Introduction
The history of use of plants for therapeutic purposes is perhaps as old as the history of human civilization on this planet. Earliest records of the usage of plants date back to 2600–1550 BC in ancient Greece, Egypt and India (Salim et al., Reference Salim, Chin, Kinghorn, Ramawat and Merillon2008; Dias et al., Reference Dias, Urban and Roessner2012). Out of about 422,000 plant species estimated to be on this planet, about 52,800 are estimated to be medicinal plants species, which, however, are not distributed evenly over all plant families; some plant families like Apocynaceae, Araliaceae, Apiaceae, Asclepiadaceae, Canellaceae, Guttiferae and Menispermaceae have higher proportion of medicinal plant species than others (Schippmann et al., Reference Schippmann, Cunningham and Leaman2003). A total of 163 drugs have been discovered from 114 plant species so far (Lahlou, Reference Lahlou2013), out of which 122 have been discovered in 94 plant species based on their ethno-medical uses (Fabricant and Farnsworth, Reference Fabricant and Farnsworth2001). The discovery of anti-cancer alkaloids in leaves of Madagascar periwinkle [Catharanthus roseus (L) G. Don], an Apocynaceous plant, is considered to be one of the most significant discoveries of drugs made so far from higher plants, which have had their therapeutic efficacy and utility proven beyond doubt (Tyler, Reference Tyler1988; Pezzuto, Reference Pezzuto1997).
The medicinal property of periwinkle has been recorded in folklores and traditional medicine literature as early as in 50 B.C. (Husain, Reference Husain1993). Different parts of this plant have been used in various forms in traditional and/home remedies all over the world for the treatment of a wide range of ailments such as diabetes, fevers, malaria, menorrhagia, hypertension, cancer, stomach ailments, heart disease, leishmaniasis, amenorrhea, dysmenorrhoea, rheumatism, liver disease, etc., (Ross, Reference Ross1999). The wide spectrum of pharmaceutical properties of this plant caught the interest of the scientific world.
During the mid-1950s, two independent research groups, one at the University of Western Ontario, Canada, and the other at Eli Lilly Company, Indiana, USA, investigating the reported folkloric use of periwinkle as an oral hypoglycaemic agent could not demonstrate hypoglycaemia in either normal or experimentally induced hyperglycaemic rabbits. They observed that extracts of periwinkle leaves produced leukopenic activity in rats and prolongation of life of DBA/2 mice infected with P1534 leukaemia, respectively (Svoboda, Reference Svoboda, Taylor and Farnsworth1975). Further phytochemical investigations led to the discovery of two alkaloids, vincaleukoblastine [vinblastine (VLB)] by Noble et al. (Reference Noble, Beer and Cutts1958) and leurocristine [vincristine (VCR)] by Eli Lilly Company, USA, possessing anti-cancer property (Svoboda, Reference Svoboda, Taylor and Farnsworth1975; Tyler, Reference Tyler1988). Interestingly, VLB and VCR were discovered in samples collected from Jamaica and Philippines although periwinkle is endemic to Madagascar (Cragg and Newman, Reference Cragg and Newman2005). Four alkaloids from periwinkle are used clinically: VLB, vinorelbine (VRL), VCR and vindesine (VDS). VLB sulphate, commercially known as Velban® is used in the treatment of Hodgkin's disease, lymphosarcoma, neuroblastoma and choriocarcinoma. VCR sulphate, commercially known as Oncovin® or Vincovin® is used in the treatment of leukaemia in children and reticulum cell sarcoma (Svoboda and Blake, Reference Svoboda, Blake, Taylor and Farnsworth1975). Two semi-synthetic bisindole alkaloids, VRL, marketed as Navelbine® and VDS marketed as Eldisine® and Fildesin® are used in the treatment of breast cancer and bronchial cancer, and acute lymphoblastic leukaemia and refractory lymphoma, respectively (Bruneton, Reference Bruneton1995; Pezzuto, Reference Pezzuto1997). These alkaloids are primarily used in combination with other cancer chemotherapeutic drugs for the treatment of a variety of cancers (Cragg and Newman, Reference Cragg and Newman2005). In 2005, the market for VCR and VLB was estimated at US$150–300 million and Oncovin® and Velban®, are sold for a total of US$100 million per year (Schmelzer, Reference Schmelzer, Schmelzer and Gurib-Fakim2007).
The discovery of ajmalicine, used in the treatment of circulatory diseases, especially hypertension, in roots of periwinkle followed their detection as an adulterant in Rauvolfia roots exported from India. Since it proved as valuable source as Rauvolfia of ajmalicine, it was readily accepted as an alternate source of this alkaloid (Krishnan, Reference Krishnan, Chadda and Gupta1995; Singh, Reference Singh, Handa and Kaul1996).
Although anti-cancer alkaloids VLB and VCR were discovered more than 50 years ago, periwinkle still remains the sole source of these alkaloids. Apart from VLB and VCR, periwinkle produces more than 130 diverse groups of alkaloids (Zhu et al., Reference Zhu, Zeng, Sun and Chen2014) and has been termed as an alkaloid engine (Dugo de Bernonville et al., Reference Duge de Bernonville, Clastre, Besseau, Oudin, Burlat, Glevarec, Lanoue, Papon, Giglioli-Guivarc'h, Benoit St-Pierre and Courdavault2015). However, the low contents of VLB and VCR (1 g and 20 mg in 1000 kg of plant material, respectively) in the plant (Tyler, Reference Tyler1988) and high costs of their extraction have led to extensive efforts towards increasing their production and reducing their costs through various approaches. As a result, periwinkle has emerged as one of the most extensively investigated medicinal plants and is regarded as model ‘non-model’ plant for the study of alkaloid metabolism in plants (Facchini and De Luca, Reference Facchini and De Luca2008). Around 3000 scientific publications have appeared on this plant during last 50 years (1963–2015) with an average of about 58 publications per year. About 80 papers have been published every year during the last three decades suggesting continued interest in this plant. About 67% of these papers are in the areas of biochemistry and physiology, cell and tissue culture, molecular biology and genetic engineering, and phytochemistry. The remaining 33% of the publications are in the areas of botany, crop production and management, plant pathology, pharmacology, entomology and nematology, genetics and plant breeding (Fig. 1). While during early years (1963–1974) after the discovery of these alkaloids, botany and phytochemistry were the top two researched areas; biochemistry and physiology, cell and tissue culture emerged as the two most researched areas during the next two decades, i.e. 1975–1984 and 1985–1994. However, during the last two decades, the highest numbers of publications have appeared in the area of molecular biology and genetic engineering (Fig. 2). This is in conformity with the general trend observed in the most actively pursued areas of plant sciences during these five decades. Thus, it is apparent that although periwinkle can be easily cultivated in tropical regions, relatively very little attention has been paid to field production of this plant as a source of these important alkaloids as compared with their production in vitro. This may be because cancer is regarded as afflicting mainly developed countries (Cragg and Newman, Reference Cragg and Newman2005), i.e. mostly in temperate regions, where periwinkle does not thrive well. Therefore, extensive efforts have been made to produce these alkaloids through in vitro systems. On the other hand, in tropical areas where periwinkle can be easily cultivated, very little work has been done on increasing the yield of periwinkle alkaloids through improved agronomy, probably, because measurement of alkaloids in the plant material requires sophisticated and expensive instruments, technically trained personnel and is also time consuming.

Fig. 1. Distribution (%) of publications on periwinkle in different subject areas during the period 1963–2015 (search with key words C. roseus or V. roseus at websites http://newcrops.com.au/; http://www.ncbi.nlm.nih.gov/pubmed and https://scifinder.cas.org/scifinder).
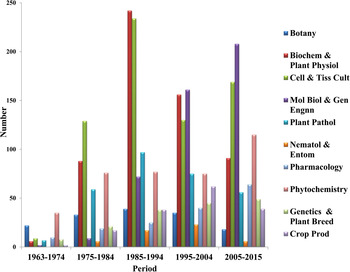
Fig. 2. Number of publications on periwinkle in different subject areas during the period 1963–2015.
More than 2600 patents have been granted on various inventions relating to use of this plant after the grant of first patent in 1961 (GB870723 19610621) on isolation of alkaloid Vincaleucoblastine from Vinca roseus. There has been a noticeable increase in number of patents granted from the year 2004, with the number of patents granted exceeding the number of publications suggesting increased commercial interest in periwinkle both as a medicinal and a horticultural plant (Supplementary Fig. 1S). Patents have been granted on a wide variety of inventions such as, use of its herb or its chemical constituents as medicine in various formulations or as drugs, including drug carrier, for novel methods of extraction or synthetic processes for its chemical constituents/ alkaloids or and their derivatives, use of tissue culture and hairy root cultures for production of anti-cancer alkaloids, methods to improve flower colour, methods for enhancement of alkaloid production by using polyploid cells, methods for plant male sterility, enhancement of plant biomass, salt and low-temperature resistance, new plant varieties and so on.
