Introduction
The genus Lactuca comprises about 100 species. Cultivated lettuce (Lactuca sativa L.) is the best known member of this genus since it represents a common food species. Wild Lactuca species are considered to be an important source of disease resistance genes, and this has driven the need to establish ex situ collections of the wild species. During this process, accessions of other Lactuca species have become commonplace in many genebank collections (Lebeda et al., Reference Lebeda, Doležalová and Astley2004). The number of wild Lactuca accessions within collections has increased not only by the incorporation of newly collected material but also by the exchange of seeds among genebanks and other donors, thus partially duplicating specific genotypes. The appropriate maintenance of germplasm collections following current international standards demands considerable financial and human resources. Since these resources are limited, there is a need to avoid redundancy. To reduce redundancy, the degree of duplication among accessions needs first to be estimated. This is facilitated both by the development of crop-specific databases and by the application of molecular techniques.
Intensive research has been conducted in recent years to identify duplicate accessions and gather the information needed to rationalize germplasm collections. While the identification of lettuce duplicates has up to now relied on a combination of RAPD (Random Amplification of Polymorphic DNA) and morphological data, in other crop species, such as rice, barley and cabbage, the AFLP technique has been preferred, since it delivers more robust markers than does RAPD (Spooner et al., Reference Spooner, Van Treuren and De Vicente2006). AFLP has been employed in lettuce to analyse phylogenetic relationships and population structure, but not as yet for the detection of genotypic duplicates. In the course of an EU-funded project (www.gene-mine.org), duplicate accessions of wild Lactuca species were identified based on passport data (especially identical collection number ID and identical donor), and then by a morphological trait analysis. Since passport data can be erroneous and environmental conditions can influence morphology, and since the latter is not always sufficient to differentiate between closely related materials, a sample of accessions from defined duplication groups has now been genotyped by AFLP.
Material and methods
The identification of duplication groups
The study sample comprised 78 accessions from 12 Lactuca species, provided by 6 genebanks (Table 1). Putative duplicate accessions of wild Lactuca species were identified based on passport data held at the Centre for Genetic Resources (CGN) in Wageningen, The Netherlands, and by a search of the Lactuca database ILDB (The International Lactuca Database, www.plant.wageningen-ur.nl/cgn/ildb). Where passport data were scarce and the number of accessions limited, all accessions of a species were included. This exercise resulted in the identification of 33 duplication groups. Morphological trait analysis was then carried out for the putative duplicate accessions or ‘duplication groups’ at the Department of Botany of Palacký University (PU) and the Gene Bank Department of the Research Institute of Crop Production (RICP) in Olomouc, Czech Republic.
Table 1 Accessions of wild Lactuca used for morphological and AFLP analyses

DG, duplication group; NLD, The Netherlands; CZK, Czech Republic; USA, United States of America; DEU, Germany.
Morphological characterization
Twenty-five seeds per accession were sown in sterile Agroperlite (EP AGRO, PERLIT Ltd, Šenov u Nového Jičína, Czech Republic), to produce 16 vigorous individuals per accession. At the 5–7 fully developed leaf stage, the plants were transplanted into containers filled with garden soil and cultivated under standard greenhouse conditions (day/night temperature range, 18–30/13–16°C). Drip irrigation and chemical protection against powdery mildew and spider mites were provided. The visual assessment of plants was performed at various developmental stages. Twenty quantitative and qualitative characters were assessed (Doležalová et al., Reference Doležalová, Krístková, Lebeda, Vinter, Astley and Boukema2003), eight of which were informative to define similarity/dissimilarity (Table 2). Based on the vegetative and generative characteristics, the accessions were then taxonomically verified (Feráková, Reference Feráková1977; Dostál, Reference Dostál1989; Iwatsuki et al., Reference Iwatsuki, Yamazaki, Boufford and Ohba1995).
Table 2 Eight most discriminatory morphological characteristics that determine similarity/dissimilarity of Lactuca accessions

Numbers in parentheses according to Doležalová et al. (Reference Doležalová, Krístková, Lebeda, Vinter, Astley and Boukema2003).
