations, and (4) induced oxidative stress. This ultimately leads to low vigour, poor seed germination, lower water uptake capacity that affects plant growth, with various biochemical, physiological and morphological processes being affected.
Under salinity stress we are still lagging behind in terms of genetic improvement in wheat grain yield mainly due to the limited genetic diversity available in wheat for salinity tolerance (Wyn Jones and Gorham, Reference Wyn Jones, Gorham, Acevedo, Fereres, Giménez and Srivastava1991; Dreccer et al., Reference Dreccer, Ogbonnaya and Borgognone2004). Likewise, slight consideration is given to the salinity tolerance per se in wheat improvement programmes (Rauf et al., Reference Rauf, Adil, Naveed and Munir2010). Although greater variation for salinity is present in various Triticeae gene pools (Trethowan and Mujeeb-Kazi, Reference Trethowan and Mujeeb-Kazi2008; Mujeeb-Kazi et al., Reference Mujeeb-Kazi, Kazi, Dundas, Rasheed, Ogbonnaya, Kishi, Bonnett, Wang, Xu, Chen, Mahmood, Bux and Farrukh2013), however preference is given to Aegilops tauschii due to its close resemblance with the wheat D-genome and wide genetic proximity (Sohail et al., Reference Sohail, Shehzad and Kilian2012; Ogbonnaya et al., Reference Ogbonnaya, Abdalla, Mujeeb-Kazi, Kazi, Steven, Gosman, Lagudah, Bonnett, Sorells and Tsujimoto2013). Synthetic hexaploid wheats (SHWs), derived from crossing durum cultivars with Ae. tauschii accessions have been reported to reveal great variation for many important agronomic traits particularly abiotic stresses such as salinity, drought and heat tolerance. There is a wide array of SHWs produced globally (Ogbonnaya et al., Reference Ogbonnaya, Abdalla, Mujeeb-Kazi, Kazi, Steven, Gosman, Lagudah, Bonnett, Sorells and Tsujimoto2013); however, very limited numbers have been evaluated for salinity tolerance (Dreccer et al., Reference Dreccer, Borgognone, Ogbonnaya, Trethowan and Winter2007).
Fast and effective screening methods that can find genetic variation for salinity tolerance in wide array of genetic resources are very important (Munns and James, Reference Munns and James2003). It is quite difficult to screen such a large population of genotypes for salinity tolerance because of soil's spatial heterogeneity, physical/chemical properties plus seasonal rainfall irregularities and fluctuations. It was reported after the field study in Syria by utilizing ICARDA's progressive durum wheat breeding lines that tolerance against salinity might be possible due to presence of genetic variation, but the baffling occurrence of stress against drought made it inflexible to recognize genotypes with salinity tolerance (Srivastava and Jana, Reference Srivastava, Jana, Staples and Toenniessen1984). Germination speed, emergence and subsequent seedling vigour are very significant factors for good crop establishment and ensure high productivity. Salinity stress reduces the germination percentage, rate of germination and seedling vigour in crops (Yildirim et al., Reference Yildirim, Dursun, Guvenc and Kumlay2002). Seeds may be extra delicate to stress than developed plants, because of exposure to the active environment near the soil surface (Dodd and Donovan, Reference Dodd and Donovan1999).
The differences in genotypic response in controlled salinity stress conditions can be assessed through germination percentage (G%) and seedling growth under saline conditions. This type of information is vital for proposing appropriate germplasm resources for saline soils. Therefore, this study was conducted to determine the performance of 226 SHWs under saline conditions by measuring germination indices as a proof-of-concept of a critical preliminary screening method to identify promising genetic resources and reliable traits for implementation of future genetic dissection studies.
Material and methods
Plant material
An association panel comprising of 226 SHWs was phenotyped for the current experiment (online Supplementary Table S1). These SHWs were derived from combinations of 196 Ae. tauschii accessions and 44 durum wheat cultivars that had been previously characterized for grain morphology (Rasheed et al., Reference Rasheed, Xia, Ogbonnaya, Mahmood, Zhang, Mujeeb-Kazi and He2014), biotic stress resistances (Joukhadar et al., Reference Joukhadar, El-Bouhssini, Jighly and Ogbonnaya2013; Mulki et al., Reference Mulki, Jighly, Ye, Emebiri, Moody, Ansari and Ogbonnaya2013) and grain quality (Emebiri et al., Reference Emebiri, Oliver, Mrva and Mares2010). The salt tolerant variety ‘S-24’ (Ashraf, Reference Ashraf2010) and the salt-sensitive cultivar ‘PBW-343’ were used as standard checks in the germination test. The 1% sodium hypochlorite solution was used to sterilize the seeds for 3 min before the germination study. Seeds were rinsed with sterilized water and were air-dried.