Literature on biosynthesis of periwinkle alkaloids, their production in vitro through cell and tissue culture, and metabolic engineering is voluminous and has been periodically reviewed (van der Heijden et al., Reference van der Heijden, Verpoorte and Ten Hoopen1989; Moreno et al., Reference Moreno, van der Heijden and Verpoorte1995; Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006; El-Sayed and Verpoorte, Reference El-Sayed and Verpoorte2007; Zárate and Verpoorte, Reference Zárate and Verpoorte2007; Zhao and Verpoorte, Reference Zhao and Verpoorte2007; Zhou et al., Reference Zhou, Shao and Tang2009; Verma et al., Reference Verma, Mathur, Srivastava and Mathur2011; Moudi et al., Reference Moudi, Go, Yien and Nazre2013; Salim and De Luca, Reference Salim, De Luca and Giglioli-Guivarc'h2013; Zhao et al., Reference Zhao, Sander and Shanks2013; Duge de Bernonville et al., Reference Duge de Bernonville, Clastre, Besseau, Oudin, Burlat, Glevarec, Lanoue, Papon, Giglioli-Guivarc'h, Benoit St-Pierre and Courdavault2014; Matsuura et al., Reference Matsuura, Rau and Fett-Neto2014; Zhu et al., Reference Zhu, Zeng, Sun and Chen2014). The biosynthesis of terpenoid indole alkaloids (TIAs) in periwinkle is a multi-step complex process consisting of more than 50 biosynthetic events involving many genes, enzymes, regulators, intracellular transporters, organs, tissues and cell organelles and is regulated by ontogenic, environmental, organ- and cell-specific factors (Roepke et al., Reference Roepke, Wu, Salim, Thamm, Murata, Ploss, Boland and De Luca2010; Zhu et al., Reference Zhu, Zeng, Sun and Chen2014). It is highly compartmentalized with different portions of the pathways occurring in chloroplasts, the cytosol, the endoplasmic reticulum, the nucleus and vacuoles (Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006; Guirimand et al., Reference Guirimand, Guihur, Poutrain, Héricourt, Mahroug, St-Pierre, Burlat and Courdavault2011). These TIAs are synthesized from secologanin (a monoterpenoid) and tryptamine (an indole) derived, respectively, from geranyl diphosphate via plastidial methyl erythritol phosphate pathway, and tryptophan via plastidial shikimate pathway. Tryptamine is synthesized from tryptophan by the enzyme tryptophan decarboxylase (TDC). The condensation of secologanin and tryptamine by the enzyme strictosidine synthase (STR) yields strictosidine, the central intermediate of all TIAs. Enzymatic deglucosylation of strictosidine through strictosidine-β-D-glucosidase (SGD) results in 4, 21-dehydrogeissoschizine, which is then catalysed by many enzymes in different branches to produce diverse TIAs. One of these branches leads to formation of cathenamine. Cathenamine is further converted into ajmalicine, serpentine, stemmadenine and the monomeric precursors (vindoline and catharanthine) of anti-cancer alkaloids, VLB and VCR, through different sub pathways (van der Heijden et al., Reference van der Heijden, Jacobs, Snoeijer, Hallard and Verpoorte2004; Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006). At present, no genes, enzymes or intermediates involved in the catharanthine pathway have been characterized (Saiman, Reference Saiman2014) except for the identification of an unique catharanthine transporter gene (CrTPT2) that is expressed predominantly in the epidermis of young leaves and is responsible for accumulation of catharanthine entirely in the wax exudates on the leaf surface (Yu and De Luca, Reference Yu and De Luca2013). Biosynthesis of vindoline involves six enzymatic steps starting from the intermediate tabersonine (synthesized from strictosidine-aglycone), with last two steps of vindoline biosynthesis occurring in specialized cells, laticifer and idioblast cells, of aerial leaf mesophyll tissues (Salim and De Luca, Reference Salim, De Luca and Giglioli-Guivarc'h2013). Spatial separation of vindoline and catharanthine, the precursors of VLB and VCR, thus provides a clear explanation for the low levels of VLB and VCR in intact plants (Yu and De Luca, Reference Yu and De Luca2013). The coupling of vindoline and catharanthine to form anhydrovinblastine occurs in the vacuole through a peroxidase-like enzyme (Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006).
Attempts to produce VLB and VCR through callus and cell culture have not been successful. Although catharanthine is produced in cell cultures, vindoline is not synthesized in undifferentiated cell and callus cultures. Vindoline biosynthesis requires shoot formation and the last biosynthetic step in vindoline biosynthesis occurs synchronously with shoot formation (Zhao et al., Reference Zhao, Hu, Guo and Zhu2001; Wink et al., Reference Wink, Alfermann, Franke, Wetterauer, Distl, Windhövel, Krohn, Fuss, Garden, Mohagheghzadeh, Wildi and Ripplinger2005; Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006; Campos-Tamayo et al., Reference Campos-Tamayo, Hernandez-Dominguez and Vazquez-Flota2008; Salim and De Luca, Reference Salim, De Luca and Giglioli-Guivarc'h2013). Organ/shoot cultures are, however, difficult to grow in large-scale bioreactors and the yields of these anti-cancer alkaloids have been low and, therefore, do not provide an economically viable alternative to field-grown periwinkle plants as a source of these alkaloids (Wink et al., Reference Wink, Alfermann, Franke, Wetterauer, Distl, Windhövel, Krohn, Fuss, Garden, Mohagheghzadeh, Wildi and Ripplinger2005; Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006; Roepke et al., Reference Roepke, Wu, Salim, Thamm, Murata, Ploss, Boland and De Luca2010).
Significant advances have been made recently in metabolic engineering of biosynthetic pathway of TIAs in heterologous organisms. The secologanin pathway is considered to be the rate-limiting step in TIA production in C. roseus cell cultures (van der Heijden et al., Reference van der Heijden, Jacobs, Snoeijer, Hallard and Verpoorte2004). Miettinen et al. (Reference Miettinen, Dong, Navrot, Schneider, Burlat, Pollier, Woittiez, van der Krol, Lugan, Ilc, Verpoorte, Oksman-Caldentey, Martinoia, Bouwmeester, Goossens, Memelink and Werck-Reichhart2014) discovered the last four missing enzymes in the secologanin pathway and demonstrated heterologous production of strictosidine by reconstituting the entire TIA pathway up to strictosidine in Nicotiana benthamiana. Similarly, Brown et al. (Reference Brown, Clastre, Courdavault and O'Connor2015) demonstrated production of strictosidine in Saccharomyces cerevisiae by engineering 14 known monoterpene indole alkaloid pathway genes. Since strictosidine is the central intermediate of all TIAs, its production in S. cerevisiae and N. benthamiana is a significant step in achieving heterologous production of periwinkle TIAs such as VLB and VCR. However, economic viability of further production of valuable periwinkle alkaloids, VLB and VCR, and or their precursors, vindoline and catharathine, vis-à-vis their extraction from field-grown plants still requires to be evaluated. At present, no economically viable alternative to field-grown plants is available for large-scale production of these alkaloids. Therefore, increasing yield of these alkaloids through appropriate crop management by identifying environmental and regulatory factors influencing alkaloid production in field-grown plants (Pasquali et al., Reference Pasquali, Porto and Fett-Neto2006) and breeding periwinkle varieties with higher concentrations and yield of these periwinkle alkaloids (Sharma et al., Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012; Chaudhary et al., Reference Chaudhary, Pandey, Sharma, Tripathi and Kumar2013) have been suggested. In this paper, published information on botany, cytology, genetics and breeding of periwinkle as a medicinal plant has been reviewed to gain an understanding of the constraints and possibilities of increasing the contents and yields of alkaloids in periwinkle at the plant level.
Botany
Origin and distribution
Periwinkle (C. roseus) is considered to be a native of the West Indies but was originally described from Madagascar (Ross, Reference Ross1999). It was introduced into Paris in 1757 and has now become naturalized in continental Africa, America, Asia, Australia and Southern Europe and on some islands in the Pacific Ocean (van der Heijden et al., Reference van der Heijden, Jacobs, Snoeijer, Hallard and Verpoorte2004). It is commonly grown as an ornamental plant throughout tropical and subtropical regions of the world by virtue of its wide adaptability, ever blooming nature and variously coloured flowers.
Taxonomy
The genus Catharanthus consists of eight species, seven of them viz., C. roseus (L.) G. Don, C. ovalis Markgraf, C. trichophyllus (Baker) Pichon, C. longifolius (Pichon) Pichon, C. coriaceus Markgraf, C. lanceus (Bojer ex A. DC.) Pichon, C. scitulus (Pichon) Pichon, indigenous to Madagascar and one viz., C. pusillus (Murray) G. Don, indigenous to India (Stearn, Reference Stearn, Taylor and Farnsworth1975).
Periwinkle (C. roseus) was designated variously earlier as Vinca rosea L., Lochnera rosea Rchb. and Ammocallis rosea Small. In 1753, Linnaeus established the genus Vinca and included two species Vinca minor and Vinca major. In 1759, he added tropical V. rosea to the temperate genus Vinca although it did not fit his generic description with regard to stamens. Reichenbach was the first to recognize V. rosea as generically different from Vinca (Stearn, Reference Stearn, Taylor and Farnsworth1975). In 1835, George Don while describing the genera retained the name Vinca for the genus containing V. minor, V. major and Vinca herbaceae, and gave the name Catharanthus to the genus typified by V. rosea and described it as C. roseus (Stearn, Reference Stearn, Taylor and Farnsworth1975).
The Taxonomic Hierarchy of Cataharanthus as described by Interagency Taxonomic Information System (ITIS) is given below (http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=30167#).
