INTRODUCTION
Following infection by parasites, hosts often display several changes in their behavioural repertoire (Holmes and Bethel, 1972; Combes, 1991). These behavioural changes can result from adaptations of the host to resist or reduce the effects of its parasite, from adaptations of the parasite to enhance its transmission rate to its next host (=host manipulation hypothesis), or lastly from the purely pathological side-effects of the infection (see Moore, 2002 for a review). Since the formulation of the ‘host manipulation’ hypothesis (Holmes and Bethel, 1972) a multitude of changes in host behaviour following parasite infection have been attributed to parasite adaptation, often without rigorous testing (Poulin, 1995, 2000). In order to promote rigor in this field of parasitology, Poulin (1995) proposed a series of four criteria that a behavioural modification should satisfy before being qualified as manipulation of host behaviour by the parasite. First of all, the putative manipulation should increase parasite transmission (Tompkins, Mouritsen and Poulin, 2004). This criterion (I), which is the most important, has obviously to be fulfilled and if not, the hypothesis of host manipulation should be rejected. Second, the putative manipulation of host behaviour should be complex, because complex traits are unlikely to have arisen by chance during evolution (II). Third, it should fit the design that an engineer might specify for a particular function (III). Lastly, it should have evolved independently several times during evolution (IV). Contrary to the first criterion, not fulfilling the three other criteria only decreases the likelihood that the behavioural alteration is a true parasite adaptation but does not lead to a rejection of the hypothesis. Thus it appears necessary, as far as possible, to test as many informative criteria as possible to improve the reliability of our conclusions. Here we propose another criterion that can help, together with the ones proposed by Poulin (1995), to evaluate the likelihood of the manipulation hypothesis: the behavioural modification should specifically affect the behavioural component that is relevant for parasite transmission, keeping other parts of the host behavioural repertoire unchanged. Again, fulfilling this criterion increases the likelihood of the manipulation hypothesis, whereas not fulfilling it does not reject this hypothesis.
For instance, consider a typical parasite with a multi-host life-cycle, one intermediate host where the putative behavioural manipulation occurs (cancellation of the escape behaviour) and a definitive host that becomes infected through predation of the intermediate host. If one can demonstrate that, let's say foraging ability, habitat selection and sexual activity of the intermediate host are not affected by the parasite whereas escape behaviour is only cancelled when the suitable definitive host is present, this would strongly support the manipulation hypothesis. On the contrary, if foraging, habitat selection and sexual activity are strongly perturbed by infection, and that escape behaviour is cancelled whatever the predator (suitable or not for parasite development), then this would reduce the likelihood of the manipulation hypothesis. Instead, it is more likely that this parasite induces several side-effects with no adaptive significance when infecting this intermediate host. Note, however, that the strengh of this prediction is weakened if alleles involved in the manipulative process (selected in the parasite genome) have a pleiotropic effect on other host behaviour and/or if the parasite could also affect behaviours that are neutral regarding parasite transmission.
Here, we tested whether this criterion was met in a host-parasitoid system in which the parasitoid can be infected with viral particles that modify its behaviour. The Drosophila parasitoid Leptopilina boulardi (Barbotin et al.) is a solitary species, meaning that only one wasp offspring can successfully develop in one host. However, females infected by filamentous viral particles (LbFV for Leptopilina boulardi Filamentous Virus) showed a very high propensity to lay supernumerary eggs in already parasitized hosts (‘superparasitizing’ phenotype), whereas uninfected females seldom did (‘non-superparasitizing’ phenotype) (Varaldi et al. 2003, 2005a). Fifteen to 90% of wild-caught females from the French populations studied so far showed the typical ‘superparasitizing’ phenotype (measured under standard laboratory conditions), suggesting a very high prevalence of infection (S. Patot, unpublished results). This modification apparently makes sense for the virus because, within superparasitized Drosophila larvae, viral particles can be horizontally transmitted (about 55% of successful transmission) from infected embryos to uninfected ones (Varaldi et al. 2003, Varaldi et al. 2006). However, the fitness payoff from such a modification is not obvious since viral particles can also be transmitted from mother to offspring (with a very efficient rate although <100%), leading to a trade-off between horizontal and vertical transmission. Nevertheless, theoretical modelling allowing derivation of the optimal superparasitism strategy (rate at which females should accept to lay an egg in an already parasitized host) from both the parasitoid and virus viewpoints showed that the virus is always selected for increasing female's natural superparasitism tendency (which is not null, see Van Alphen and Visser, 1990), suggesting that the modification of this behaviour (everything else being equal) indeed benefits the virus (Gandon et al. 2006). The criterion of an increase in parasite transmission (criterion I) proposed by Poulin (1995) thus appears to be fulfilled in this system. Since the mechanisms underlying the behavioural modification are still unknown, the complexity criterion (II) remains difficult to assess. This behavioural modification shows purposeful design (increasing superparasitism tendency is the best way for the virus to colonize new parasitoid lines), fulfilling the third criterion. Finally, the convergence criterion (IV) has not been tested yet, but will be investigated later (does other unrelated virus infecting parasitoids induce the same change in superparasitism?). We have shown elsewhere (Varaldi et al. 2005a) that viral infection has a slight positive effect on egg load, no effect on offspring sex-ratio and female survival, a low negative impact on developmental duration and male survival, and a stronger negative effect on locomotor activity.
