Introduction
Visceral leishmaniasis (VL), a parasitic disease caused by the protozoan Leishmania infantum, found on the American continent, is considered one of the most important zoonoses, and dog is an animal reservoir of the parasite of great epidemiological importance in the urban environment (Dantas-Torres, Reference Dantas-Torres2007; Lukes et al., Reference Lukes, Mauricio, Schönian, Dujardin, Soteriadou, Dedet, Kuhls, Tintaya, Jirku, Chocholová, Haralambous, Pratlong, Oborník, Horák, Ayala and Miles2007). It has high morbidity and mortality and is of great importance in the context of One Health as it mainly affects humans and domestic dogs (WHO, 2018).
A laboratory diagnosis for dogs must be assertive, and tests with high accuracy assume a crucial role in preventing false-negative animals from perpetuating the biological cycle of the parasite. For screening canine visceral leishmaniasis (CVL), the DPP® immunochromatographic rapid test (RT) is used in epidemiological surveys by environmental surveillance agencies due to its easy handling, quick results, disposability and use of samples that can be collected with a lancet from the tip of the ear, tail or paw (Coura-Vital et al., Reference Coura-Vital, Ker, Roatt, Aguiar-Soares, Leal, Moreira, Oliveira, Machado, Maria Morais, Corrêa-Oliveira, Carneiro and Reis2014; Brazil, 2016a). When the availability of DPP® RT is limited due to insufficient production to meet the national demand, the ALERE® brand RT can be used. Currently, both involve the use of recombinant K28 proteins derived from proteins of the kinesin family (Souza-Filho et al., Reference Souza Filho, Barbosa, Figueiredo, Mendes, Da Silva, Coelho and Marcelino2016). In 2014, a recombinant protein, rKDDR (kinesin degenerated derived repeat), was developed; its optimized version, called rKDDR-plus, consists of approximately 15 repetitive motifs of 39 amino acids of the kinesin protein, increasing the immunogenic potential of the original (Dhom-Lemos et al., Reference Dhom-Lemos, Viana, Cunha, Cardoso, Mendes, Pinheiro, Siqueira, Lobo, Teles, Bueno, Guimarães-Carvalho, Bartholomeu and Fujiwara2019; Siqueira et al., Reference Siqueira, Viana, Reis Cunha, Rosa, Bueno, Bartholomeu, Cardoso and Fujiwara2021).
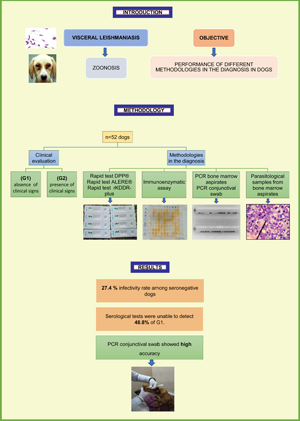
The municipality of Lavras, located in the state of Minas Gerais, Brazil, started recording cases of CVL at the end of 2013 and of human VL in 2017 and was, thus, classified as a recent transmission area (Brazil, 2016b; Lavras, 2019). Our research group has been conducting epidemiological investigations with vectors and human and canine cases of VL. This study aimed to evaluate the effectiveness of different methods for conducting the laboratory diagnosis of CVL with rK28 and rKDDR-plus proteins using molecular and parasitological tests as a reference standard in samples from dogs segregated by the presence of clinical signs (PCSs) or the absence of clinical signs (ACSs) of VL in the municipality of Lavras and the surrounding cities.
Materials and methods
Study area and sample selection
This study was carried out between August 2018 and January 2019 in the municipality of Lavras and the surrounding cities, located in the southern area of the state of Minas Gerais, Brazil.
The non-probabilistic sample consisted of 52 dogs selected without distinction of race and sex, excluding those that were under the age of 1 year and/or in a clinical state in which bone marrow puncture could pose a risk to the animal's health and those that were being treated with immunosuppressive medication. Owners who voluntarily permitted their dogs to participate in the research signed a consent form approved by the CEUA of the Federal University of Lavras (UFLA).
Procedure flowchart
The dogs received a numerical code generated by software to eliminate potential biases in the identification of the samples that could influence the results of the laboratory tests. All selected dogs underwent the following procedures: external clinical evaluation and separation into groups based on the ACSs or PCSs; collection of venous blood and plasma separation for conducting immunochromatographic RTs (DPP®, ALERE® and an RT with the rKDDR-plus antigen) and an immunoenzymatic assay (IEA) with the rKDDR-plus antigen; bone marrow puncture for direct parasitological examination and conventional polymerase chain reaction (PCR) and conjunctival swab collection for conducting conventional PCR.