Salt solution and germination test
Two different concentrations of NaCl solution (100 and 200 mM) were made by dissolving analytical grade NaCl (Merck, USA) in distilled water. A distilled water (0 mM) treatment was used for comparison as a control. Saline conditions were used by using aqueous NaCl solutions.
Five seeds per entry were surface sterilized and sown following the method described by Tlig et al. (Reference Tlig, Gorai and Neffati2008). Sterilization was done in 1% sodium hypochlorite solution for 1 min; thoroughly washed 4–5 times with distilled water and air dried before starting the germination experiment. All 226 D-genome SHWs were tested at three treatments: control (distilled water), 100 and 200 mM salt (NaCl) solutions along with the tolerant (S-24) and the susceptible (PBW-343) checks. All genotype seeds were sown in filter paper lined petri plates and allowed to germinate in the dark for 48 h at 25°C. They were then transferred to a growth chamber running at 27 ± 2°C and 10 h photoperiod with 80–85 µM/S/m2 light intensity was maintained for further growth and observations. The experimental design used was a factorial combination of treatments and genotypes organized in a totally randomized design with three replications. A seed was considered germinated when the plumule was longer than half of the length of the seed, and the radicle was equivalent to or longer than the seed length.
Traits evaluated for seeding emergence
Data were recorded on G%, germination index (GI), seedling height (SHt) and seedling weight (SWt). Seed germination was regularly noted daily according to AOSA (1990) till a continuous count was attained. Seed was considered to be germinated if plumule length exceeds half of the seed size and when radicle length surpassed 2 mm. G% was recorded for each genotype after every 24 h for 7 d by the formula adopted by Rauf (Reference Rauf2005).
 $$\eqalign{& \hbox{Germination percentage (G\%)} \cr & \quad = \displaystyle{{\hbox{No. of seeds germinated}} \over {\hbox{Total no. of sown seed}}} \times \; 100.} $$
$$\eqalign{& \hbox{Germination percentage (G\%)} \cr & \quad = \displaystyle{{\hbox{No. of seeds germinated}} \over {\hbox{Total no. of sown seed}}} \times \; 100.} $$
GI was recorded by counting the seeds germinated daily until they reached a constant. Number of seeds germinated divided by days from first seed germinated.
GI was calculated as described (Baalbaki et al., Reference Baalbaki, Elias, Marcos-Filho and McDonald2009):
 $$\eqalign{\hbox{GI} & = \displaystyle{{\hbox{No. of emerged seeds}} \over {\hbox{Days of first count}}} + \cdots \cr & \quad + \displaystyle{{\hbox{No. of emerged seeds}} \over {\hbox{Days of final count}}}.} $$
$$\eqalign{\hbox{GI} & = \displaystyle{{\hbox{No. of emerged seeds}} \over {\hbox{Days of first count}}} + \cdots \cr & \quad + \displaystyle{{\hbox{No. of emerged seeds}} \over {\hbox{Days of final count}}}.} $$
Percentage change over control was computed as under:
SHt was measured by a ruler in centimetres. The height was taken from the portion of the plant above roots to the seedling tip (Fokar et al., Reference Fokar, Nguyen and Blum1998). Three randomly selected seedling's length was recorded from each replication. Fresh SWt was also recorded by using a precision weighing balance in milligrams (Fokar et al., Reference Fokar, Nguyen and Blum1998).
At germination, salt tolerance trait index (STTI) was calculated according to the formula of Ali et al. (Reference Ali, Salam, Azhar and Khan2007):
Salt tolerance index (STI) was measured as a mean of STTIs. STIs were used to group the 226 SHW genotypes into four salt-tolerant groups. Percentage survival rate was calculated as ratio of survived and germinated genotypes under salt stress to total number of genotypes tested.
Statistical analysis
Descriptive statistics was calculated on data using MS excel and the analysis of variance (ANOVA) of the data was done using STATISTICA 7. Co-efficient of correlation between all parameters was estimated using R-package.
Results
The results revealed significant differences amongst genotypes, treatments and their interaction for all four traits (online Supplementary Table S2). Although salinity affected all traits, results were highly significant for SHt and SWt, as compared with G% and GI (Table 1, online Supplementary Table S2). Similarly, all the traits decreased significantly by increasing the salt concentration from 100 to 200 mM (Table 1). All genotypes showed 100% germination at 0 mM NaCl, except few which showed >90% germination. Therefore, this treatment was excluded for G% for further analysis. On an average, G% decreased 14.5 and 25.3% at 100 and 200 mM, while GI decreased 11 and 24% at 100 and 200 mM, respectively. SHt decreased 57.1 and 90.3% and SWt decreased 54.2 and 85.8% at 100 and 200 mM, respectively.