AFLP analysis
Seventy-eight accessions with 20 individual plants each were grown in the greenhouse, and genomic DNA was isolated in 96-well plates from leaves according to Doyle and Doyle (Reference Doyle and Doyle1990), but modified for a robotic, liquid-handling system. AFLP procedure was performed according to the following modifications: genomic DNA (100 ng) was simultaneously restricted and ligated with appropriate adapters (Table 3), with 5 U EcoRI, 1 U MseI (both from New England Biolabs, Frankfurt, Germany), 0.2 pmol EcoRI adapters, 2.0 pmol MseI adapters, 2.0 pmol NaCl, 50 μg/ml BSA, 1 × ligase buffer and 0.2 U ligase (Invitrogen, Karlsruhe, Germany). Restriction/ligation products were diluted ten times in TE buffer. Preselective amplification was performed in two steps: first with primers with two bases, and then with three selective bases (Table 3). Diluted, restricted and ligated DNA (3.5 μl) was added to 10 pmol EcoRI and MseI primers, 200 pmol dNTP, 2.25 nmol Mg(OAc)2, 1 × PCR buffer and 0.3 U Taq polymerase (Eppendorf, Hamburg, Germany). After each PCR, the template was again diluted at a ratio of 1:50 in TE. For the selective amplification, three primer combinations were used (Table 3). The products of the three selective amplifications were pooled and fragment analysis was performed on the MegaBACE 1000 sequencer (Amersham Biosciences Europe, Freiburg, Germany), following its genotyping protocol.
Table 3 List of primers and adaptors used

Primer information kindly provided by Keygene N.V., Wageningen, The Netherlands.
Data analysis
A binary matrix was created from genotyping data (peak present/absent) with Fragment Profiler 1.2 software (Amersham Biosciences). The number of polymorphic loci, Nei's original measures of genetic identity and genetic distance and genetic diversity (G st) were calculated using POPGENE software v1.32 (Yeh and Boyle, Reference Yeh and Boyle1997). Jaccard's coefficient of similarity, the neighbour-joining (NJ) tree and unweighted pair group method with arithmetic mean (UPGMA) tree were calculated by NTSYS 2.1 software (Rohlf, Reference Rohlf2002). The NJ tree was used for multiple-species analysis, since different species can have different evolutionary rates, and UPGMA was used for identifying within-species duplication. The same software was used to perform principal coordinate analysis (DCENTER and EIGENVEC procedures). An analysis of molecular variance (AMOVA) was performed with WINAMOVA 1.55 software package (Excoffier et al., Reference Excoffier, Smouse and Quattro1992). Variance components were tested for significance by a non-parametric re-sampling approach using 1000 permutated datasets. For random choice of plants in testing influence of plant number reduction on variance components, a table of 2000 random digits was used (Weir, Reference Weir1996).
Results
AFLP and morphological analysis of examined accessions
In total, 357 peaks in the range from 70 to 415 base pairs were identified, with a G st value of 0.49. The number of polymorphic fragments per accession generated by one primer combination ranged from 0 (only monomorphic fragments, detected in two duplicates) to 66. Within some of the species, the morphological test indicated probably taxonomic misidentification (Table 4), based on comparisons with herbarium specimens. Most of these errors were confirmed from the AFLP analysis (Sretenović Rajičić and Dehmer, Reference Sretenović Rajičić and Dehmer2008). Pending reclassification, we have retained the existing labelling of the accessions.
Table 4 Taxonomic re-determination within the set of wild Lactuca spp. after morphological characterization

a Original botanical name in Lactuca database.