Kingdom: Plantae – plantes, Planta, Vegetal, plants
Subkingdom: Viridiplantae
Infrakingdom: Streptophyta – land plants
Superdivision: Embryophyta
Division: Tracheophyta – vascular plants, tracheophytes Subdivision Spermatophytina – spermatophytes, seed plants, phanerogames
Class: Magnoliopsida
Superorder: Asteranae
Order: Gentianales
Family: Apocynaceae – dogbane, apocyns
Genus: Catharanthus G. Don – periwinkle
Species: Catharanthus roseus (L.) G. Don – Madagascar Periwinkle
Botanical description of the plant
Periwinkle is an annual or perennial semi-shrub growing to a height of 75 cm–1 m, sub-woody at the base and profusely branched. Stem colour – yellowish green, light pink or dark purple. Leaves, petioles and twigs contain milky latex. Leaves – oblong or ovate, opposite, short-petioled, smooth or pubescent with entire margin. Flowers – borne in pairs in axils, pedecellate, bracteate, hermaphrodite, actinomorphic, complete, hypogynous and pentamarous. Calyx – five parted, the sepals free almost to the base. Corolla – five lobed, small to large, salver-shaped, rose or white; tube cylindric, throat bearded, slender, externally swollen at the insertion of the stamens but contracted at the mouth; lobes free or overlapping, aestivation convolute. Stamens – five, attached to the middle of the corolla tube or just below the mouth, conniving over the stigma; filaments very short, not geniculate; anthers free from the stigma, dorsifixed, the connective not prolonged into an apical appendage, anther 2.5 mm long and the filament about 0.3 mm long, at anthesis. Carpels – two, distinct, narrowly triangular glands present at the base of the carpels; ovules, numerous (about 10–30) in two series in each carpel; style long, slender; clavuncle shortly cylindric, truncate at base. Carpels united only by the style at the apex. Stigma capitate, bearded at the top and furnished with a cup-shaped/‘skirt’ like membrane below, which sheaths the upper part of the style. Fruit consists of two long cylindric pointed follicles (mericarps) diverging or parallel, containing 10–30 seeds, dehiscent at maturity along the length. Seeds – numerous, small (1.5–3.0 mm long), oblong, cylindrical, not arillate, with the hilum in a longitudinal depression on one side, blackish, muriculate, the surface minutely reticulate.
Reproductive biology
Flower development
A knowledge of the morphology of the flower, its development, anthesis, pollination mechanism, mode of pollination, presence or absence of incompatibility, fruit and seed set of the plant is an essential prerequisite for understanding the breeding behaviour of the plant species. Information on these aspects is necessary for developing artificial selfing and hybridization techniques, maintenance of varietal purity and for choosing and executing appropriate breeding methodology.
Flowering in periwinkle begins when plants are about 10–15 cm tall or about 10 weeks old and continue to flower as long as plants live. Flowers appear in pairs in the alternate leaf axils and the two flowers are never in the same stage of development, with one flower opening approximately 2–3 d before the other.
The vegetative phase is very short and the adult shoot apex is associated with flowering. After many meristematic divisions in the shoot apex, sepals are initiated. Following sepal initiation, the floral apex enlarges rapidly and becomes broad and flat. Petals arise at the edge of the floral meristem, adaxial to and alternate with the sepals (Boke, Reference Boke1948). In early stages of development, the flower is polypetalous. The corolla tube begins to form shortly after carpel initiation. The upper corolla tube (part above insertion of stamens and below corolla lobes) is formed by ontogenetic union of bases of petals. The lower corolla tube (i.e. below insertion of stamens) is formed by intercalary growth in the common bases of petals and stamens. The region below the stamen continues to elongate until the flower opens (Boke, Reference Boke1948).
Stamen primordia appear soon after petal initiation. After a sequence of cell divisions, the stamen at first appears to consist of sessile anthers and later the filaments are formed. Following the stamen initiation, the floral apex flattens. The central region grows at a slow rate and later develops into carpel. The carpels are sessile and are not united with each other in the ovary region. The style remains short until the flower is nearly mature (Boke, Reference Boke1948, Reference Boke1949; Rendle, Reference Rendle1971).
Anthesis and pollination
The corolla tube grows faster than the limb up to the day of flowering (Simmonds, Reference Simmonds1960). The elongation of style corresponds with the elongation of the corolla tube, so that the relative positions of the stigma and stamens remain almost constant. As the flower approaches anthesis, epidermis of the capitate stigma forms secretory cells on the sides and a curious ‘skirt’ (a cup-shaped membrane) at its base. Based on these observations on the development of stamens and carpels, Boke (Reference Boke1949) concluded that the flower is well adapted for self-pollination.
Under South Indian conditions, anthesis generally starts from 15.00 to 16.00 h and continues until the next day. Anther dehiscence occurs just before anthesis and the pollen is shed as a sticky mass (Supplementary Fig. 2S). The stigma was found to be receptive between 06.00 and 10.00 h, and between 15.00 and 17.00 h (Sreevalli, Reference Sreevalli2002).
Sprengel (Knuth, Reference Knuth1909) and Boke (Reference Boke1949) supposed that periwinkle flower was adapted for self-pollination. Periwinkle has also been considered as a self-pollinating species in several other studies also (Flory, Reference Flory1944; Simmonds, Reference Simmonds1960; Krishnan et al., Reference Krishnan, Naragund and Vasantha Kumar1979; Levy et al., Reference Levy, Milo, Ashri and Palevitch1983; Milo et al., Reference Milo, Levy, Akavia, Ashri and Palevitch1985). As periwinkle plants were generally found to be true breeding for their flower colour, Flory (Reference Flory1944) and Simmonds (Reference Simmonds1960) considered that periwinkle is a self-pollinating species. Krishnan et al. (Reference Krishnan, Naragund and Vasantha Kumar1979) used pink flower colour as the marker trait for determining out-crossing and found that natural out-crossing in periwinkle ranged from 1.7 to 13.8% in white flowered plants. They opined that periwinkle is essentially a self-pollinating plant with not infrequent out-crossing, as flowers remain open up to 3 d for possible insect pollination.
A characteristic feature of Apocynaceae, is the disc-like or otherwise-shaped enlargement of the stigma-head with a sticky secretion and a brush of hairs on which pollen collects as it is shed. The receptive portion of the stigma is at the base of the stigma head, and owing to the position of the anthers, self-pollination is rendered almost impossible, and insect-visits are necessitated (Rendle, Reference Rendle1971). Pollination is brought about by nectar-seeking insects, which effect pollination by depositing pollen collected from the flowers during their previous visits (Knuth, Reference Knuth1909). Automatic self-pollination in periwinkle is thus excluded. Experimental studies have also shown that automatic self-pollination does not occur in periwinkle and pollinators are necessary to bring about pollination (Kulkarni, Reference Kulkarni1999; Sreevalli et al., Reference Sreevalli, Baskaran, Kulkarni and Kumar2000). Further, two pollinating butterflies (Supplementary Fig. 3S), Pachliopta hector and Catopsilia pyranthae, have been found to exhibit flower colour constancy during their flower visits and cause about phenotypic assortative mating for flower colour resulting in greater number of intra-flower colour matings than inter-flower colour matings. This phenotypic assortative mating for flower colour combined with geitonogamy could result in plants appearing to be breeding true for their flower colour on open-pollination (Kulkarni, Reference Kulkarni1999). Many pollinators Bombylius discolor, B. major, Anthophora pilipes, Apis mellifica, Bombus agrorum, B. hortorum, B. hypnorum, B. pratorum, B. terrester, B. vestalis, Osmia fusca, O. rufa and thrips have been observed in the genus Vinca (Knuth, Reference Knuth1909).
Some self-pollinating strains have, however, also been found in periwinkle. In these strains, self-pollination occurs after anthesis due to continuous elongation of either ovaries or styles until pollination by overcoming spatial separation of stigma and stamen. Thus, both types of pollination occur in the periwinkle (Kulkarni et al., Reference Kulkarni, Sreevalli, Baskaran and Kumar2001, Reference Kulkarni, Sreevalli and Baskaran2005a).
Fertilization and embryology
The mature megagametophyte is normal or polygonum type with ephemeral type of antipodal cells. The microsporangium contains 92–96% viable pollens. The pollen tube reaches the megagametophyte within 12–24 h after pollination. Fertilization is porogamous type and usually occurs 48–72 h after pollination. Endosperm is of nuclear type and endosperm wall formation starts from the periphery towards the centre. The embryo development is caryophyllad type (Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1977).
The corolla remains persistent generally for 2–3 d but generally falls off before fruit set becomes visible. Fruit (follicle) matures in 4 to 5 weeks. The follicles are dehiscent and shed seeds as they dehisce by the ventral suture. Seeds generally exhibit a period of dormancy of about 3–4 weeks and have been found to remain viable for about 12–18 months at room temperature under tropical – subtropical conditions.
Artificial hybridization and selfing techniques
Artificial hybridization and selfing techniques have been employed for carrying out genetic studies. Flower buds have been emasculated 1 d before anthesis (Kulkarni et al., Reference Kulkarni, Sreevalli, Baskaran and Kumar2001) or 2–3 d before anthesis (Levy et al., Reference Levy, Milo, Ashri and Palevitch1983; Sevestre-Rigouzzo et al., Reference Sevestre-Rigouzzo, Nef-Campa, Ghesquiere and Chrestin1993) by making a partial cut, about 1 mm, above the base of the throat of the corolla tube. The top portion of the flower bud was then removed along with the undehisced anthers. (Kulkarni et al., Reference Kulkarni, Sreevalli, Baskaran and Kumar2001). Pollination of emasculated flower buds was carried out on the same day of emasculation (Kulkarni et al., Reference Kulkarni, Sreevalli, Baskaran and Kumar2001) or 2–3 d after emasculation (Levy et al., Reference Levy, Milo, Ashri and Palevitch1983) or twice a day for 3 d after emasculation (Sevestre-Rigouzzo et al., Reference Sevestre-Rigouzzo, Nef-Campa, Ghesquiere and Chrestin1993). Pollinated flower buds were protected by paper bags or pieces of plastic straw (closed at the top end) to prevent accidental pollination and seed loss at the maturation stage. The per cent fruit set ranged from 90 to 100% (Sevestre-Rigouzzo et al., Reference Sevestre-Rigouzzo, Nef-Campa, Ghesquiere and Chrestin1993; Kulkarni, Reference Kulkarni1999).