Table 1. Descriptive statistics for germination, GI, seedling height and weight, and their STTI at various salt stress conditions
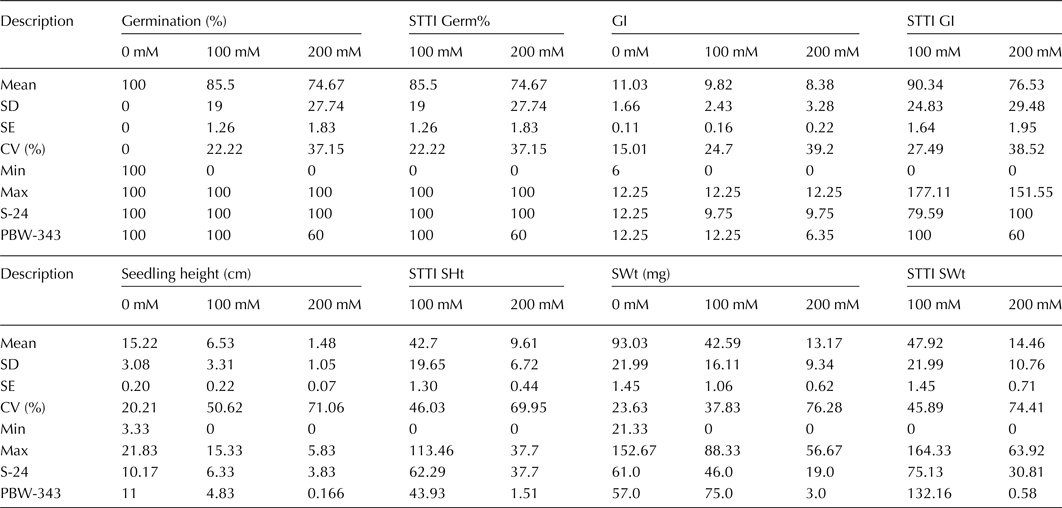
STTI, salt tolerance trait index; GI, germination index; SHt, seedling height; SWt, seedling weight.
In our experiment, we used two check cultivars, which showed expected results. In tolerant standard (S-24), G% was not affected at both 100 and 200 mM, while GI decreased 20.4% at both 100 and 200 mM, SHt decreased 37.7 and 62.3%, and SWt decreased 24.5 and 68.8% at 100 and 200 mM, respectively. S-24 is derived from the reciprocal crossing of two high-salt-tolerant genotypes ‘LU26 S’ and Indian spring wheat landrace ‘KHARCHIA’ (Ashraf, Reference Ashraf2010). S-24 is known to maintain high K+/Na+ ratio in the plant tissue and possesses good characteristics of agronomic importance, including 1000-kernel weight (TKW) of 42 g and grain yield. Therefore, this control was a stringent challenge to the accessions evaluated in our panel and ranked third based on STI.
STTI calculated for all traits provided an estimate for salinity effect on each trait and correlated well with the relevant traits (Fig. 1). STTI of each trait was averaged together to calculate STI. In correlation analysis, the four traits studied were positively and significantly (P ≤ 0.01) correlated with each other (online Supplementary Table S3). Strongest correlation was between G% and GI and (r = 0.846) and between SHt and SWt (r = 0.86). STI has significant positive correlation with SHt (r = 0.75), SWt (r = 0.69), G% (r = 0.79) and GI (r = 0.77). Additionally, the data indicated that the variation is continuous for SHt and SWt as compared with G% and GI (Fig. 1), indicating SHt and SWt are more reliable traits for genetic analysis.

Fig. 1. Frequency distributions, co-efficient of correlations and relationship between all traits and their STTI. The values are based on data averaged over all treatments (see footnote of Table 1 for trait abbreviations).
Discussion
Although, salinity suppressed germination, but SHt and SWt were more prone to salinity stress as compared with germination indices. Therefore, the selection criteria based on germination indices should be complemented with other quantitative traits including SHt and SWt for reliable selection of tolerant accessions. Munns and James (Reference Munns and James2003) also concluded that seedling-based screening techniques are effective and could identify genetic variations for salinity tolerance in a wide array of genetic resources. The genotypic difference for biomass production or leaf elongation (SHt in our case), was due to decrease in growth rate imposed by the osmotic effect of salt. These traits correlated well with Na+ exclusion (Munns and James, Reference Munns and James2003).