Clinical evaluation
All dogs underwent clinical evaluation by a veterinarian to assess their general condition and check for the presence of possible external clinical signs that could be associated with CVL. The verified signs were those adopted according to the Ministry of Health's VL Surveillance and Control Manual (Brazil, 2014).
The animals were grouped based on the ACSs or PCSs. A score (1 or 2) was used to classify the animals according to the assessment of their clinical signs. Dogs that attained a final total score of 0–3 points were classified as having no clinical signs, and those that had a final total score of >3 points were classified as having clinical signs, as shown in Table 1. The classification of animals into those that did or did not present with clinical signs with a score is based on other previous studies that assessed the accuracy of CVL diagnostic tests (Quinnell et al., Reference Quinnell, Courtenay, Davidson, Garcez, Lambson, Ramos, Shaw, Shaw and Dye2001; Borja et al., Reference Borja, Coelho, De Jesus, De Queiroz, Celedon, Zachin, Silva, Ferreira, Krieger, Veras and Fraga2018).
Table 1. Standardized score values for clinical signs related to CVL and classification as to the final score

a Final score 0–3: animal with no clinical signs. Final score >3: animal with the PCSs.
Conducting immunochromatographic tests
After centrifugation and separation of blood cells, plasma collected from the dogs was used to perform the immunochromatographic tests. The DPP® and ALERE® RTs, both with the K28 protein, and the RT with the rKDDR-plus protein were carried out according to the manufacturers' specifications.
IEA with rKDDR-plus antigen
The protocol used for the enzyme immunoassay was standardized according to Dhom-Lemos et al. (Reference Dhom-Lemos, Viana, Cunha, Cardoso, Mendes, Pinheiro, Siqueira, Lobo, Teles, Bueno, Guimarães-Carvalho, Bartholomeu and Fujiwara2019), differing only in the dilution of the antibody conjugated with peroxidase, which was 1:2500 in the current study.
The cutoff point was calculated using the area under the curve through the construction of the receiver operating characteristic curve using the plasma of uninfected dogs (according to the negative parasitological examination and bone marrow PCR) as the negative control. As a positive control, plasma of the dogs with confirmed L. infantum according to parasitological examination and bone marrow PCR was used.
Direct parasitological examination of bone marrow puncture
Four smears were prepared on a microscopic slide with the bone marrow aspirates from all dogs. After drying the smears, the slides were stained with the haematological dye Panotic Rapid® (Laborclin, Brazil) following the procedure described by the manufacturer. A minimum of 200 fields were observed under an optical microscope in the immersion objective for the observation of Leishmania amastigote forms.
DNA extraction
DNA was extracted from bone marrow samples according to the procedures described in the ‘Genomic DNA from tissue’ user manual from the NucleoSpin® Tissue kit (Macherey-Nagel, Germany), with modifications only in the DNA elution phase, where 50 μL ultrapure water, rather than 100 μL buffer BE solution from the kit, was added to the microtube, and the incubation time at room temperature was changed to 10 min.
For DNA extraction from the conjunctival swab, a DNeasy Blood & Tissue® kit (QIAGEN, Germany) was used.
After extracting the DNA from the bone marrow and swab samples, it was measured in a spectrophotometer at 260/280 nm and 260/230 nm ratios.
Conventional PCR
Each sample had its DNA adjusted to a concentration of 20–100 ng μL−1. The reactions were conducted according to the protocol of Lachaud et al. (Reference Lachaud, Marchergui-Hammami, Chabbert, Dereure, Dedet and Bastien2002). For PCR, specific primers for the subgenus Leishmania were used, which target the DNA of the minicircle kinetoplast (kDNA) of the organisms (Table 2). The microtubes were placed in an automatic thermocycler with the following thermal cycle conditions: initial denaturation at 94°C for 5 min; followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s and extension at 72°C for 30 s; then final extension at 72°C for 7 min.
Table 2. Primers used in PCR reactions with their respective amplified product sizes and sequences

Source: Adapted from CARDOSO et al. (Reference Cardoso, Bento, De Almeida, De Castro, Reis-Cunha, Barbosa, De Souza, Brasil, Valdivia and Bartholomeu2019).