Cytology
Chromosome number
Periwinkle is a diploid plant species with a chromosome number of 2n = 16 (Janaki Ammal and Bezbaruah, Reference Janaki Ammal and Bezbaruah1963; Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1968, Reference Dnyansagar and Sudhakaran1970; Stearn, Reference Stearn, Taylor and Farnsworth1975; De Padua et al., Reference De Padua, Barrion, Casal and De La Cruz1992). Out of eight pairs of chromosomes, four pairs were found to have submedian centromeres and two each subterminal and median centromeres (De Padua et al., Reference De Padua, Barrion, Casal and De La Cruz1992; Jia et al., Reference Jia, Dai, Xu, Jin, Zhang, Chen and Wang2008; Guimarães et al., Reference Guimarães, Cardoso, Oliveira, Santos, Duarte and Sottomayor2012). Few abnormal cells with 2n = 12 and 14 chromosomes were also observed (De Padua et al., Reference De Padua, Barrion, Casal and De La Cruz1992). The average length of chromosomes ranged from 4.784 to 8.627 µm (Guimarães et al., Reference Guimarães, Cardoso, Oliveira, Santos, Duarte and Sottomayor2012).
Based on karyomorphological studies of C. pusillus and six varieties of C. roseus, Rani and Kumar (Reference Rani and Kumar2011) concluded that C. pusillus was evolutionarily more primitive and white-flowered variety of C. roseus was most advanced. They further observed secondary constrictions on short arm of three out of eight pairs of chromosomes of C. pusillus but none in C. roseus. However, Guimarães et al. (Reference Guimarães, Cardoso, Oliveira, Santos, Duarte and Sottomayor2012) observed the presence of the nucleolar organizer region in chromosome 6.
Dnyansagar and Sudhakaran (Reference Dnyansagar and Sudhakaran1968) studied chiasma frequency, type of bivalents at diakinesis and metaphase I of meiosis in pink and white varieties of periwinkle. The percentage of ring bivalents was found to be 73% at diakinesis and 72% at metaphase I. The chiasmata per bivalent varied from 2.8 to 1.7% from late diplotene to metaphase I. The mean number of chiasmata per cell at the early metaphase I was 13.7 and did not vary between varieties.
Genome size
The nuclear DNA content (1C) of periwinkle has been estimated to be 0.70 and 0.76 pg corresponding to 696 and 738 Mbp by Zonneveld et al. (Reference Zonneveld, Leitch and Bennett2005) and Guimarães et al. (Reference Guimarães, Cardoso, Oliveira, Santos, Duarte and Sottomayor2012), respectively. The quantity of DNA and molecular size of chromosomes of C. roseus ranged from 0.070 to 0.127 pg and 69 to 124 Mbp, respectively (Guimarães et al., Reference Guimarães, Cardoso, Oliveira, Santos, Duarte and Sottomayor2012).
The complete plastome of C. roseus (154,950 bp in length) has been sequenced and 41 C. roseus-specific plastome (SSR) markers with potential utility for C. roseus breeding and phylogenetic analyses have been identified. Complete plastome sequence could be used to engineer the C. roseus plastome to accelerate synthesis of isopentenyl pyrophosphate (IPP), the rate limiting step for alkaloid accumulation which occurs in plastids (Ku et al., Reference Ku, Chung, Chen and Kuo2013).
Genetics
Genetics of corolla colour
Flower or corolla colour is the most conspicuous trait for which variation is easily apparent in periwinkle populations. Periwinkle owes its horticultural importance to its variously coloured flowers. Flower colour was, therefore, the first trait whose inheritance was studied. In natural populations, mainly three basic corolla colour patterns are observed viz., pink, red-eyed (white corolla with red centre) and white. Flory (Reference Flory1944) attributed epistatic interaction between two genes R and W to explain flower colour differences in these three phenotypes. In a cross between pink and white flowers, he observed a ratio of nine pink: three red-eyed: four white-flowered plants; with genotypes R–W being pink, R-ww red-eyed and rrW- and rrww being white flowered. Red pigmentation in stems of pink-flowered plant was associated with flower colour. Thus, stem colour indicated flower colour prior to flowering. According to Simmonds (Reference Simmonds1960), two more genes (A and B) are involved in the determination of flower colour. Gene A is a basic colour gene, which is complementary to gene R of Flory (Reference Flory1944), without both of which the flower colour is white. Gene B is a co-pigmentation gene, which blues the pigment in pink- and red-eyed flowers resulting in violet- and purple-eyed flowers, respectively. In addition to the above three corolla colours, Milo et al. (Reference Milo, Levy, Akavia, Ashri and Palevitch1985) identified another flower colour namely, pale pink centre and attributed this corolla colour to another gene I which is also epistatic to gene R like the gene W. The gene I produces pigment in the centre of the corolla but in smaller amounts than gene W.
Mode of inheritance of other corolla colours viz., orange-red corolla and magenta corolla and the presence or absence of red eye has also been studied (Sreevalli et al., Reference Sreevalli, Kulkarni and Baskaran2002; Kulkarni et al., Reference Kulkarni, Baskaran and Sreevalli2005b, Reference Kulkarni, Baskaran and Sreevalli2008). Epistatic interactions between independently inherited genes R, W, B, I, O, Om, J and E were found to be responsible for the production of pink, white, violet, pale pink, orange-red, magenta, scarlet-red and rose corolla with or without red eye (Supplementary Fig. 4S). Gene E determines the presence or absence of red eye, i.e. R allele produces pigment in the eye region only in the presence of allele E; in its absence flowers have white eye. Allelic genes O and Om produce orange-red and magenta corolla, respectively, only in the absence of W allele. Heterozygotes with OOm alleles at O locus produce scarlet-red corolla. Rose corolla is produced by inhibitory interaction between Om and J alleles.
A large number of cultivars with a range of corolla colours have been developed by horticulturists (Snoeijer, Reference Snoeijer2001).
Genetics of resistance to die-back disease
Die-back disease, caused by Pythium aphanidermatum, is a devastating disease in the rainy season in the tropical and subtropical regions. High mortalities up to 70–80% have been reported (Pareek et al., Reference Pareek, Singh, Srivastava, Mandal, Maheshwari and Gupta1981; Kulkarni, Reference Kulkarni1984). The pathogen also causes collar and root rot resulting in the death of plants. A die-back resistant variety named ‘Nirmal’ was developed as a pure line selection from a single plant that survived in a severe die-back epidemic (Kulkarni et al., Reference Kulkarni, Baskaran, Chandrashekara and Kumar1999). Inheritance of resistance to die-back was studied using a die-back resistant dwarf mutant of variety, Nirmal, with a green stem, and a susceptible accession, ‘OR’, with a purple stem. From both quantitative and qualitative analyses of data, resistance to die-back appeared to be governed by a single gene (with a broad-sense heritability of 0.85) and was inherited independently of genes governing dwarfness and stem pigmentation. Resistance of variety, Nirmal to dieback has remained durable for the last nearly 30 years (Kulkarni and Baskaran, Reference Kulkarni and Baskaran2003).
Genetics of mechanisms of pollination
The structure of periwinkle flower is of typical reverse herkogamy where the stigma is below the anthers and automatic intra-flower self-pollination is excluded. Pollination occurs through nectar-seeking insects. However, self-pollinating strains have also been found. Studies on the mechanism of self-pollination in these strains revealed two different mechanisms, one in which self-pollination is brought about by the continuous elongation of the style, and the other by the continuous elongation of the ovary till pollination (Supplementary Fig. 5S) The two mechanisms were found to be governed by duplicate alleles recessive to allogamy (SP 1 SP 1 SP 2 SP 2), with alleles (spo 1 spo 1 spo 2 spo 2) governing ovary elongation being dominant to alleles (sps 1 sps 1 sps 2 sps 2) governing style elongation (Kulkarni et al., Reference Kulkarni, Sreevalli and Baskaran2005a). However, self-pollination in these strains occurred 1–2 d after anthesis and thus, out-crossing is not ruled out under conditions of open pollination. Nevertheless, genes governing self-pollination can be transferred to desirable genotypes and their genetic purity maintained through seeds produced by autonomous self-pollination in the absence of pollinators or under isolation.
Development of cleistogamy
Self-pollination, brought about by increase in the length of gynoecium (ovary or style) in self-pollinating strains described above, was found to occur after 1–2 d after anthesis. Therefore, maintenance of genetic purity is not automatically ensured in these self-pollinating strains. Cleistogamy, if developed in periwinkle, would facilitate maintenance of genetic purity without manual selfing and seed production without dependence on pollinators. It would also ensure pollen containment which is an important requirement in the development of transgenics. An ethyl methanesulphonate (EMS)-induced mutant, in which corolla abscised before opening of the corolla, i.e. at the flower bud stage was identified. By crossing this mutant with self-pollinating strains described in the previous section, cleistogamous strains were developed (Supplementary Fig. 6S). These strains constitute pyramiding of recessive alleles at four loci, closed corolla, normal plant height (or a tightly linked gene inhibiting closed corolla) and self-pollination (Kulkarni and Baskaran, Reference Kulkarni and Baskaran2013a).
Breeding
Assessment of genetic variability and genetic divergence
Information on the breeding system of the plant, germplasm resources available, extent of genetic variability for traits of interest, their heritabilities, inter-trait correlations and genetic divergence in the germplasm is the basic pre-requisite for initiating breeding work in any plant species of economic interest.