For an accurate screening of large germplasm collections, very fast and specific techniques will be needed. Several immediate experiments and their failure are debatable for salt stress screening, because the preliminary reaction to salt stress is considered as an osmotic effect of the salt, recognizing that the salt is externally present outside the root (Munns, Reference Munns1993). Germination is an appropriate trial for big populations of genotypes as described previously, but slight or no association was seen between genotypic alterations in germination and later development in saline conditions for species as diverse as barley (Norlyn and Epstein, Reference Norlyn, Epstein and Pietro1982), bread wheat (Ashraf and McNeilly, Reference Ashraf and McNeilly1988) and durum wheat (Almansouri et al., Reference Almansouri, Kinet and Lutts2001). Many species such as barley and wheat have the potential to germinate at highly saline conditions (over 300 mM NaCl), but the radicle emerging from seed could not develop further at that high salinity level. The difference present among species to germinate under saline conditions can be well elucidated by physicochemical nature of the swelling phenomenon in germination. For example, glycophytes are no more salt tolerant than halophytes at germination, even though haplophytes rapidly express their higher tolerance in the initial stage of hypocotyl enlargement (Malcolm et al., Reference Malcolm, Lindley, O'Leary, Runciman and Barrett-Lennard2003). The salt-specific effect takes time to develop. Therefore, we additionally used SHt and SWt parameters and combined all these parameters in STI to use this index as a decisive selection tool. SHt and SWt have more significance because the genotypes, which do not exclude salt effectively from the transpiration stream, salt build up reaches to a toxic level in the leaves that have been transpiring the longest (Munns, Reference Munns1993). The speed at which they die is relative to the speed at which new leaves are produced and is critical. This is probably because the cell driven expansion processes during germination and during subsequent growing are completely different. Uptake of water and elongation that permits imbibition and development of the radicle is a physicochemical procedure, in comparison with the molecular and biochemical processes that motivate successive cell division and extension. External to the roots the osmotic stress of the salt reduces the development rate of new leaves and rate of production of tillers (Munns, Reference Munns2002). Consequently, the salt's effect can be calculated in weight and height of plant. The development of a leaf usually responds in linear proportion to the osmotic strength of the soil solution (Rawson et al., Reference Rawson, Richards and Munns1988), with few species being more sensitive than the rest (Cramer, Reference Cramer2003).
Top 20 tolerant genotypes along with the tolerant check S-24 with their STTI of all four studied parameters were ranked according to best STI (online Supplementary Table S3), which could be promising resources for use in breeding. SHWs were grouped into four categories. The tolerant (21) SHWs are ample for use in breeding, irrespective whether they are superior to the check cultivars. Their uniqueness is in the fact that diverse Ae. tauschii accessions are in their combinations. The relationship between both stress treatments (100 and 200 mM) was stronger for SHt (R 2 = 0.38) and SWt (R 2 = 0.31), as compared with G% (R 2 = 0.12) and GI (R 2 = 0.25) (online Supplementary Fig. S1). This again implies that germination indices solely are insufficient to categorize genetic variations for salinity tolerance.