In this paper, we tested the specificity of the behavioural modification induced by the virus on its parasitoid host's behaviour by studying the effect of viral infection on several behavioural components of the parasitoid that are not directly linked to viral horizontal transmission: organization of circadian activity, perception of host volatils and trajectometric paths of females. Furthermore, testing this criterion requires knowledge of all possible transmission modes of the virus. We thus tried to estimate whether viral particles could have other horizontal transmission routes that could modify our interpretation of the viral modification of wasp behaviour. Finally, since investigations on infected individuals that are not able to transmit infection can be informative on parasite strategy, we conducted experiments on males (which do not transmit viral infection, Varaldi et al. 2003). We have some indications that males are also infected, since males from infected lines suffer costs on developmental time and survival compared to males from uninfected lines (Varaldi et al. 2005a). Males from infected and uninfected lines were compared for their activity rhythms and their ability to perceive female pheromones.
MATERIALS AND METHODS
Strains, rearing and experimental conditions
To investigate the specificity of viral horizontal transmission and the specificity of the behavioural modification induced, we need to compare lines that differ only in the presence or absence of the viral particles. Starting from an uninfected and inbred line (8 generations of brother-sister mating, 82% of homozygosity, origin: Sienna, Italy), we derived an infected line by injecting in the Drosophila larvae ovary extracts containing the superparasitism inducing virus (Varaldi et al. manuscript in preparation). Thus, comparisons were made using 2 parasitoid strains sharing the same genetical background, but either infected (‘superparasitizing’ phenotype, S) or uninfected (‘non-superparasitizing’ phenotype, NS). Experiments on locomotor activity were performed on 2 other lines, also originating from Sienna, either infected (S) or not (NS), and also sharing the same nuclear background, but obtained in a different way. Briefly, the non-superparasitizing NS uninfected line was obtained by repeatedly crossing NS females with S males during 5 generations of back-crosses. The introgressed line conserved NS phenotype demonstrating the maternal transmission of the trait (mean number of eggs per parasitized host±S.E., introgressed line=1·00±0·00, n=15, infected line=3·23±0·33, n=14, P<0·0001). The infection status of each line was checked once, using electron microscopy (Varaldi et al. manuscript in preparation) as no molecular tool is available for the moment. Drosophila melanogaster larvae fed with standard diet (David, 1962) were used as hosts for experiments and rearing (25 °C).
Impact of viral infection on the perception of host kairomones by females
Drosophila parasitoids are known to use aggregation pheromones released by Drosophila adults to localize their hosts (Hedlund, Vet and Dicke, 1996). These pheromones are emitted during mating and oviposition. In this experiment, our aim was to test whether viral infection modifies parasitoid females' perception of such Drosophila pheromones (called kairomones since here they are used by another species, for its own benefit). Patches of agar (6 mm diameter and 3 mm deep) coated with baker's yeast were marked by these kairomones by placing 5 patches in contact with 20 adult Drosophila (10 males+10 females 4–5 days old) in a vial (2·6 cm diameter and 10·1 cm length) for a night (19.00 to 09.00). Control patches were also made by placing them in the same conditions without Drosophila. Patches were used the same day they were obtained. Any Drosophila eggs that were laid were removed before the experiment (see Komeza et al. 2001 for details).