Although periwinkle attained prominence as a source of anti-cancer alkaloids in the late 1950s, studies on genetics and breeding aspects of this plant were few and sporadic till the late 1990s. In perhaps, the first study, Levy et al. (Reference Levy, Milo, Ashri and Palevitch1983) reported marked differences for yields of leaves and roots and for contents of ajmalicine in roots of three unrelated pure lines representing three flower colour types: pink corolla, white corolla and white corolla with red eye. The differences between lines varied according to developmental stage of the plant. They also observed 29 and 24% significant and positive heterosis over better parent for leaf and root dry yields per plant, respectively, in the F1 hybrid involving the parental lines, pink corolla, and white corolla with red eye. However, no heterosis was observed for ajmalicine content in roots. They suggested breeding of pure line cultivars with high yields of leaves and roots and high contents of alkaloids in leaves and roots till the availability of male sterile lines for exploiting the observed heterosis for the leaf and root yield. The absence of heterosis for ajmalicine content in roots did not favour the development of hybrid cultivars of periwinkle for use by the pharmaceutical industry.
Virk et al. (Reference Virk, Singh and Bhullar1988) identified two genotypes with high yields of leaf and root alkaloids for direct cultivation after 2-year evaluation of 17 genotypes collected from diverse sources such as, Poland, the then USSR and different parts of India. Significant genotype × year interaction was also found for a majority of the traits studied by them, implying that ranking of genotypes was dependent on the year of evaluation. Genetic variability, heritability of different traits, genetic advance and genetic diversity among selected 20 M8 lines and six other selected lines were determined by Dwivedi et al. (Reference Dwivedi, Singh, Singh, Sharma, Uniyal and Kumar1999, Reference Dwivedi, Singh, Singh, Sharma, Uniyal and Kumar2000). Wide variation was observed for plant height, number of branches per plant, leaf yield, and for the contents of total alkaloids, vindoline, catharanthine, VCR and VLB. High broad-sense heritabilities (83–91%) were observed for leaf yield and for the contents total alkaloids, vindoline, catharanthine, VCR and VLB suggesting that the differences between the studied genotypes were mainly due to genetic causes. However, recently Sharma et al. (Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012) found moderate to moderately high heritabilities for leaf yield, root yield and for contents of vindoline, VLB, catharanthine in leaves (40–79%) and low heritabilities for contents of total alkaloids in leaves and roots (5 and 13%). The 26 entries evaluated by Dwivedi et al. (Reference Dwivedi, Singh, Singh, Sharma, Uniyal and Kumar2000) fell in to nine clusters, I–IX. Wide diversity was observed between clusters VIII and IX, and clusters VI and VIII, suggesting hybridization between entries belonging to these clusters for realizing greater magnitude of heterosis and wide genetic variability in the segregating generations.
In a relatively larger study, Mishra et al. (Reference Mishra, Uniyal, Sharma and Kumar2001) evaluated 32 accessions collected from wide geographical areas such as different regions of the Indian sub-continent, Sri Lanka, Madagascar, Singapore and Malaysia for 53 growth, development and alkaloid yield-related characters over two seasons. Large differences were observed between accessions for six morphological and 14 agronomic traits; the differences were 3, 80 and 15-fold for the main alkaloid yield components viz., leaf dry matter yield, VLB and VCR contents, respectively. The 32 accessions fell into seven clusters by principal component analysis. Five accessions from tropical areas formed separate cluster (cluster 1). The largest cluster (cluster 2) had 16 accessions from semi-tropical to semi-temperate geographical areas. However, four accessions also from semi-tropical to semi-temperate geographical areas separated into cluster 3. Four accessions from heavy rainfall and humid areas formed cluster 5. One accession each from Andaman Islands and Singapore, and a mutant cultivar separated from each other as well as from rest of the accessions. Hierarchical UPGMA analysis grouped the accessions into five clusters. In general, the genetic base of periwinkle population from the Indian subcontinent and surrounding Asian region appeared to be narrow as half of the accessions from wide geographical distances were placed in the same clusters by multivariate and hierarchical UPGMA analyses.
Diversity among 32 C. roseus accessions obtained from different geographical regions of India, Sri Lanka, Mozambique and private seed companies from India and Sweden, and one accession each of C. trichophyllus, C. pusillus, Vinca minor, Thevetia peruviana and Nerium indicum was assessed for the first time using sequence-tagged microsatellite site (STMS) markers (Shokeen et al., Reference Shokeen, Sethy, Kumar and Bhatia2007). Non-Catharathus species separated clearly from Catharathus cluster. Catharathus trichophyllus, C. pusillus separated from other C. roseus accessions within Catharathus group. Within the Catharathus group, the accessions were generally grouped according to their geographical origin (Shokeen et al., Reference Shokeen, Sethy, Kumar and Bhatia2007)
Inter-trait correlations
Information on inter-trait correlations is essential to know the effect of selection for one trait of interest on other unselected traits, and to know the possibility of carrying out indirect selection for characters of interest which are difficult or time consuming to measure, or are less heritable. In periwinkle, estimation of contents of total alkaloids and specific alkaloids viz., VLB, VCR, vindoline, catharanthine and ajmalicine is time consuming and limits the number plants that can be evaluated in a breeding programme. Any trait with high heritability and a strong correlation with contents of these alkaloids could be useful for preliminary screening for content of alkaloids as well as for indirect selection for these important traits.
Leaf yield and root yield, leaf yield and leaf alkaloid yield, root yield and root alkaloid yield were found to be positively correlated suggesting that simultaneous improvement for these pairs of traits should be possible through selection (Mishra et al., Reference Mishra, Uniyal, Sharma and Kumar2001; Sharma et al., Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012). Leaf yield and root yield were not correlated with leaf alkaloid concentration and root alkaloid concentration, respectively (Mishra et al., Reference Mishra, Uniyal, Sharma and Kumar2001). Therefore, it should be possible to combine high yield of these two plant parts with high concentrations of alkaloids in them. The content of total alkaloids in leaves was positively correlated with contents of catharanthine, vindoline and VLB in leaves (Sharma et al., Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012) suggesting that selection for total alkaloids in leaves should be effective in improving contents of catharanthine, vindoline and VLB in leaves. As expected, the contents of catharanthine, vindoline and VLB in leaves were positively correlated (Sharma et al., Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012; Chaudhary et al., Reference Chaudhary, Pandey, Sharma, Tripathi and Kumar2013).
No relationship was found between flower colour and contents of vindoline and catharanthine in 50 horticultural cultivars which had been bred for flower colour. However, one of the cultivars had low content of vidoline and ten times lower tabersonine-16-hydroxylase activity as compared with C. roseus cv. Little Delicata (Magnotta et al., Reference Magnotta, Murata, Chen and De Luca2006).
Interspecific hybridization and exploiting wild species
Although the genus Catharanthus consists of eight species (including C. roseus), no attempt has been made to evaluate and exploit interspecific variability. Catharanthus roseus is considered to be incompatible with other Catharanthus species. However, natural hybridization between periwinkle species has been observed in Madagascar and most of these hybridizations were between C. longifolius and C. roseus. Reciprocal differences were observed in crossability between C. roseus and C. trichophyllus. Catharanthus roseus as female parent failed to form fruits and therefore, no introgressions were found from C. trichophyllus to C. roseus (Sevestre-Rigouzzo et al., Reference Sevestre-Rigouzzo, Nef-Campa, Ghesquiere and Chrestin1993). In reciprocal cross, however, up to 100% seed set with good germinability was found. Alkaloid profiles of C. trichophyllus and C. roseus differed with absence of serpentine in leaves of C. trichophyllus and catharanthine in roots of C. roseus. Hybrids contained serpentine in leaves such as leaves of C. roseus and catharanthine in roots such as roots of C. trichophyllus. Further, significant heterosis was found for the contents of ajmalicine, catharanthine and serpentine both in leaves and roots and for the content of vindoline in leaves. The hybrids also had higher leaf and root yields than the parental species. Therefore, they suggested development of hybrids coupled with micro-propagation to exploit observed heterosis for alkaloid production.
Induced autotetraploidy
Induced autopolyploids generally have larger and thicker leaves, stems, roots, flowers and fruits. Autopolyploidy has also generally been found to enhance production of secondary metabolites (Dhawan and Lavania, Reference Dhawan and Lavania1996; Lavania, Reference Lavania2005; Lin et al., Reference Lin, Zhou, Zhang, Lu, Zhang, Shen, Wu, Chen, Wang and Tang2011; Dehghan et al., Reference Dehghan, Häkkinen, Oksman-Caldentey and Ahmadi2012; Lavania et al., Reference Lavania, Srivastava, Lavania, Basu, Misra and Mukai2012; Madani et al., Reference Madani, Hosseini, Dehghan and Rezaei-chiyaneh2015). In plant species in which vegetative parts are economically important and where very little genetic improvement work has been done, induced autopolyploidy could be a rapid method of increasing the yield of their vegetative parts. Further, plant species which have lower chromosome number are considered to be better suited for development of autotetraploids superior to their diploids than those which have higher chromosome number. It is likely to be less successful in plant species where seed is the commercial product and is the only method of propagation. Periwinkle has a low chromosome number and its vegetative parts, leaves and roots, and not seeds, are of economic importance. Therefore, many attempts have been made to induce and evaluate autotetraploids.
There are numerous reports on induced autotetraploidy in periwinkle (Janaki Ammal and Bezbaruah, Reference Janaki Ammal and Bezbaruah1963; Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1970, Reference Dnyansagar and Sudhakaran1977; Mohan Kumar, Reference Mohan Kumar1980; Kulkarni et al., Reference Kulkarni, Chandrashekar and Dimri1984, Reference Kulkarni, Rajagopal, Chandrashekar, Dimri, Suresh and Rao1987; Krishnan et al., Reference Krishnan, Chandravadana, Mohan Kumar and Ramachander1985). To induce tetraploidy, either seeds or apical buds of young seedlings were treated with different concentrations of colchicine solutions ranging from 0.01 to 1.0%. Colchicine treatment of apical buds was more effective in inducing tetraploidy than seed treatment. Increase in the length of stomata and pollen grain diameter have been observed as the two characteristic effects tetraploidy in periwinkle also.
