Introduction
Reduction in the population size (Bottleneck) has detrimental consequences (Garza and Williamson, Reference Garza and Williamson2001), including the loss of genetic variation, increase in inbreeding (Nei et al., Reference Nei, Maruyama and Chakraborty1975), loss of adaptive potential (Chan et al., Reference Chan, Anderson and Hadly2006) and, to the extreme case, extinction through natural demographic genomic sweeps (Ellstrand et al., Reference Ellstrand, Prentice and Hancock1999). The study of population bottleneck is thus useful to provide information for conservation of plant genetic resources (Luikart and Cornuet, Reference Luikart and Cornuet1998). If populations had undergone recent bottlenecks, they may still not have had adequate time to adapt to the problems often caused by small population size (Luikart et al., Reference Luikart, Allendorf, Cornuet and Sherwin1998) and the deleterious effects of a bottleneck can be avoided or minimized through different effective conservation measures. Microsatellites are the markers of choice for estimating effective population size (N e) and detecting bottlenecks (e.g. Cornuet and Luikart, Reference Cornuet and Luikart1996), and they were applied for a number of plant and animal species (e.g. Thuillet et al., Reference Thuillet, Bataillon, Poirier, Santoni and David2005; Busch et al., Reference Busch, Waser and DeWoody2007; Lawler, Reference Lawler2008; Wang et al., Reference Wang, Zhu, Barkley, Chen, Erpelding, Murray, Tuinstra, Tesso, Pederson and Yu2009).
There are many ways by which population bottlenecks could be deduced from allele frequency data. These include lack of allelic diversity and increased genetic differentiation due to drift (Chan et al., Reference Chan, Anderson and Hadly2006). Genetic tests have also been developed for identifying recently ‘bottlenecked’ populations using allele frequency data, when no information exists on the current or historical population size. These are the heterozygosity excess (Cornuet and Luikart, Reference Cornuet and Luikart1996), the mode shift (Luikart and Cornuet, Reference Luikart and Cornuet1998) and the M-ratio test (Garza and Williamson, Reference Garza and Williamson2001). Similarly, the two commonest methods of estimating N e are based on two extreme assumption models of microsatellite evolution (the infinite allele model, IAM (Ohta and Kimura, Reference Ohta and Kimura1973); and the stepwise mutation model, SMM (Hartl and Clark, Reference Hartl and Clark1989)). Therefore, the expected heterozygosity under mutation drift equilibrium (H et E q) is simulated using IAM or SMM, depending on how mutations are introduced into the population (Lawler, Reference Lawler2008). However, due to the extremeness of the two models, the true N e should lie in between (Busch et al., Reference Busch, Waser and DeWoody2007). This is the underlying reason for introducing the two phase model (TPM) of microsatellite evolution (Di Rienzo et al., Reference Di Rienzo, Peterson, Garza, Valdes, Slatkin and Freimer1994).
The primary centre of origin and diversity for sorghum is the area extending from Ethiopia to Lake Chad (Aldrich et al., Reference Aldrich, Doebley, Schertz and Stec1992). Members of the wild S. bicolor ssp. verticilliflorum are the immediate progenitors of the domesticated sorghum (de Wet, Reference de Wet1978) and are dry savanna plants that were most probably domesticated west of the Ethiopian highlands (Stemler et al., Reference Stemler, Harlan and De Wet1977). As Ethiopia is within the probable region of the centre of origin for sorghum, diversified forms of the crop and its wild relatives represent possible sources of germplasm for crop improvement, yet information is lacking about the population biology of wild sorghum, including bottlenecks. The objectives of this study were: (1) to investigate the occurrence of recent bottlenecks in two wild sorghum populations in Ethiopia due to habitat change; (2) to estimate their long-term N e; (3) to generate useful information for their long-term conservation.