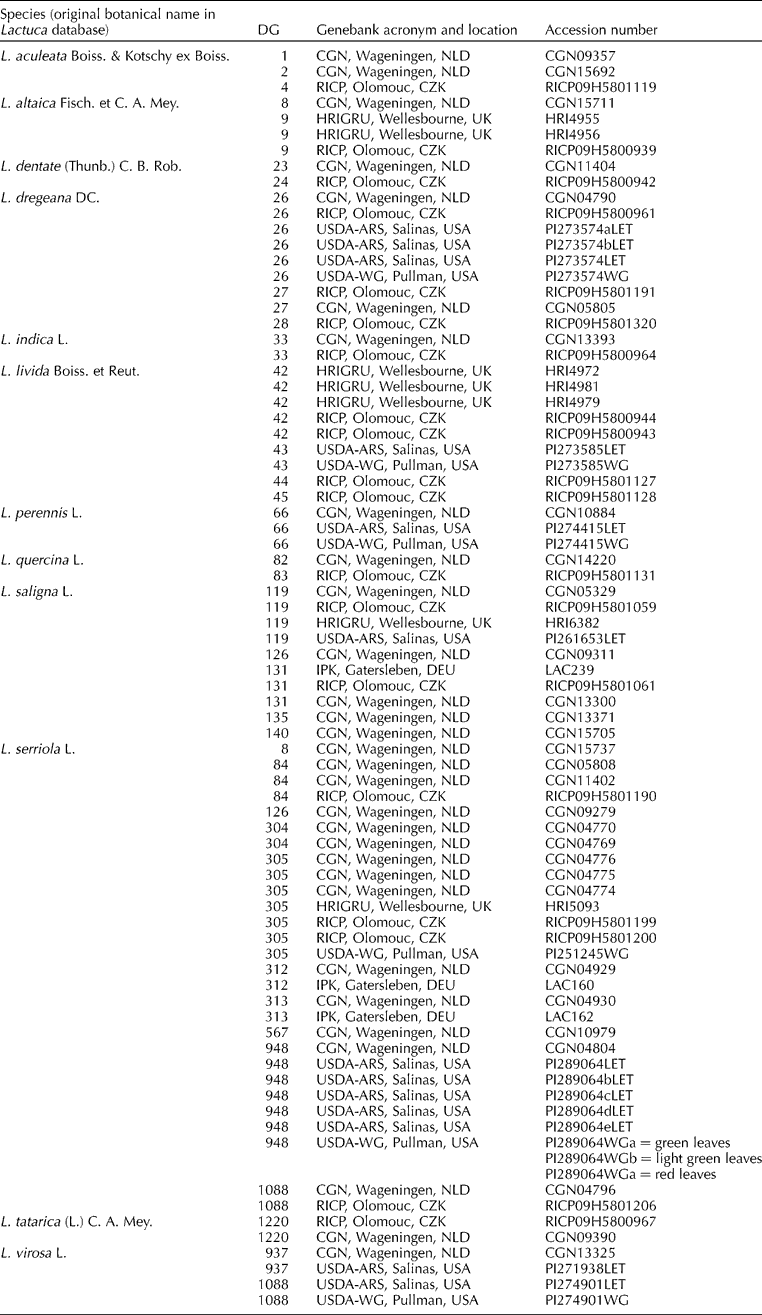
All the available genebank accessions were included in the analyses for the five rarely collected species (Lactuca aculeata, Lactuca dentata, Lactuca dregeana, Lactuca livida and Lactuca quercina). The genetic diversity within these species can be illustrated in PCO plots (Fig. 1). Within L. livida (Fig. 1a), accessions RICP09H5801127, RICP09H5801128 and HRI4979 differed from the others. The former two are morphologically L. dregeana (Table 4), while among the remaining L. livida accessions, some duplicates were found. More diversity was detected in L. dregeana (Fig. 1b): accession RICP09H5800961 was very outlying, and PI273574bLET and PI273574WG probably need to be taxonomically re-identified. The third mislabelled accession PI273574LET was grouping within L. dregeana species. The accessions of L. aculeata, L. dentata and L. quercina were genetically dispersed (Fig. 1c). Most of the diversity (more than 80%) is contained within the first two principal components (Fig. 1).