Autotetraploids were found to be more vigorous in growth with broader leaves, larger stomata, flowers, pollen grains and embryos but had low pollen fertility, poor fruit set and low seed production as compared with diploids (Janaki Ammal and Bezbaruah, Reference Janaki Ammal and Bezbaruah1963; Mohan Kumar, Reference Mohan Kumar1980). In autotetraploids, pollen fertility was low (32–43%) leading to poor seed set (17.5–22.5%). Pre-fertilization abnormalities such as undeveloped ovules, lack of normal organization or delayed organization of the embryosac and delayed fertilization were responsible for the breakdown of seed formation in the autotetraploids (Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1977). Polyploidization did not affect the normal pattern of development of embryo but increased the rate of growth of embryo and endosperm. Embryo grew faster during the early period, while in later stages the growth seemed to slow down, whereas endosperm development was slow initially and faster later. Tetraploid seeds took longer time (28–32 d) to mature than the diploid seeds (24 d). Seed development in autotetraploids was similar to that in diploids; however, polyploidization altered the growth of embryo and endosperm therein. The embryo size was larger in tertaploids than in diploids (Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1977).
No consistent effects of autotetraploidy have been found on leaf yield, root yield and content of total alkaloids in leaves and roots in periwinkle. In some studies (Dnyansagar and Sudhakaran, Reference Dnyansagar and Sudhakaran1970; Mohan Kumar, Reference Mohan Kumar1980), autotetraploids had significantly higher leaf yield, root yield and content of total alkaloids than diploids, while in other studies (Kulkarni et al., Reference Kulkarni, Chandrashekar and Dimri1984; Krishnan et al., Reference Krishnan, Chandravadana, Mohan Kumar and Ramachander1985) they were found to be on par with diploids for leaf yield and root yield but had lower ajmalicine content and harvest index. Goswami et al. (Reference Goswami, Tyagi, Rani, Uniyal and Kumar1996), however, observed that tetraploids, generally, had lower leaf yields but some of them had higher contents of catharanthine, vindoline and VCR than their diploid parents. The observed differential effects of induced autotetraploidy on different traits may have been due to differences in the genetic makeup of diploid parental genotypes used.
Tetraploids performed better than diploids at close plant spacings (30 × 30 cm2), especially in the absence of nitrogen application suggesting that tetraploids could be grown on soils with low fertility as a rain-fed crop for obtaining higher yields than diploids (Kulkarni et al., Reference Kulkarni, Rajagopal, Chandrashekar, Dimri, Suresh and Rao1987). Further studies showed that tetraploids had higher nitrogen utilization efficiency than diploids (Kulkarni et al., Reference Kulkarni, Chandrashekara and Chadrashekara1995). Induced autotetraploids were also found to be highly resistant to die-back, and collar and root rot (devastating diseases in rainy season) and yielded four and five times more leaf and root total alkaloids, respectively, than diploids. Their resistance was equivalent to the protection provided by 5.76 kg/ha of a fungicide Captafol or soil solarization for a susceptible variety (Kulkarni and Ravindra, Reference Kulkarni and Ravindra1988, Reference Kulkarni and Ravidra1997; Kulkarni et al., Reference Kulkarni, Kalra and Ravindra1992).
In a recent study, the contents of vindoline, catharanthine and VLB were found to be higher in tetraploid lines than in diploids and corresponded with higher levels of expression of the genes of tdc, g10h, sls, str, dat and prx1 in the tetraploid lines as compared with the diploids (Xing et al., Reference Xing, Guo, Wang, Pan, Tian, Liu, Zhao, Wang, Sun and Tang2011).
Mutation breeding
Mutation breeding is generally adopted to create novel variation when genetic variation for desirable traits is not available in the germplasm or is available in undesirable genetic backgrounds. It is a powerful and effective tool in the hands of plant breeders for improvement of crops having narrow genetic base (Micke, Reference Micke1988). The role of mutation breeding in increasing the genetic variability for desired traits in various crop plants has been proved beyond doubt. It is also perhaps the most rapid method of variety development. As per International Atomic Energy Association (IAEA) mutant variety database, 3220 mutant varieties have been released in 170 different plant species in more than 60 countries (Pathirana, Reference Pathirana2011; Mba, Reference Mba2013) and of these, about 2700 have been developed directly from induced mutants (Pathirana, Reference Pathirana2011; Mba, Reference Mba2013; Roychowdhury and Tah, Reference Roychowdhury, Tah, Hakeem, Ahmad and Ozturk2013).
In industrial crops, such as medicinal plants, the content of the economically important metabolite is more important than the yield of the plant parts containing the metabolite because it determines the cost of extraction of the metabolite. Mutation breeding is one of the most promising approaches for the development of ‘ideochemovars’ (Swaminathan, Reference Swaminathan1972; Levy, Reference Levy1982).
Mutation breeding has been adopted more frequently in self-pollinating crops than in cross-pollinating ones, due to failure of recessive mutations to express in cross fertilizing systems without manual selfing or sib-mating. Periwinkle, although a herkogamous species, was earlier considered to be a self-pollinating species because of geitonogamy and the need for artificial selfing was not realized. Nevertheless, periwinkle has been subjected to induced mutagenesis and several mutants affecting different traits including contents of alkaloids, with direct or indirect utility through hybridization, have been isolated. Estimation of contents of alkaloids is time consuming. So far no rapid methods for estimation of contents of alkaloids are available for use in breeding programme. Although radio-immunoassays were developed long ago (Arens et al., Reference Arens, Stockigt, Weiler and Zenk1978) for rapid, accurate and reliable quantitative estimation of periwinkle alkaloids, to the best of our knowledge, we are not aware of their use subsequently in breeding programmes. In the absence of rapid methods for screening plants for their alkaloid contents, macro-mutants with altered morphology have been evaluated for identifying mutants for altered alkaloid contents. Induced macro-mutants in periwinkle include those with altered plant height, leaf morphology, floral traits, reproductive traits, and those with tolerance to salt, heat and water stress.
Mutants with altered plant height
Plant height mutants are one of the most commonly observed groups of mutants in induced mutagenesis programmes in different crop plants. Plant height is an important trait which along with shoot branching and inflorescence morphology determines plant architecture and crop yield (Wang and Li, Reference Wang and Li2006). Among plant height mutants, those with reduced plant height have generally been observed more frequently than those with increased plant height and have also been studied with greater interest due to their resistance to lodging and response to fertilizers. In fact, the ‘Green Revolution’ is attributed to the discovery of dwarfing genes ‘Norin 10’ and ‘Dee-Gee-Woo-Gen’ in wheat and rice, respectively.
Three distinct reduced plant height mutants, ‘dwarf’, ‘semi-dwarf’ and ‘bushy’, respectively, about 60, 40 and 30% shorter than their parental variety, Nirmal have been reported in periwinkle (Kulkarni et al., Reference Kulkarni, Baskaran, Chandrashekara and Kumar1999, Reference Kulkarni, Baskaran, Shyamaprasad and Kulkarni2009). The ‘dwarf’ and ‘semi-dwarf’ mutants were due to monogenic recessive genes (dw 1 and dw 2, respectively) which were allelic to each other and had significantly higher content of root alkaloids than parental variety. The ‘bushy’ mutant which was governed by an independently inherited non-allelic recessive gene (by), however, had similar contents of leaf as well as root alkaloids as the parental variety, Nirmal. The double-mutant recombinant (bydw 1 ) was 30% shorter than the shorter of the parental mutants and exhibited 20% higher content of root alkaloids than the better parent. All the three mutants and the double-mutant recombinant (bydw 1 ) had similar contents of leaf alkaloids. Higher root alkaloids content has been found to be related to thin root morphology in hairy root cultures of periwinkle (Palazon et al., Reference Palazon, Cusido, Gonzalo, Bonfill, Morales and Pinol1998). An extremely tall mutant, about 90% taller than the parental variety, Nirmal and controlled by epistatic inhibitory interaction between two independently inherited dominant genes has also been reported. The mutant, however, had similar leaf and root yields as well as contents of leaf and root alkaloids as the parental variety (Kulkarni and Baskaran, Reference Kulkarni and Baskaran2013b).
Mutants with altered leaf morphology
As economically important alkaloids are present in the leaves, altered leaf morphology may suggest altered alkaloid contents. Three leaf mutants, viz. wavy leaf margin, ‘necrotic leaf’ (a lesion mimic mutant) and ‘nerium leaf’ (resembling leaf lamina of another Apocynaceous plant, Nerium oleander) exhibited higher contents of leaf alkaloids than their respective parents (Kulkarni et al., Reference Kulkarni, Baskaran, Chandrashekara and Kumar1999; Baskaran et al., Reference Baskaran, Srinivas and Kulkarni2013). Further, enhanced contents of leaf alkaloids of ‘necrotic leaf’ and ‘nerium leaf’ mutants over their parental variety were found to be due to recessive alleles at different loci, and 13 out 14 double-mutant recombinants for parental mutant traits ‘necrotic leaf’ and ‘nerium leaf’ developed by crossing the two mutants had significantly higher content of leaf alkaloids than parental mutants (Kulkarni and Baskaran, Reference Kulkarni and Baskaran2014).