The samples were subjected to electrophoresis on a 2% agarose gel stained with ethidium bromide (0.5 μg mL−1), using a 100 base pair molecular weight standard. DNA extracted from promastigote forms of L. infantum (MHOM/BR/74/PP75) and ultrapure water were used as a positive control and a negative control, respectively.
Samples with negative results in PCR in bone marrow samples, but with positive results in PCR with a conjunctival swab were subjected to the restriction fragment length polymorphism (RFLP) technique for identification of species. Conventional PCR was carried out using the initiators (LITSR: 5′CTGGATCATTTTCCGATG3′ and L5.8S: 5′TGATACCACTTATCGC ACTT3′) addressed to the ITS I (internal transcribed spacer I) target at 10 μ m. The samples were digested with the enzyme ‘HaeIII’ (10 U μL−1) (Promega, USA). The digestion reaction solution was prepared for a final volume of 15 μL containing 1 μL ‘HaeIII’ (10 U mL−1), 1.5 μL enzyme buffer, 2.5 μL ultrapure water and 10 μL PCR product. The mixture was incubated at 37°C for 2 h and the restriction profiles were analysed on 4% agarose gel and compared with the following Leishmania profiles: L. amazonensis (IFLA/BR/67/PH8), L. braziliensis (MHOM/BR/75/M2903) and L. infantum (MHOM/BR/74/PP75).
Reference standard
The reference or gold standard for the evaluation of the diagnostic tests consisted of the visualization of amastigote forms of the parasite in the bone marrow smear; the presence of Leishmania sp. DNA as detected by PCR in bone marrow samples and the presence of Leishmania sp. DNA as detected by PCR in conjunctival swab samples.
The animal was considered uninfected (true negative) when all tests included in the reference standard were negative and infected (true positive) when at least one positive result was obtained from any of the standard reference tests.
Data and statistical analysis
Each diagnostic test was evaluated to obtain its sensitivity, specificity and accuracy. For the analysis of the agreement between the diagnostic tests, the kappa test was used, and the values were interpreted according to Landis and Kockh (Reference Landis and Koch1977).
For comparison between the absolute frequency of positivity of the diagnostic tests with the reference standard, the differences were assessed by using the McNemar test and considered statistically significant when P < 0.05.
Results
In the current study, 59.62% (n = 31) of the animals had physical clinical signs compatible with CVL. Among the dogs in the group with no clinical signs (n = 21), 52.4% had a score of 0; that is, none of the clinical signs given in Table 1 was present in these dogs according to the evaluation of the veterinarians.
It was possible to verify the presence of rounded shapes, measuring approximately 3.0 μm and demonstrating nuclei and kinetoplasts, compatible with Leishmania amastigotes in only six of the smears prepared from the bone marrow of the animals.
Of the 52 dogs included in the current sample, 50 were classified as infected and two as not infected according to the results of the standard reference tests.
The main parameters used to assess the performance of the different techniques used in the diagnosis of CVL are shown in Table 3. Among all the immunographic techniques, the DPP® RT showed the highest sensitivity (76%) and accuracy (76.9%). All serological tests showed lower sensitivity values when evaluated the group with no clinical signs (ACS) (42.9–57.1%) than in the group with clinical signs (PCS) (79.3–93.1%). Direct parasitological examination of the bone marrow showed very low values of sensitivity and accuracy for all groups evaluated. The molecular techniques showed high-sensitivity and accuracy values for all groups evaluated, and PCR with bone marrow aspirate samples showed higher values compared with PCR with conjunctival swab samples. All techniques showed 100% specificity.
Table 3. Parameters for evaluating the performance of various techniques for the diagnosis of VL in 52 dogs from the municipality of Lavras and the surrounding cities

RT, rapid test; IEA, immunoenzymatic assay; PCR, polymerase chain reaction; ACS, absence of clinical signs; PCS, presence of clinical signs; SENS, sensitivity; CI, confidence interval; AC, accuracy.
All serological tests for the 52 dogs showed an agreement classified as mild by the McNemar test, and the differences in the absolute frequencies of positivity between the tests and the reference standard were all statistically significant. For the serological tests, only the DPP® RT showed a greater agreement with the reference standard in the PCS group. In this same group, the frequency of positivity between the tests and the reference standard was not significantly different, except for the ALERE® RT.
Among the tests comprising the reference standard, PCR with bone marrow aspirate samples showed a substantial agreement and even perfect agreement when evaluated only for dogs presenting with clinical signs. The P values obtained with the McNemar formula indicated that among the tests comprising the reference standard, only the direct parasitological examination yielded a significant difference.