Materials and methods
Sample collection sites and plant materials
Wild sorghum sampling was performed in situ in October and November in 2008 from two diverse geographical zones in Ethiopia, Gibe River Valley (coded as G, Latitude 8°11′, Longitude 37°33′, altitude 1425 m) and Awash National Park (coded as AW, Latitude 8°56′, Longitude 40°05′, altitude 1044 m) (Fig. 1). Both populations belong to S. bicolor subsp. verticilliflorum with unidentified races and were found isolated from the crop. Each wild sorghum population consisted of 20 individuals, which is reportedly adequate to achieve reasonably high power for this kind of study (Cornuet and Luikart, Reference Cornuet and Luikart1996). Habitats of Awash National Park and Gibe River Valley were chosen for the study because they were presumed to be more vulnerable to loss of wild sorghum due to the direct effect of anthropogenic and environmental factors including wild life depredation. Gibe area is a fertile settlement area for people from other drought-affected regions of the country and hence the wild sorghum could be threatened by modern agricultural and human settlement activities. On the other hand, Awash National Park is one of the ten national parks in Ethiopia, but received little protection for wild life. This park is located in the Afar pastoral area, where there has been recurrent infiltration of livestock. In Gibe, the wild sorghum occurred along the roadsides, in the forest and along the Gibe river. In Awash, they occurred along the main highway to Djibouti.

Fig. 1 Google Earth map of Ethiopia showing the two sites where the study was conducted (their position is shown in reference to the capital city Addis Ababa (N09° 01′ E38° 44′).
DNA extraction
DNA was extracted in situ using the Whatman FTA card and later purified following the procedure developed by the manufacturer and optimized for sorghum by Adugna et al. (Reference Adugna, Snow and Sweeney2011). DNA extraction and the subsequent molecular marker-based analyses were performed at the Stanley J. Aronoff laboratory, Department of Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, Ohio, USA.
Polymerase chain reaction and genotyping
Polymerase chain reactions (PCRs) were run using 12 highly polymorphic neutral sorghum genomic microsatellite loci with dinucleotide repeats (neat and compound) that were previously mapped and distributed in the ten sorghum linkage groups (Brown et al., Reference Brown, Hopkins, Mitchell, Senior, Wang, Duncan, Gonzalez-Candelas and Kresovich1996; Taramino et al., Reference Taramino, Tarchini, Ferrario, Lee and Pe1997; Bhattramakki et al., Reference Bhattramakki, Dong, Chhabra and Hart2000; Li et al., Reference Li, Yuyama, Luo, Hirata and Cai2009) (Table S1, available online). PCRs were carried out following the QIAGEN® multi-master mix kit protocol for SSR multiplex. These loci were selected based on their high polymorphism and compatibility for multiplexing in previous studies (Adugna et al., Reference Adugna, Snow, Sweeney, Bekele and Mutegi2013). Forward primers were labelled with different fluorescent dyes: FAM (6-carboxyfluorescein), HEX (hexachloro-6-carboxyfluorescein) or NED (2,7,8′-benzo-5′-fluoro-2′,4,7-trichloro-5-carboxyfluorescein) (PE-Applied Biosystems Inc., Foster City, CA). PCR was carried out in 12 μl total volume of reaction mixture containing 1 μm of each primer pair in a multiplex, 1 μl of template DNA, 2.6 μl of sterile ddH2O and 6 μl of QIAGEN® Multiplex PCR 2X Master mix. PCRs were run in a Master cycler (Eppendorf™) with an initial denaturation step of 15 min at 95°C, followed by 35 cycles of 30 sec at 94°C, 90 sec at 58°C, 60 sec at 72°C and 30 min at 60°C, and held at 4°C according to the QIAGEN® protocol for microsatellite multiplexes. Three of the 12 primer pairs used did not amplify most of the DNA samples of these populations and were excluded from final analyses.
For determination of microsatellite fragment sizes, 2 μl of the PCR product were diluted with 14 μl of ddH2O and then 2 μl of the diluted PCR product were added to 14 μl of 36:1 Hi-Di-Formamide: GenScan™/350 Rox™ size standard in a 96-well microtitre plate and denatured at 95°C for 5 min and cooled on ice for approximately 5 min. Allele size scoring of the PCR fragments was performed by ABI 3100 Genetic Analyzer (DNA sequencer) and sizes were read using the associated GeneMapper 3.7 software (Applied Biosystems Inc., CA, USA).
Data analysis
Detection of population bottlenecks using SSR allelic polymorphism
Observed alleles and polymorphism information content (PIC) (Botstein et al., Reference Botstein, White, Skolnick and Davis1980) were computed using the PowerMarker software v3.25 (Liu and Muse, Reference Liu and Muse2005). Nei's (Reference Nei1973) unbiased gene diversity and average observed heterozygosity were computed using the FSTAT software (Goudet, Reference Goudet2002). GENEPOP 4.0 (Rousset, Reference Rousset2008) was used for Maximum likelihood estimation of null allele frequencies using the EM algorithm (Dempster et al., Reference Dempster, Laird and Rubin1977). An allele, which has the highest number for a given locus in each population, is considered by Genepop as a null allele in that population. The rarefaction method, which was first used by Hurlbert (Reference Hurlbert1971) and implemented in the HP-Rare 1.1 software (Kalinowski, Reference Kalinowski2005), was used to calculate allelic richness (R s) and private allelic richness (R p).