No studies have been carried out on linkage between leaf alkaloids content and these morphological mutant traits. However, it appears that ‘necrotic leaf’ trait could be used as a seedling marker trait for enhanced content of leaf alkaloids. Extract of Pythium (a soil borne pathogen of periwinkle) is well known to be an elicitor of alkaloid production in cell and tissue cultures of C. roseus (Nef et al., Reference Nef, Rio and Chrestin1991). Therefore, constitutive expression of self-defence reactions in the ‘necrotic leaf’ mutant may have induced enhanced production of alkaloids similar to that elicited by Pythium. Transgenic tobacco (Nicotiana tobacum) plants expressing TDC exhibited necrotic lesions on leaves due to accumulation of high levels of tryptamine in the chloroplasts, which is poisonous to chloroplasts (Di Fiore et al., Reference Di Fiore, Li, Leech, Schuster, Emans, Fischer and Schillberg2002). Many induced lesion-mimic mutants with enhanced resistance to pathogens have been obtained in crop plants using different mutagens. Wu et al. (Reference Wu, Bordeos, Madamba, Baraoidan, Ramos, Wang, Leach and Leung2008) obtained 21 independently induced lesion-mimic mutants using gamma rays, fast neutrons and diepoxybutane. Three of them were dominant, 17 recessive and one was dominant and maternally inherited. Two of these mutants showed enhanced resistance to multiple strains of rice blast (Magnaorthe oryzae) and bacterial blight (Xanthomonas oryzae pv. oryzae) pathogens. Further, double-mutant recombinant of two independent lesion-mimic mutants showed higher level of resistance to bacterial blight pathogen than parental mutants indicating synergistic effect of parental mutations. The frequency of lesion-mimic mutants appears to be high in plants and more than 200 lesion-mimic genes have been estimated in maize alone (Johal et al., Reference Johal, Hulbert and Briggs1995). Since these lesion-mimic mutants can be easily identified at seedling stage, screening populations for lesion-mimic mutants following treatment with different mutagens could be one simple way of identifying mutants with enhanced contents of alkaloids. It should be possible to further enhance the contents of alkaloids by developing double-mutant recombinants. However, not all lesion-mimic mutants have been found to exhibit enhanced resistance to diseases and, also, not all leaf mutants in periwinkle have been found to have altered contents of alkaloids.
Mutants with altered floral and reproductive traits
Significant heterosis for leaf and root yields was found in one of the earliest genetic studies in periwinkle (Levy et al., Reference Levy, Milo, Ashri and Palevitch1983). However, for commercial exploitation of heterosis, male sterility is required for large-scale production of hybrid seeds. A patent has been granted for the method for developing hybrids in Cathranthus using male sterility (Bowman, Reference Bowman2000). Streptomycin was used to develop genetic male sterile line ‘13861–1’ with msGS gene, which does not set selfed seed. The gene did not show any undesirable pleiotropic effect.
Two other types of male sterile mutants viz., indehiscent anthers (functional male sterility) and pollen-less anthers governed by a single recessive allele and duplicate recessive alleles, respectively, have also been reported (Sreevalli et al., Reference Sreevalli, Baskaran and Kulkarni2003; Kulkarni and Baskaran, Reference Kulkarni and Baskaran2008). The mutant with indehiscent anthers had relatively smaller anthers and about 30% lesser number of pollen grains but was otherwise phenotypically normal, had high pollen fertility and showed high seed set on artificial selfing. The possibility using this mutant for hybrid seed production was studied by determining fruit- and seed-set in a small seed production plots planted with one row of the mutant surrounded by two rows of its parental variety. Good fruit-set and number of seeds per follicle were observed in the mutant line used as female parent. All the seedlings raised from a sample of hybrid seeds produced were found to be true hybrids. Since the mutant carried recessive genes for three early expressing traits, use of a dominant allele at one or more of these loci in the male parent was suggested to facilitate easy identification of true hybrids at seedling stage for transplantation in the field (Sreevalli et al., Reference Sreevalli, Baskaran and Kulkarni2003). The mutant could be easily multiplied by manual selfing, vegetatively through stem cuttings or micro-propagation. The utility of the mutant in hybrid seed production would, however, be determined by economics of its multiplication vis-à-vis heterotic advantage of the hybrid. In contrast to these two male sterile mutants, another partially sterile mutant with in situ pollen germination has also been reported (Mishra and Kumar, Reference Mishra and Kumar2001).
Mutations affecting the gynoecium have also been reported. In periwinkle flower, the stigma is about 0.5 mm below the anthers, typical of reverse herkogamy. Mutants with short style (about one-third length of normal style) and long style with stigma 2.5 mm above the tip of the cone of anthers, with partial and high pollen sterility, respectively, have been reported (Mishra and Kumar, Reference Mishra and Kumar2003; Kulkarni and Baskaran, Reference Kulkarni and Baskaran2008). The short-styled mutant trait was inherited as a recessive trait, while the long-styled mutant constituting ‘pin’ flower in contrast to ‘thrum’ flower in normal plants appeared to be under the control of inhibitory, epistatic interaction between two independently inherited genes P and T, with gene T being inhibitory to gene P. Accordingly, genotypes P-T-, T-pp, or pptt produce normal ‘thrum’ flowers whereas P-tt produce mutant ‘pin’ flowers.
A recessive mutant producing heterocarpous flowers, with one (3%), two (82%) and three (15%) carpels and high fertility has been reported with a possibility of genetic engineering for fruit size, carpel number and seed number per plant (Rai and Kumar, Reference Rai and Kumar2001).
As periwinkle is also valued as an important garden plant because of its variously coloured flowers, mutants affecting flower colour, flower density, floral persistence etc., would also be of interest. A mutant described as ‘leafless inflorescence’, in which flowers are borne on nodes without leaves, has been reported and the locus ‘lli’ has been mapped (Chaudhary et al., Reference Chaudhary, Sharma, Prasad, Bhatia, Tripathi, Yadava and Kumar2011). The mutant produced more number of flowers per plant than its parent and further enhancing its horticultural value. New improved horticultural genotypes were developed by crossing this mutant with other genotypes with different flower colours and plant habit (Kumar et al., Reference Kumar, Chaudhary, Kumari, Sharma and Kumar2012).
Another novel mutant with caducous closed corolla (corolla abscising before anthesis) inherited as a monogenic recessive, was isolated after mutagenesis with EMS (Supplementary Fig. 7S). The trait was used for development of cleistogamy, a new trait, in periwinkle (Kulkarni and Baskaran, Reference Kulkarni and Baskaran2013a).
Mutants with altered contents of alkaloids
There are not many studies where mutagenized populations have been screened for the contents of alkaloids. To the best of our knowledge, in only one study (Thamm, Reference Thamm2014), 3600 EMS mutagenized C. roseus plants were screened and one plant with high ajmalicine, and low catharanthine and vindoline contents was identified. RNA sequencing and comparative bioinformatics of mutant and wild-type plants showed up-regulation of SGD and the transcriptional repressor, Zinc finger Catharanthus transcription factor (ZCT1) in the mutant line. The increased SGD activity in mutants seemed to yield a larger pool of uncharacterized SGD reaction products that are channelled away from catharanthine and vindoline towards biosynthesis of ajmalicine when compared with the wild-type.
Mutants with tolerance to salt, water and heat stress
It is largely believed that plant secondary metabolites are synthesized in response to various kinds of abiotic and biotic stresses. Therefore, it would be of interest to study alkaloid accumulation in mutants exhibiting tolerance to these stresses. Eight mutants (gsr1 to gsr8), tolerant to salinity (250 mM NaCl) or high-temperature (45 °C) stress have been isolated (Rai et al., Reference Rai, Luthra and Kumar2001, Reference Rai, Luthra and Kumar2003; Kumari et al., Reference Kumari, Sharma, Sharma and Kumar2013a, Reference Kumari, Yadav, Sharma, Sharma and Kumarb). These mutants (gsr 1 to gsr 6) accumulated more proline and glycine betaine constitutively as well as under water stress, and transpired lower amounts of water under water stress than their parental variety. The contents of catharanthine, VLB, VCR and serpentine in two of these gsr mutants (gsr3 and gsr6) were found to be higher than those in their parental variety and correlated well with the expression profiles of TIA biosynthetic pathway genes, strictosidine synthase, desacetoxyvindoline 4-hydroxylase and deacdetyl vindoline 4-O-acetyl transferase (Dutta et al., Reference Dutta, Batra, Pandey-Rai, Singh, Kumar and Sen2005). Although their most conspicuous mutant morphological traits were inherited as monogenic recessive traits, the mutants exhibited pleiotropic effects for several other traits (Rai et al., Reference Rai, Luthra and Kumar2003). Three of these mutations were thought to be in loci/genes that have very large and wide regulatory roles in the regulatory gene network of C. roseus for metabolism, development and adaptation to environment. The pleiotropies displayed by the mutants were considered to result from changes in gene expression affecting various kinds of functions responsible for achievement of plant morphology. The mutants were found to be hypomethylated at repeat sequences, up-regulated and down-regulated for many genes resulting in pleiotropic alterations for several traits (Kumari et al., Reference Kumari, Sharma, Sharma and Kumar2013a, Reference Kumari, Yadav, Sharma, Sharma and Kumarb).
In recent years, mutations have been induced in Japan and China using high-energy (220 MeV) and low-energy (30 keV) ion beams, respectively, and many ornamental crop cultivars with unique colour characteristics have been developed. The spectrum of mutations induced by ion beam radiation was found to be different from that of gamma rays, and novel mutants have been identified in ornamental plants (Pathirana, Reference Pathirana2011).