Fig. 1 PCO plots of individual accessions from five Lactuca species. (a) Lactuca livida accessions. 1, RICP09H5801127; 2, RICP09H5800943; 3, HRI4981; 4, RICP09H5800944; 5, HRI4979; 6, HRI4972; 7, PI273585LET; 8, RICP09H58001128; 9, PI273585WG. (b) Lactuca dregeana accessions. 1, PI273574bLET; 2, PI273574LET; 3, RICP09H5801191; 4, PI273574aLET; 5, CGN04790; 6, RICP09H5801320; 7, CGN05805; 8, PI273574WG; 9, RICP09H5800961. (c) acu, Lactuca aculeata (CGN09357, RICP09H5800942, CGN15692); den, Lactuca dentata (RICP09H5800942, CGN11404); que, Lactuca quercina (RICP09H5801131, CGN14220).
Redundancy determination
Seventeen accessions (covering L. livida, Lactuca saligna and Lactuca serriola) formed seven groups (Fig. 2; a more detailed analysis is given elsewhere; Sretenović Rajičić and Dehmer, Reference Sretenović Rajičić and Dehmer2008). Coefficients of similarity among those 17 accessions are presented in Table 5. Only one pair of accessions (HRI6382 and PI261653LET) showed 100% similarity on the basis of AFLP profiling.

Fig. 2 Duplication groups found after AFLP analyses presented on the NJ tree with Nei's genetic distance coefficient.
Table 5 Nei's coefficient of genetic identity (above diagonal) and genetic distance (below diagonal) among 17 highly similar accessions

Numbers on the top of each column correspond to the accessions labels given at the beginning of each row.
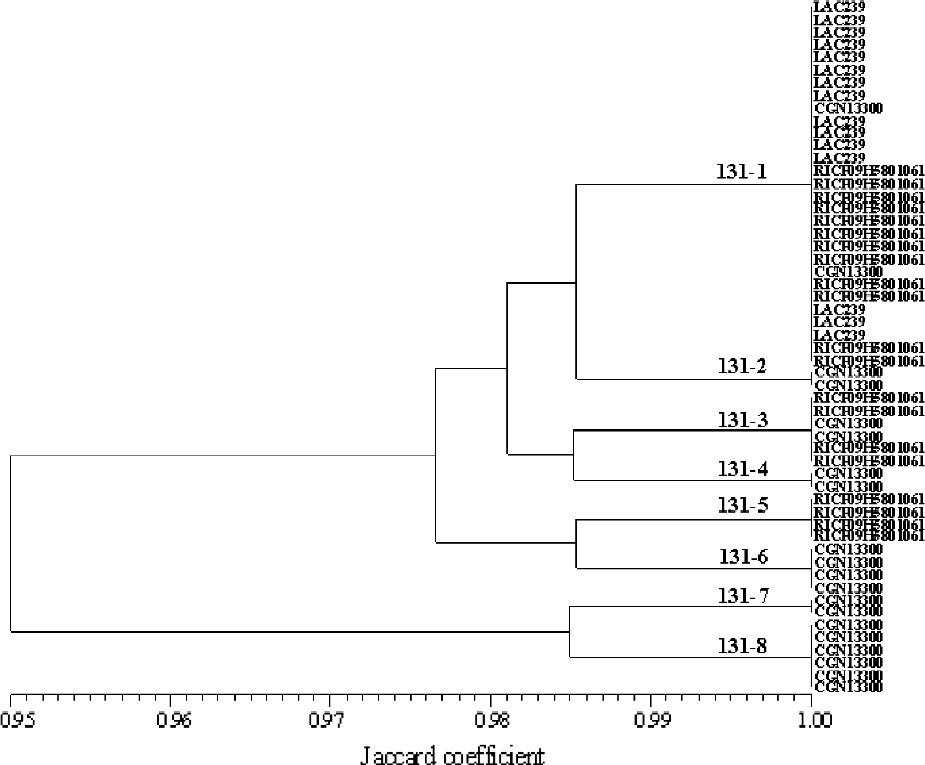
An additional layer of redundancy was determined by investigating how many distinct genotypes are present within any one accession or duplication group (Table 6). All plants that displaying the same AFLP profile were scored as an identical genotype, and these were arrayed in duplication-group-specific phenograms (Fig. 3). Genotypic variability, which should relate to accession diversity, differs widely among the duplication groups. For example, a minimum of five distinct genotypes were found within duplication group 119, and up to 18 in duplication groups 304/305 (Table 6).