Detection of population bottlenecks using heterozygosity excess and mode shift methods
Two methods of detecting population bottlenecks, namely heterozygosity excess and the mode shift, were applied. In this experiment, the program BOTTLENECK 1.2.02 (Cornuet and Luikart, Reference Cornuet and Luikart1996) was used to detect bottleneck in the wild sorghum populations. The program estimates the number of alleles per locus and from which it generates the expected heterozygosity (H e) and the H et E q. Variances for the TPM were set to 30 and the proportion of SMM in TPM was 70% by default in the program. Significance tests for the heterozygosity excess/deficiency were based on 10,000 iterations. The statistical tests that the BOTTLENECK program applies include the sign test, the standardized differences test, the Wilcoxon signed-rank test and mode shift under the three mutation models (IAM, TPM and SMM). However, for the standardized differences test to work, the number of markers should be at least 20. As only nine SSR markers were used, this test was not applied and the most appropriate tests were the Wilcoxon signed-rank test and the mode-shift test. The Sign test and the Wilcoxon signed-rank tests were used to test the deviation from 50:50 heterozygosity deficiency-to-excess ratio (Cornuet and Luikart, Reference Cornuet and Luikart1996). In the mode-shift test, the allele frequency distribution histogram deviates from the normal L-shape at selectively neutral loci in the presence of a bottleneck. This is because bottlenecks reduce the number of low-frequency ( < 0.1) alleles rather than alleles at intermediate allele frequency class (e.g. 0.1–0.2), the effect of which is likely to be detectable for only a few dozens of generations and consequently only recent bottlenecks are likely to be detected by this test (Luikart et al., Reference Luikart, Allendorf, Cornuet and Sherwin1998).
Estimation of effective population size
N e was estimated using two methods on the basis of the BOTTLENECK program output. The first method was the one described by Hartl and Clark (Reference Hartl and Clark1989), which is based on H e and μ computed using the following equation:
where H e is the expected heterozygosity and μ the rate of microsatellite mutation. This equation assumes the IAM of microsatellite mutation (Kimura and Crow, Reference Kimura and Crow1964). The second method was the one developed by Ohta and Kimura (Reference Ohta and Kimura1973), which assumes the SMM (Nei, Reference Nei1987) as follows:
As the rate of mutation of microsatellites in sorghum is not available from the literature, that of maize, which belongs to the same tribe, andropogoneae was used. The value is 5.1 × 10− 5 (Vigouroux et al., Reference Vigouroux, Jaqueth, Matsuoka, Smith, Beavis, Stephen, Smith and Doebley2002).
Results
Analyses of bottlenecks
SSR allelic polymorphism and genetic diversity
The SSR locus, Sb6-57, was found to be monomorphic in Awash population (Table 1). The remaining loci were polymorphic with PIC values ranging from 0.09–0.72 and 0.43–0.85 in Awash and Gibe populations, respectively. The Gibe population showed higher SSR allelic diversity than the population from Awash National Park in terms of average observed alleles per polymorphic locus (AW/G = 3.6/6.3), overall allelic richness (2.95/4.87), overall private allelic richness (1.38/2.55) and PIC (0.38/0.67) (Table 1). It also had higher observed heterozygosity (H o= 0.22) than the Awash population (H o= 0.08). The expected heterozygosity in the Gibe population was also higher (H e= 0.71) than that of the Awash population (H e= 0.41). Null alleles were observed in both populations, but the frequency of these alleles was higher in the Awash population.
Table 1 SSR allele diversity indices of the wild sorghum populations

N a, average observed alleles per polymorphic locus; f NA, frequency of null alleles; R s, allelic richness; R p, private allelic richness; PIC, polymorphism information content; H o, average observed heterozygosity; H e, gene diversity; NA, not available.