When the McNemar test was carried out for the ACS group, a statistically significant difference in the frequency of positivity between the tests and the reference standard was found for all tests except for the molecular techniques, in which the P value obtained was >0.05.
According to the compatibility of the band pattern obtained in the gel electrophoretic analysis of the RFLP technique, it was possible to verify only the L. infantum species among all tested samples.
Among the dogs in the studied sample, three found in the municipality of Nepomuceno/MG, a city close to the municipality of Lavras, were diagnosed with VL and confirmed with samples sent to the state's public reference laboratory, FUNED (Fundação Ezequiel Dias), which identified L. infantum using the RFLP technique. The dogs were reported to the competent environmental surveillance bodies as the first autochthonous cases of CVL in the municipality of Nepomuceno.
Discussion
The correct diagnosis of CVL is fundamental for the adoption of preventive and control measures such as canine vaccination, euthanasia and treatment. Public control policies directed towards the different municipalities will vary according to the classification each receives in terms of the number of reported cases, which also depends on a correct diagnosis.
In the current study, the diagnosis of CVL was based on the detection of the parasite with different techniques that composed of a rigorous reference standard, which improves the reliability of the results. Thus, through the adopted diagnosis, it is possible to evaluate the characteristics of the sampled dogs and the effectiveness of the different laboratory diagnostic techniques.
The adoption of a score for classifying groups of animals with or without clinical signs helps reducing subjectivity in the classification, increasing the reliability of the group designations. In the current study, only signs physically observed during clinical evaluation were adopted, as these are the signs that can be perceived by the guardian of the dog. Thus, if the animal does not show any clinical signs, its guardian would not seek veterinary medical care. Other studies have also adopted classification into groups only by assessing physical signs compatible with CVL (Quinnell et al., Reference Quinnell, Courtenay, Davidson, Garcez, Lambson, Ramos, Shaw, Shaw and Dye2001; Grimaldi et al., Reference Grimaldi, Teva, Ferreira, Santos, Pinto, Azevedo and Falqueto2012; Laurenti et al., Reference Laurenti, Santana Leandro, Tomokane, De Lucca, Aschar, Souza, Silva, Marcondes and Da Matta2014; Borja et al., Reference Borja, Coelho, De Jesus, De Queiroz, Celedon, Zachin, Silva, Ferreira, Krieger, Veras and Fraga2018).
When evaluating the performance of the rKDDR-plus protein, the sensitivity and accuracy of the RT and IEA were unsatisfactory, with low agreement in relation to the reference standard. Better results were obtained in the group presenting with clinical signs; however, the agreement was still considered moderate. Siqueira et al. (Reference Siqueira, Viana, Reis Cunha, Rosa, Bueno, Bartholomeu, Cardoso and Fujiwara2021) also evaluated the effectiveness of an IEA with the rKDDR-plus protein in the diagnosis of CVL. The selected dogs were not evaluated for clinical status. High-sensitivity (96.67%) and accuracy (97.22%) values were obtained for the IEA, with an excellent agreement with the reference standard. The difference in relation to the current study is that in the Siqueira study, the samples were obtained from a serum bank, where there was already previous knowledge of the results (intrinsic validation), whereas in the current study, there was no sample tracking, and the results of the true positives and negatives were obtained after the application of the reference standard tests, featuring an exploratory methodology (extrinsic validation).
In the evaluation of the performance of the different techniques in the studied samples, the DPP® RT showed a higher sensitivity and accuracy compared with the other RTs. The ALERE® RT showed the lowest sensitivity and accuracy and the lowest agreement in the group of dogs with clinical signs, showing a statistically significant difference in the frequency of positivity with the results of the reference standard.
It appears that the RTs provide heterogeneous data, and thus it is necessary to be careful and rigorous when choosing an RT for screening. Overall, the sensitivity of all immunochromatographic RTs was considered low. When a screening test has a relatively low sensitivity value, the number of false-negative results can be high, when ideally, the screening test should be able to detect all cases of the disease (Franco and Passos, Reference Franco and Passos2005).