Space-flight environment has been found to induce mutations (‘space breeding’). Spaceships/satellites have been used to expose seeds to space-flight environment. Both genetic and epigenetic changes and dominant mutations indicative of a different spectrum mutations have been detected in plants derived from seeds exposed to space flight (Yu et al., Reference Yu, Wu, Wei, Cheng, Xin, Huang, Zhang and Sun2007; Ou et al., Reference Ou, Long, Wu, Yu, Lin, Qi and Liu2010). Use of these novel mutagens in periwinkle can, therefore, be expected to yield novel useful mutants. Desired level of specific metabolite may also be achieved via targeted mutagenesis (Belhaj et al., Reference Belhaj, Chaparro-Garcia, Kamoun and Nekrasov2013), as realized for reduced content of nornicotine in tobacco (Julio et al., Reference Julio, Laporte, Reis, Rothan and Dorlhac de Borne2008). Saika et al. (Reference Saika, Oikawa, Matsuda, Onodera, Saito and Toki2011) succeeded for the first time in producing a novel rice plant, with 230 times higher levels of tryptophan than normal plants, through site-targeted mutagenesis via gene targeting (GT) that could not have been obtained from conventional mutagenesis. They further suggested that it may be possible to achieve higher levels of various kinds of tryptophan-derived secondary metabolites, such as indole alkaloids, in plants obtained similarly from site-targeted mutagenesis via GT. Similarly, vindoline and catharanthine-rich mutants may be obtained if serpentine route gene functions can be knocked out (Singh et al., Reference Singh, Rai, Pandey-Rai, Srivastava, Mishra, Sharma and Kumar2008).
Detection of mutations is the first and most critical step in mutation breeding. Historically, mutants have been identified phenotypically, in large mutagenized populations, for easily recognizable characters such as, altered plant height and architecture, early or late flowering and maturity, altered flower, fruit and seed characteristics, resistance to diseases that can be screened easily in natural or artificial epiphytotics, and for biochemical quality traits for which low-cost, high-throughput and rapid evaluation methods are available. To increase efficiency of mutation breeding, high-throughput DNA technologies for mutation screening such as TILLING (Targeting Induced Limited Lesions IN Genomes), ECOTILLING, and high-resolution melt analysis (HRM), have been developed and used in crop plants (McCallum et al., Reference McCallum, Comai, Greene and Henikoff2000; Comai et al., Reference Comai, Young, Till, Reynolds, Greene, Codomo, Enns, Johnson, Burtner, Odden and Henikoff2004; Henikoff et al., Reference Henikoff, Till and Comai2004; Mackay et al., Reference Mackay, Wright and Bonfiglioli2008; Xin et al., Reference Xin, Wang, Barkley, Burow, Franks, Pederson and Burke2008). These reverse genetics techniques can be used for discovering allelic variation in natural or mutagenized populations using large number of mutants isolated and TIA pathway genes already cloned in periwinkle.
Genomic resources, genetic linkage maps, quantitative trait loci (QTLs) and marker-assisted selection
There were no genomic resources or ESTs reported from this plant till 2006. Murata et al. (Reference Murata, Bienzle, Brandle, Sensen and De Luca2006) sequenced two cDNA libraries from leaf base and root tips to generate 5023 ESTs of which 3553 could be annotated. This work served as the first platform to mine markers (microsatellite). With the rapid growing EST databases, efforts were initiated to develop molecular markers for Catharanthus. Medicinal plant genomic resource consortium presents 19899 ESTs clustering to 20460 contigs, 115 GSS and 749 nucleotides sequences (http://medicinalplantgenomics.msu.edu, as on 14th May, 2010). Currently 22867 ESTs, 88543 genomic sequences and 118 GSS and 46 transcriptome of Catharanthus are available in public domain (http://www.ncbi.nlm.nih.gov/date as on 19 December, 2014) which can be utilized for development of SSR, SNP and candidate gene markers. Comprehensive microsatellite and STMS marker resources (423) have been developed from genomic-enriched libraries (Shokeen et al., Reference Shokeen, Sethy, Choudhary and Bhatia2005, Reference Shokeen, Sethy, Kumar and Bhatia2007, Reference Shokeen, Choudhary, Sethy and Bhatia2011). A set of 350 unigene derived microsatellite markers of which 80 were found to be co-transferable to other Apocynaceses species such as Rauwolfia serpentina, R. tetraphylla, R. vomitoria, Nerium and Tabernomontana were developed (Jhang et al., Reference Jhang, Gautam, Shukla, Fatayal, Annula and Kulkarni2012). Similarly microsatellite marker resources from EST databases have been described (Joshi et al., Reference Joshi, Kar and Nayak2011; Mishra et al., Reference Mishra, Gangadhar, Yu, Kim and Park2011). Transcriptome sequencing of 26 d old seedlings has been used recently to mine 2520 SSR markers of which a subset of 48 markers have been validated (Kumar et al., Reference Kumar, Shah, Garg and Bhatia2014).
The first framework genetic linkage map of C. roseus was constructed by Gupta et al. (Reference Gupta, Pandey-Rai, Srivastava, Naithani, Prasad and Kumar2007). Six morphological markers and 125 DNA markers (79 RAPD, 7 ISSR, 2 EST-SSR, 37 other PCR-based DNA markers) mapped in the study resulted in 14 linkage groups generating a total map length of 1131.9 cM, with an average map length of 80.9 cM. The distance between two markers was 8.6 cM. Second genetic linkage map was developed using 423 co-dominant markers using 111 F2 individuals derived from a cross CrN1 (Nirmal) × CrN82 (Kew), of which 134 were polymorphic between the parental genotypes. A total of 114 markers were mapped on eight linkage groups that spanned a 632.7 cM region of the genome with an average marker distance of 5.55 cM (Shokeen et al., Reference Shokeen, Choudhary, Sethy and Bhatia2011).
A genetic linkage framework map consisting of 172 DNA markers and one morphological marker (leaf-less inflorescence) was constructed by Chaudhary et al. (Reference Chaudhary, Sharma, Prasad, Bhatia, Tripathi, Yadava and Kumar2011) and further advanced to include six additional markers (Sharma et al., Reference Sharma, Chaudhary, Srivastava, Pandey and Kumar2012). Twenty QTLs for seven alkaloid yield related traits viz., contents of alkaloids in leaves, stems and roots, three harvest indices for leaves, stems and roots and total dry matter weight were identified and were found to be associated with five linkage groups, LG1, LG2, LG3, LG4 and LG6. Subsequently, they identified 20 QTLs, five for concentration of catharanthine in leaves, four each for concentrations of vindoline in leaves, catharanthine and serpentine in roots, two for ajmailicine in roots and 1 for VLB in leaves, located in four linkage groups, LG1, LG3, LG4 and LG6, by applying single marker analysis, simple interval mapping and composite interval mapping (Chaudhary et al., Reference Chaudhary, Pandey, Sharma, Tripathi and Kumar2013). They further demonstrated the feasibility of marker-assisted selection for alkaloid yield related traits the first time in periwinkle. However, QTLs may not show the same effects in other genetic back grounds and environments and need to be validated. According to Brumlop and Finckh (Reference Brumlop and Finckh2011), ‘the more important a crop economically is, the more likely markers are applied in the breeding process’, which aptly explains the late and slow developments in this area in periwinkle.
Conclusions
It is apparent from above review that periwinkle is an extensively investigated medicinal plant during the last half-a-century. As a result, it has emerged as a model ‘non-model’ plant for the study of alkaloid biosynthesis in plants. It is still the sole source of anti-cancer alkaloids, VLB and VCR, and its precursors, vindoline and catharanthine, in spite of extensive efforts to produce these alkaloids more economically ex vivo, through chemical synthesis, cell, tissue or organ cultures, or metabolic engineering than through extraction from field-grown plants.
Periwinkle is a diploid self-compatible, ever-blooming perennial plant species with a small chromosome number and a relatively short seed-to-seed cycle. It is thus ideally suited for genetic, cytogenetic research and for genetic enhancement through plant breeding. However, as it is a herkogamous and entomophyllus species, the need for pollination control (artificial self-pollination for advancing generations and maintenance of genetic purity), non-synchronous fruit maturity, dehiscent fruits, seed dormancy, short period of seed viability (about 12–18 months) and high susceptibility to die-back disease coupled with lack of availability of rapid methods for quantitative estimation of alkaloids amenable to plant breeding programmes appear to be some of the constraints in breeding of periwinkle.
In general, the genetic base of periwinkle populations from the Indian subcontinent and surrounding Asian region has been found to be narrow. Therefore, the need for germplasm collection from tropical and sub-tropical regions, particularly from its the centre of origin, cannot over emphasized. Mutation breeding using new mutagens, such as low- and high-energy ion beams and space-flight environment, could be explored as they have been reported to have induced higher frequency and a different spectrum of mutations (both genetic and epigenetic) than conventional mutagens.
A large number of loci are known to be involved in the production of lesion-mimic mutants in plants. As an induced lesion-mimic mutant in periwinkle exhibited high contents of leaf alkaloids, lesion-mimic mutants could used as early expressing morphological markers to identify mutants with high contents of alkaloids in populations derived after mutagen treatment. Double-mutant recombinants could be developed from non-allelic lesion-mimic mutants exhibiting enhanced contents of alkaloids to further enhance the alkaloid contents. Site-targeted mutagenesis via targeting genes involved in the secologanin pathway, considered to be the rate-limiting step in TIA production, could be attempted. The TILLING approach could be adopted to discover allelic variation in mutagenized populations using mutant libraries.
A beginning has just been made for marker-assisted selection for alkaloid content and yield by identification of QTLs for these traits. Validation of these QTLs in different genetic back grounds and their use for simultaneous improvement in contents and yields of economically important alkaloids could be expected in the near future.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262116000150.
Acknowledgements
The authors thank the Director CSIR-CIMAP, Lucknow, India for library and internet facilities.