Detection of population bottlenecks using heterozygosity excess and mode-shift methods
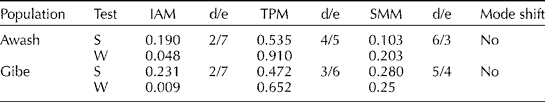
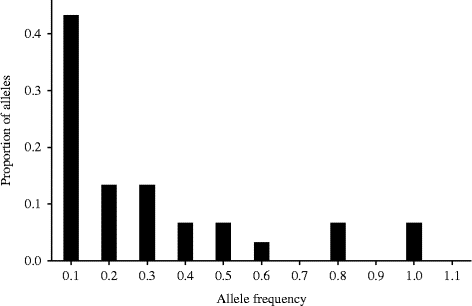
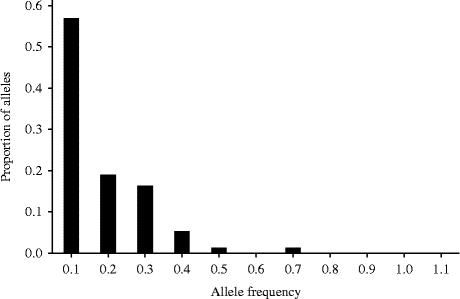
The mode-shift method did not detect any signature of bottlenecks in both of the studied populations (Table 2; Figs 2 and 3). The frequency of SSR alleles plotted against the allele probability depicted a normal L-shaped distribution (Figs 2 and 3). The sign test did not detect any departure from the 50:50 heterozygosity deficiency-to-excess ratio in all of the three mutation models (IAM, TPM and SMM). However, the Wilcoxon signed-rank test detected significant deviation from the 50:50 heterozygosity deficiency-to-excess ratio from IAM, but not from TPM and SMM (Table 2). Moreover, allelic diversity was not compromised in the Gibe population. Despite the low allele diversity, there was no signature of bottlenecks in the Awash population in terms of both heterozygosity excess and the mode-shift methods.
Table 2 Sign (S), Wilcoxon (W) and mode-shift tests for existence of bottleneck in the studied wild sorghum populations (two tail for H excess and deficiency)
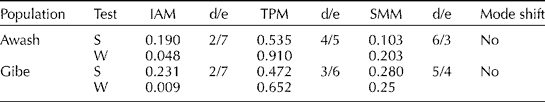
d/e, the ratio of the number of microsatellite loci exhibiting heterozygosity deficiency to excess.
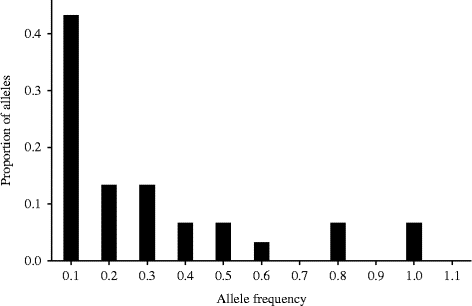
Fig. 2 Allele frequency histogram of the Awash population showing the normal L-shaped distribution, an indication of the absence of bottleneck. Figures along the x-axis show the frequency of alleles from all of the nine SSR loci and figures along the y-axis show their proportion.

Fig. 3 Allele frequency histogram of the Gibe population showing the normal L-shaped distribution, an indication of the absence of bottleneck. Figures along the x-axis show the frequency of alleles from all of the nine SSR loci and figures along the y-axis show their proportion.
Contemporary effective population size
In general, the N e in both wild sorghum populations was higher under the more conservative SMM model than the IAM. Although IAM is more vigorous, SMM is reported to be more appropriate for microsatellites. Therefore, compared with the populations from Awash National Park, which had N e values of 9103 (IAM) and 43,252 (SMM), the effective size of Gibe populations was much higher (13,874 = IAM and 78,431 = SMM) (Table 3).
Table 3 Estimated long-term effective population size (N e) based on IAM and SMM microsatellite mutation models in the two wild sorghum populations in Ethiopia

H et E q, expected equilibrium heterozygosity.
Discussion
Bottlenecks and effective population size
Studying the historical demography (expansion or reduction) of populations is vital because the patterns of genetic variation in a population could be greatly influenced by historical changes in demographic population size (Björklund, Reference Björklund2003). It is even more serious in this case, given that the species under the present investigation are in their primary centres of origin and diversity. Moreover, detection of bottlenecks and estimation of N e are of paramount importance for the conservation of plant genetic resources and evolutionary biology. In general, smaller N e is equivalent to greater susceptibility to differentiation by drift (Hoelzel, Reference Hoelzel, Bertorelle, Bruford, Hauffe, Rizzoli and Vernesi2009) and it can be the result of a population bottleneck, a long-term small population size, or a recent selective sweep (Beerli, Reference Beerli, Bertorelle, Bruford, Hauffe, Rizzoli and Vernesi2009).