When the sensitivity was assessed in the two groups, the performance was even lower for the dogs without clinical signs, with an average accuracy of 49.24%, differently from the group with clinical signs, which presented an average accuracy of 87.07%. This means that approximately 51% of infected dogs without clinical signs cannot be detected by RTs. This fact is very important because dogs that do not present with clinical signs may also have high transmissibility, indicating their role in the maintenance and spread of the parasite in endemic areas (Laurenti et al., Reference Laurenti, Rossi, Da Matta, Tomokane, Corbett, Secundino, Pimenta and Marcondes2013), although most studies suggest that only a small proportion of dogs with symptomatic infection and high parasitic load on the skin is responsible for the majority of transmission events for sandflies (Courtenay et al., Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002, Reference Courtenay, Carson, Calvo-Bado, Garcez and Quinnell2014). Thus, there is no consensus on the exact relevance of the clinical landscape in the parasite transmission cycle, and it is prudent to consider that dogs that do or do not present with clinical signs can be considered a source of infection for vectors and that both must be correctly diagnosed so that the actual prevalence of the infection is considered in the adoption of control measures by public policies (Ribeiro et al., Reference Ribeiro, Michalick, Da Silva, Dos Santos, Frézard and Da Silva2018).
We cannot infer whether an infected dog is sick. As a rule, asymptomatic dogs are parasitized, but may not have developed the disease yet. In areas endemic for CVL, the prevalence of infection is greater than that of the disease, and the percentage of seronegative-infected dogs is relatively high (Solano-Gallego et al., Reference Solano-Gallego, Morell, Arboix, Alberola and Ferrer2001). However, these non-sick infected dogs can act as a reservoir, perpetuating the cycle, and/or evolving into a clinical picture of VL.
Peixoto et al. (Reference Peixoto, De Oliveira and Romero2015) conducted a systematic review and meta-analysis with articles published until February 2013 on serological diagnosis for CVL and demonstrated that the values for sensitivity found in the articles were 83.5% for the DPP® RT, which was higher than that found in the current study (76%). Among the methodological problems identified in this review, the majority of studies used dogs previously screened for the presence or absence of the infection through other serological tests, overestimating their accuracy. This study differs from the studies in the above review in the use of molecular tests to classify dogs as true positive and true negative. When another serological test is used as the gold standard for assessing the performance of the DPP® RT, to obtain positive results, seroconversion is required in the dog, which can take an average of 3–5 months and can generate a false negative if the animal has not produced detectable antibodies or even in cases of anergy or immunosuppression, even if the animal is already infected (Moreno and Alvar, Reference Moreno and Alvar2002).
A major challenge for diagnostic test validation studies for CVL is the definition of the reference standard. Traditionally, parasitological techniques have been employed, but following the emergence of molecular techniques, infection can be detected in animals that have not yet been diagnosed by parasitological and serological examinations. Thus, so long as adequate samples, well-designed primers and standardized methodologies are implemented, these techniques can be used to compose the reference standard, as they can overcome the limitations of serological and parasitological diagnoses.
Similar to the current study, Lopes et al. (Reference Lopes, Sevá, Ferreira, Nunes, Keid, Hiramoto, Ferreira, Oliveira, Bigotto, Galvis-Ovallos, Galati and Soares2017) used molecular tests as the reference standard (PCR with blood, lymph node and conjunctival swab samples) and compared their results with those from the DPP® RT for 975 dogs in an endemic region, concluding that one in five seronegative dogs was infected. This result is in accordance with those of the current study, where it can be observed that the RTs failed to detect the infection in an average of 27.4% of infected dogs. Teixeira et al. (Reference Teixeira, Silva, Vital, Nitz, De Carvalho, Hecht, Oliveira, Oliveira, Rabello and Romero2019), who also included molecular tests as the reference standard, found that the sensitivity values in several serological tests, such as the DPP® RT (21.74%), Biomanguinhos IEA (11.59%) and enzyme-linked immunosorbent assay rK39 (37.68%), were too low.
In the current study, performance was evaluated separately for each technique that composed of the proposed reference standard. The results helped justify the use of more than one technique as the gold standard. If only the parasitological technique was used as the gold standard, for example, more than 80% of infected dogs would not have been diagnosed. The combination of two different samples to assess the performance of PCR increased the sensitivity of the reference standard, because some dogs yielded negative PCR results with the bone marrow aspirate but positive results with the conjunctival swab. This can be due to the difficulty in collecting bone marrow, where aspiration can be accompanied by a large volume of blood, influencing the quantification of extracted DNA because studies have already demonstrated the low sensitivity of PCR with blood samples (Quaresma et al., Reference Quaresma, Murta, Ferreira, Da Rocha-Lima, Xavier and Gontijo2009).