Table 6 Genotypes within the duplication groups (DG) determined by AFLP analysis

Numbers of individuals per genotype are indicated in parentheses.

Fig. 3 Phenogram of genotypes found in duplication group 131, based on Jaccard's (Reference Jaccard1908) coefficient of similarity, as an example for genotypes existing within duplication groups. Genotype labels are on the branches.
Within duplication group 119, CGN05329 and RICP09H5801059 formed a pair of highly similar accessions (Nei's coefficient of genetic identity 0.999; Table 5). If the basis for duplication reduction is to eliminate all but one member of groups of accessions that have the same genotype, then accession RICP09H5801059, with three of the four genotypes present in accession CGN05329, is redundant and should be discarded. Similarly, HRI6382 and PI261653LET contain the same genotype, and one of these should be eliminated. Same approach is used for all duplication groups. Overall, therefore, one accession is probably redundant in each of the groups 42/43, 131 and 304/305, while in group 119, two of the four accessions should be conserved, and in group 312, none of the accessions are redundant (Table 6).
In all the groups except 119, within-accession variation is higher than that between groups. With a reduction in plant number from 20 to 10, the number of identifiable genotypes was reduced by at least one in four of the five groups, whereas this only occurred once when plant number was reduced from 20 to 15 (Table 7).
Table 7 Analyses of molecular variance in duplication groups: cases with different numbers of plants analysed

Reduction in number of plants has been performed randomly by choosing 10 or 15 plants (n, number of plants), according to the random numbers from the ‘tables of 2000 random digits’ (Weir, Reference Weir1996). Analyses have been performed with the two hierarchical levels: between accessions and within accessions belonging to a certain duplication group. P values are derived from permutation tests and present probability of observing larger variance components at random.
Discussion
Rarely collected species
We have particularly attempted to examine in detail some of the more rarely collected Lactuca species. Those species labelled as L. livida appear to form three separate gene pools. Duplication within one of these has most likely occurred as a result of exchange of materials among genebanks. Duplication due to exchange was also expected for the other rarely collected Lactuca species, but there was no evidence for this. In L. dentata, the two accessions were distant enough from one another not to be considered as duplicates. The grouping of L. dregeana accessions indicates that they most probably did not result from the exchange of the same material. Some accessions of L. dentata and L. quercina thought to be duplicates proved to be genotypically quite distinct from one another.
Redundancy determination
The use of molecular markers to determine the redundancy in a germplasm collection is not a trivial activity. Small differences between (and within) accessions can be expected to arise as a result of a number of reasons. First of all, there can be an error, noise, in the genotyping. For example, when duplicate samples were employed to check the robustness of the DNA profiles in an AFLP-based diversity analysis of Populus nigra, the identity level was from 96 to 100% (Winfield et al., Reference Winfield, Arnold, Cooper, Le Ray, White, Karp and Edwards1998). But still diversity that was not yet mendeled out and point mutations can cause small differences to occur. This was shown by Waycott and Fort (Reference Waycott and Fort1994) who used morphological analysis and RAPDs to identify duplicates within L. sativa, leading to similarity coefficients between nearly identical accessions of >92%.
However, since Lactuca species are mostly inbreeding with only sparse evidence of spontaneous hybrids (Lindqvist, Reference Lindqvist1960), it may be tempting to define as duplicates those accessions showing 100% similarity, as has been done for some autogamous and clonal crops (Virk et al., Reference Virk, Newbury, Jackson and Ford-Lloyd1995; McGregor et al., Reference McGregor, Van Treuren, Hoekstra and Van Hintum2002).