For the experiments, an isolated female (NS or S) was placed on a glass plate where 2 patches (one with kairomone and one control) were deposited, and covered with a circular plastic dish (2·5 cm diameter, 1 cm height). The position of each patch was noted and the trajectory of the female was recorded for 8 min with an automatic video-tracking system (see Komeza et al. 2001 for details). In total, 18 NS females and 21 S females (1 or 2 days old) were individually tested in a climate chamber (T=23 °C). We defined an individual choice index as the proportion of time spent on the marked patch of total time spent on both patches. This choice index was calculated over the 8-min period, and also every minute, to follow the kinetics of kairomone perception. Since this index could not be calculated for some time intervals (when the female spent no time at all on any patch during 1 min), we were not able to use repeated measure ANOVA on this index. Instead, classical ANOVA was used (time, infection status and interaction). The proportion of time spent outside both patches was also calculated over the 8-min period, and also every min. This last statistic was analysed using repeated measure ANOVA. Data were arcsine square root transformed prior to statistical analysis.
Impact of viral infection on trajectometric parameters of parasitoid females
Characteristics of walking paths are an important feature that influence the fitness of foraging females (Chassain and Boulétreau, 1987; Bell, 1990; Wajnberg and Colazza, 1998) and can be subject to modifications when the central nervous system is perturbed, for instance by sublethal doses of insecticide (Delpuech et al. 1998). Using trajectory data collected in the previous experiment, we compared walking paths of infected and uninfected females by calculating the distance covered during the 8-min period (cm), the proportion of time spent immobile during the 8 min, the mean duration of pauses (s), the linear speed (cm/s) and the angular speed (deg/s).
Impact of viral infection on locomotor activity and activity rhythms
Individual locomotor activity rhythms were monitored using a video-tracking and image analysis system that allowed automatic continuous measurement of 120 insects over several days (see Allemand et al. 1994 for details). Individuals were isolated in experimental circular glass arenas (diameter 1·5 cm) without hosts but with honey as food (temperature 23 °C). The position of arenas containing individuals from infected or uninfected lines was randomized over the system. Females were 3–4 days old and males 4–5 days. The locomotor activity of each individual was quantified every 3 min by binary data (1 if the wasp had moved during a 2-sec video-recording and 0 if not). Hourly activity was calculated as the proportion of active recordings among the 20 records/h. Pictures were taken under infrared light, to which insects are not sensitive, allowing for observation also in the dark. Individuals (30 of both sexes) from infected and uninfected lines were studied 2 days under LD 12[ratio ]12 (white light from 08.00 to 20.00). The daily pattern of activity was studied for each individual by calculating the mean proportion of active recordings/h (rate of locomotor activity RLA). The plot of RLA against time was used to study the temporal organization of activity. From this study, it was clear that 2 major peaks of activity (around 07.00 and 14.00) were detectable for almost every individual (males and females). The timings of these 2 peaks were calculated for each individual to characterize and test the overall rhythmical organization of activity. RLA were arcsine square-root transformed prior to repeated measure ANOVA analysis. To simplify plotting of RLA profiles, we chose to calculate the average confidence interval (aci) of rhythm profiles which is the mean of confidence intervals of all points of each curve (Fleury et al. 2000).
Specificity of horizontal transmission
It has been shown that some viral particles infecting lepidopteran hosts can be transmitted from one host to another through viral contamination of parasitoid ovipositors (Hochberg, 1991; Lopez et al. 2002). L. boulardi, like every Drosophila parasitoid, needs to sting a Drosophila larva to assess whether or not it is parasitized (see Van Alphen and Vet, 1986), leading to potential contamination of the ovipositor. An NS female that stings and rejects an S parasitized host may thereafter infect its own offspring. Another possible transmission route for the virus would be through food sharing between infected and uninfected females. Four experiments were designed to test these hypotheses. First we tested the possibility of horizontal transmission without hosts, through contact between NS and S adults feeding on the same food (‘food sharing’). We then tested the possibility of horizontal transmission through contact of the ovipositor with Drosophila larvae previously parasitized by S females (‘hosts sharing’). For each experiment, food or hosts were either given sequentially to S first and then NS females (‘sequential exploitation’) or at the same time (‘simultaneous exploitation’).