Although PCR with the bone marrow sample was more sensitive according to the results of current research, it requires more invasive collection and an experienced professional to perform the procedure. This is a limitation for its use in the field and in epidemiological surveys.
On the contrary, the collection of cells from the conjunctival mucosa is not invasive, it can be easily performed in the field, and, according to the current research, it showed higher values of sensitivity compared with the serological tests, with no statistically significant difference in the frequency of positivity with the reference standard, even for the group presenting with no clinical signs. Lombardo et al. (Reference Lombardo, Pennisi, Lupo, Migliazzo, Caprì and Solano-Gallego2012) also presented satisfactory results for the diagnostic accuracy of PCR with conjunctival swabbing, corroborating the results of the current research. Ferreira et al. (Reference Ferreira, Leite, Ituassu, Almeida, Souza, Fujiwara, De Andrade and Melo2012), in a study investigating 80 dogs naturally infected by L. infantum, obtained higher frequencies of positive results by conjunctival swabbing than by blood collection and bone marrow aspiration and concluded that the ‘conjunctival swab’ can yield a clinical sample suitable for the qualitative molecular diagnosis of CVL regardless of the clinical status of the dog.
The use of PCR with conjunctival swabs as a screening test can serve as an alternative in the diagnosis of animals without clinical signs of VL to the use of serological methods, which are the techniques recommended by the Ministry of Health today.
This was the first exploratory study on the extrinsic evaluation of tests for the diagnosis of CVL in the municipality of Lavras, which used molecular techniques as a reference standard.
According to the results, further research should assess the prevalence of CVL infection in Lavras and its micro-region with a larger sample size through the use of conjunctival swab PCR, given the limitations and difficulties of bone marrow aspiration.
This research reported the first confirmed cases of CVL in the municipality of Nepomuceno, neighbouring the Lavras territory, demonstrating the spread of the disease.
Further studies with xenodiagnosis and/or specific and sensitive markers of infectivity are still needed to ascertain the true role of asymptomatic dogs in the transmission to vectors.
The identification of the species according to the polymorphisms of DNA fragments found only L. infantum in the selected samples (those that showed positive results with PCR using conjunctival swabs but negative results using marrow aspirations). This identification was essential for excluding the possibility of dogs being parasitized by other species of the subgenus Leishmania. Dogs that had positive PCR results with bone marrow samples were not included for identification of species because it is more likely that L. infantum is present in this type of sample and because the primer used in the PCR is specific for the subgenus Leishmania. However, this cannot be stated confidently, because some studies have reported possible visceralization of another species of the subgenus Leishmania in a lymph node, L. amazonensis (Tolezano et al., Reference Tolezano, Uliana, Taniguchi, Araújo, Barbosa, Barbosa, Floeter-Winter and Shaw2007).
The results obtained reinforce existing concerns with the use of serological tests to identify dogs lacking clinical signs, given that this methodology is the main tool used to control CVL in Brazil. The results of this study reinforce the need to improve screening tests to assist in resolving diagnoses and establishing control measures.
Acknowledgements
The authors acknowledge the Veterinary Hospital of UFLA for allowing the procedures to be carried out and the partnerships with the Institute of Biological Sciences of the Federal University of Minas Gerais (UFMG) and Lavras City Hall.
Author contributions
Carolina Novato Gondim is the main author and participated in all stages of the study. Joziana Muniz de Paiva Barçante and Sidney de Almeida Ferreira conceived and designed the study. Beatriz Ketelin Sousa Vasconcelos and Flademir Wouters conducted sample collections on the dogs. Ricardo Toshio Fujiwara and Joseane Camilla de Castro contributed to the implementation of the study methodology techniques.
Financial support
This study was financed by the National Council for Scientific and Technological Development (CNPq); the Coordination for the Improvement of Higher Education Personnel (Capes) and by the federal universities UFLA and UFMG. JCC has a PhD fellowship provided by the Capes and Post-graduation Program in UFMG. BKSV is a research fellow from the CNPq and graduation in UFLA.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
A minimum number of animals was used to produce statistically reproducible results. All procedures that contributed to this study are in accordance with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals in accordance with the provisions of Law 11794 of 8/10/2008 and of Decree 6899 of 15/07/2009 and with the standards issued by the National Council for the Control of Animal Experimentation (CONCEA) of the Ministry of Science, Technology and Innovation (MCTI). The Ethics Committee on the Use of Animals (CEUA) of the UFLA approved the project on 22/12/17, as stated in certificate 77/17.