SHWs and their advanced derivatives have been well assessed for drought (Lopes and Reynolds, Reference Lopes and Reynolds2010; Tang et al., Reference Tang, Yang, Wu, Li, Li, Zou, Chen and Mares2010; Ali et al., Reference Ali, Arshad, Naqvi, Ahmad, Sher, Fatima, Kazi, Rasheed and Mujeeb-Kazi2014; McIntyre et al., Reference McIntyre, Rattey, Kilian, Dreccer and Shorter2014) and heat stress (Gororo et al., Reference Gororo, Eagles, Eastwood, Nicolas and Flood2002; Trethowan et al., Reference Trethowan, Reynolds, Sayre and Ortiz-Monasterio2005), however, less information is available for their performance under salt stress. Although it is well known that modern durum wheat cultivars have less tolerance to salinity as compared with bread wheat (Munns et al., Reference Munns, Richard and Lauchli2006), therefore it is most likely that tolerance in these SHWs is contributed by the Ae. tauschii parent and not the durum parent. This has been partially confirmed from the pedigree analysis of current SHWs that accessions with same durum parents were observed in tolerant and susceptible groups indicating the sole contribution of Ae. tauschii for salt tolerance (online Supplementary Table S2). For example, among the 24 SHWs derived from durum cultivar Croc_1 three were susceptible, one tolerant, ten moderately susceptible and ten moderately tolerant (online Supplementary Table S4). Such trends can be observed for other SHWs derived from same durum parents, i.e. Decoy-1 and Altar-84. Trethowan and Mujeeb-Kazi (Reference Trethowan and Mujeeb-Kazi2008) had also observed such tolerance contributions of the Ae. tauschii accessions. Previously, we observed similar trend for tolerance to boron toxicity in 45 SHWs derived from Decoy-1 (Ilyas et al., Reference Ilyas, Mahmood, Ali, Babar, Rasheed and Mujeeb-Kazi2015), and we could only identify one SHW {Decoy_1/Ae. squarrosa (466)} tolerant to both salinity and boron toxicity. Similarly, Yang et al. (Reference Yang, Zhao, Zhang, Yang, Wang, Wen, Zhang, Rustgi, von Wettstein and Liu2014) concluded that morpho-physiological traits remained dramatically augmented and many of these traits of synthetic wheats became more similar to the Ae. tauschii parent than to the durum parent, signifying that the salinity stress has improved functionality of the D-genome in the SHW, giving it the property of salt tolerance. This significant capability of instantly rearranging functionality of the sub-genomes in response to different growing environment is obviously an exceptional property for preferential use of SHWs in breeding (Yang et al., Reference Yang, Zhao, Zhang, Yang, Wang, Wen, Zhang, Rustgi, von Wettstein and Liu2014). Formerly, evaluation of the D-genome chromosome replacement stocks for the A and B genome chromosomes of Triticum turgidum cv. Langdon and D-genome-based synthetic hexaploid germplasm has shown us the chromosomal link of the trait responsible for salinity (Gorham et al., Reference Gorham, Hardy, Wyn Jones, Joppa and Law1987; Shah et al., Reference Shah, Gorham, Forster and Jones1987). These studies confirmed that the D-genome has a trait situated on chromosome 4D with the ability to enhance K+: Na+ perception. This subsequently led to ascribing the trait to the 4DL arm of chromosome 4 with the locus designated as ‘kna1’ (Byrt et al., Reference Byrt, Platten, Spielmeyer, James, Lagudah, Dennis, Tester and Munns2007).
Ogbonnaya et al. (Reference Ogbonnaya, Abdalla, Mujeeb-Kazi, Kazi, Steven, Gosman, Lagudah, Bonnett, Sorells and Tsujimoto2013) validated the effective transmission of salt stress tolerance in SHW measured as Na+ exclusion into a top Australian well-known wheat variety, Yitpi with few of SBLs displaying considerably improved Na+ exclusion in comparison with either the SHW or the persistent local wheat variety. This was also confirmed by an independent study where the SBL genotype ranked 3rd out of 150 lines evaluated for salinity tolerance using a hydroponic system at ICARDA. All the parameters studied showed positive correlation with each other that tells us that these traits were strongly linked with salinity tolerance and could be used to evaluate SHW genotypes in salt stress. All the genotypes with high survival rate indicated that they have resilience to deal with salinity stress inhibiting the wheat establishment at early germination stage. Overall, SHWs showed the potential to provide good plant stand establishment and biomass production at early seedling stage, which are essential to get high production under high-salt stress.
Conclusion and future prospects
SHWs have shown sufficient diversity for seedling emergence traits under salt stress and have the potential to be used for genetic dissection of loci underpinning salt tolerance. Recognizing that SHWs are to be important resources for inducing salinity tolerance through breeding we could also envision that the SHWs will not only allow us to exploit the D-genome-tolerant salinity trait, but also enable us to exploit the variability of the A and B genomes of the durum parent and thus widen the span of variability that would enhance overall productivity with simultaneous infusion of the ‘intraspecific’ breeding option integrated with the ‘interspecific’. We will be applying genetic dissection studies using association mapping on these SHWs using the DArT marker data available (Rasheed et al., Reference Rasheed, Xia, Ogbonnaya, Mahmood, Zhang, Mujeeb-Kazi and He2014) on this panel, which will identify the potential genomic regions associated with adaptability and stability of seedling vigour under salt stress conditions.
Supplementary material
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S1479262116000198
Acknowledgements
The facilitation extended by Abdul Aziz, Zahid Mahmood and Muhammad Jamil at Wheat Wide Crosses Program NARC (Pakistan) over the study phases is gratefully recognized and appreciated.
Author contribution
JQ and AM-K designed the research; ZK conducted the research; ZK and AR analysed the data and wrote the paper, JQ and AM-K checked and edited the manuscript. All authors have read and approved the final manuscript.