The question remains: what to do with accessions that are very similar but not identical. The proposed approach is to analyse the genotypes within duplication groups prior to a decision about redundancy. The accessions that are more diverse, i.e. within which more genotypes can be observed, should be retained unless there are indications that contamination has occurred. In case duplicates are identical (have the same fingerprint), the most original one according to genebank documentation, should be maintained.
From the 78 accessions that were studied, grouped into 22 duplication groups identified on the basis of passport and morphological analysis, 17 within 7 duplication groups (approximately 21% of the analyzed material) presented a similarity coefficient above 0.995. In total, only five pairs of accessions showed identical genotypes (6% of the analyzed material) and therefore could easily be considered as redundant. This allows a first reduction from 78 to 73 accessions (6.4%). In the case of L. serriola, the most common wild Lactuca species, 7 out of 28 accessions (25.0%) were highly similar to others and one (3.6%) was identical to another. Much stronger tendency towards duplication was found in samples labelled as L. livida, where three out of seven accessions (42.8%) were highly similar and one was identical (14.3%). These results imply considerable redundancy in the tested material.
Implications for genebank management and conservation
The presented molecular findings allow some recommendations about wild Lactuca conservation in genebanks. First of all, given the number of wrongly classified material, the taxonomic status of all accessions should be verified by experts in this field. Second, to avoid further duplication of genebank material, the global diversity across genebanks should be assessed prior to the planning of future collection activities, as was also suggested by Guarino et al. (Reference Guarino, Ramanatha Rao and Reid1995).
Duplication analysis can hint at problems in genebank management. Reproduction cycles with suboptimal regeneration and maintenance conditions might cause slight deviations in the genetic structure of accessions. If comparison of material regenerated at different sites shows that the diversity after regeneration changed at only one site, then the maintenance system of that respective site should be examined more closely.
Reduction of redundancy improves the cost efficiency of conservation, but will also introduce the risk of losing low frequency but potentially important diversity (Van Hintum et al., Reference Van Hintum, Boukema and Visser1996; Van Treuren et al., Reference Van Treuren, Van Soest and Van Hintum2001). With 20 plants in the sample, the probability of observing a genotype that occurs with a frequency of 0.10, 0.05 or 0.01 is 0.88, 0.64 and 0.18, respectively. If the number of plants is reduced to 15, these probabilities decrease to 0.79, 0.54 and 0.14, respectively. Similar considerations are valid in regard to the number and kind of markers applied: diversity of important traits might not be sampled by the marker system used; the higher the number or polymorphism of the marker system used the larger the chance of detecting differences between accessions. However, as noted before, differences between and within accessions are expected to occur, and decisions about redundancy have to be based on the scale of these differences.
On top of this are economic considerations; does the investment in the redundancy analysis pay off in terms of savings of capacity or increased access? Redundancy that exists in wild Lactuca germplasm consumes significant capacity available for the preservation of these accessions. Tracing and reducing such redundancy can, however, consume even more capacity. When appropriate data are available for reduction of redundancy, this should, obviously, be done. However, investments in tracing these redundancies should be weighed against the saving resulting from these investments.
In any case, it is therefore important to avoid duplication of germplasm prior to the inclusion of accessions in the genebank, whenever possible.
Acknowledgements
This work is a part of the EU-funded GENE-MINE project (QLK5-CT-2000-00 722), and the work of Palacký University was partly supported by the grant MSM 6198959215. The authors would like to thank Drs Eva Křístková and Ivana Doležalová, Department of Botany, Palacký University in Olomouc, Czech Republic, for taxonomic determination and morphological evaluation of Lactuca accessions; Keygene N.V., Wageningen, The Netherlands, for providing information about the primer combinations; and to all genebanks mentioned above, for providing seeds. We would also like to thank to Dr Elena Potokina, School of Biosciences, University of Birmingham, UK, and Drs Frank Blattner and Andreas Graner, IPK, Gatersleben, Germany, for fruitful discussions.