Most methods that have been implemented to detect bottlenecks require sex ratio and other information on the historical background of the populations. However, the methods followed in this study do not require prior information on historical population size (Cornuet and Luikart, Reference Cornuet and Luikart1996). Lack of allelic diversity and increased genetic differentiation due to drift are among the signs of bottlenecks in populations. In the present study, however, none of these was evident. The total number of alleles observed per polymorphic locus was in the range of 3.6–6.3. Population bottlenecks are known to reduce allelic richness faster than heterozygosity (Kalinowski, Reference Kalinowski2004). Although heterozygosity can be used as a measure of the capacity of the population to respond to selection immediately after a bottleneck, the impact of bottlenecks is more predictable on allelic diversity than heterozygosity (Leberg, Reference Leberg1992). Thus, in the present study, the overall allelic richness and private allelic richness in Awash and Gibe populations were 2.95 and 4.87 and 1.38 and 2.55, respectively. In bottlenecked populations, it is also expected that genetic variability in alleles, especially rare ones, is lost and heterozygosity is lowered (Parisod et al., Reference Parisod, Trippi and Galland2005), which was not evident in this study. In both populations, the IAM detected heterozygosity excess, which was not detected by the other two mutation models (TPM and SMM), indicating that the inference from heterozygosity excess was highly influenced by the mutation model. SMM is reported to be a more appropriate model for microsatellite evolution than the IAM (Ellegren, Reference Ellegren2000), since most microsatellites are assumed to follow SMM (Gaggiotti et al., Reference Gaggiotti, Lange, Rassmann and Gliddon1999).
As the immediate progenitors of cultivated sorghum are indigenous to Ethiopia, studying their demographic status is timely for undertaking conservation and management policies or measures. In this study, both the heterozygosity excess (mainly the TPM, which is known to be more reliable) and mode-shift methods did not detect any recent bottlenecks in the studied populations. Muraya et al. (Reference Muraya, Sagnard and Parzies2010) conducted a similar study with two populations of wild relatives of sorghums from different eco-geographical regions in Kenya and found no signature of bottlenecks and the N e was higher than that expected. Endangered plant populations are known to have very low estimated N e (Zietsman et al., Reference Zietsman, Dreyer and Esler2008). However, the effective size of the two populations used in the present study showed no risk of population reduction.
Implications for conservation and management policies or measures
Plant conservation biologists employed N e estimates to explore the importance of population bottlenecks in genetic diversity and in reconstructions of historical anthropogenic effects on gene flow and genetic diversity (Espeland and Rice, Reference Espeland and Rice2010). The results of the present study indicated that the allopatric wild sorghum populations of Ethiopia can survive by occupying patches by the roadsides and fences, and areas within abandoned farm lands, forests, etc., which shows that their wild characteristics of adaptation have been adequate for them to survive from extinction. However, this guarantees neither the survival of these populations nor the survival of other populations that have not been sampled, for the future and ex situ conservation is suggested to maintain the population diversity. Even though no bottlenecks were observed in both populations, compared with the Gibe population, the population from Awash National Park showed smaller N e probably because there was lack of polymorphism at Sb6-57 locus for this population or their introduction into the area and expansion was a recent founder effect. In recent years, the park is also being grazed by livestock from local Afar nomads because of laxity in enforcing security measures to protect it. Probably, the reduced N e is a sign of being getting into or recovering from a bottleneck, which may require ex situ conservation effort. It is clear that the rate of recovery after a bottleneck is very slow as it takes time for new alleles to occur by mutation (Felsenstein, Reference Felsenstein2007). The Gibe population, on the other hand, maintained larger private allelic richness than the Awash population, indicating the possibility of possessing valuable genes for future crop improvement programmes.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262115000015
Acknowledgements
This study was supported by a grant from the Biotechnology and Biodiversity Interface (BBI) programme of the United States Agency for International Development (USAID) to Professor Allison A. Snow, Department of Evolution, Ecology, and Organismal Biology, Ohio State University, Columbus, Ohio. The authors thank Professor Allison Snow for her interest and follow-up of this research.