Simultaneous exploitation
Pairs of mated females (1 S, 1 NS) 2 to 5 days old and marked with acrylic paint, were enclosed in a Petri dish (diameter 5·5 cm) filled with agar and a yeast spot in the presence of a drop of honey (‘simultaneaous food sharing’) or in the presence of 20 first-instar Drosophila larvae (‘simultaneous hosts sharing’). Females were kept enclosed for 48 h. Thereafter, NS females were individually placed on a batch of unparasitized Drosophila larvae so that they could lay their eggs, in order to obtain the offspring of each NS female. Four and 5 pairs of S and NS females were used respectively for ‘simultaneous food sharing’ and ‘simultaneous hosts sharing’.
Sequential exploitation
Pairs of mated females (1 S, 1 NS) 2 to 5 days old, were sequentially (S female from 13.00 to 18.00, NS female from 18.00 to 10.00 next day) enclosed in a Petri dish (diameter 5·5 cm) filled with agar and a yeast spot in the presence of a drop of honey (‘sequential food sharing’) or in the presence of 20 first-instar Drosophila larvae (‘sequential hosts sharing’). After removal of the S female and before adding the NS female, 2 Drosophila larvae were taken in each Petri dish and dissected to ensure that hosts had been parasitized by the S female. This was indeed the case (all 88 collected larvae were either parasitized or superparasitized). Just after introduction of the NS female, we checked that all females started foraging, ensuring that they will sting the parasitized hosts previously parasitized by S females. After S and NS passages, NS females were individually placed on a batch of unparasitized Drosophila larvae so that they could lay their eggs, in order to obtain the offspring of each NS female. 21 and 23 pairs of S and NS females were used respectively for ‘sequential food sharing’ and ‘sequential hosts sharing’.
Estimation of the success of horizontal transmission
The success of the potential contamination was determined using the superparasitism phenotype of the offspring of each NS female tested, as no molecular tools are available (neo-infected offspring are expected to express the ‘superparasitizing’ phenotype). Female offspring (1 or 2 days old) of each NS female were individually provided with 10 Drosophila larvae from 17.00 to 10.00 (as described by Varaldi et al. 2003), and their superparasitism behaviour was estimated by dissecting 3 Drosophila larvae from each batch. This set-up ensures that females encounter each host several times (Varaldi et al. 2005b). Superparasitism behaviour of each female was quantified as the mean number of parasitoid eggs laid per Drosophila larva (excluding Drosophila larvae without any parasitoid egg). Superparasitism phenotypes of 66, 76, 117 and 133 female offspring were scored in the ‘simultaneous food sharing’, ‘simultaneous hosts sharing’, ‘sequential food sharing’ and ‘sequential hosts sharing’ experiments, respectively, in addition to those of S and NS controls (n=22 and n=36 respectively). Experiments were all conducted at 25 °C under LD 12[ratio ]12 (light from 08.00 to 20.00).
Comparison of sexual pheromone perception by males from infected or uninfected lines
To estimate the effect of infection on the perception of sexual pheromones by males, we used an automatic video-tracking system that allowed us to follow the trajectory of males (from infected/ uninfected lines) in a 3 cm diameter by 1 mm deep arena. Arenas were made in punched Plexiglass sheets covered on both sides with a glass sheet. For each arena, a piece of filter paper was sandwiched between the glass and the Plexiglass plate. Arenas were divided in the middle by a silicon bar (chemically inert). For each arena, a virgin NS female (1–2 days old) was allowed to explore one half of the arena for 5 min. After removing the female and the silicon bar, a male (from S or NS line, 1–3 days old) was placed in the arena and its path was recorded for 8 min with a computerized video-tracking device (for details see Delpuech et al. 1998). Video-tracking was performed in a climate chamber (T=23 °C) on 28 ‘NS’ males and 40 ‘S’ males. To characterize the perception of female pheromones by males, we calculated the proportion of time spent in the marked half of the arena during the whole 8-min period for each male, and also the same proportion, calculated on 20-sec intervals, to visualize the kinetics of pheromone perception. These last data were analysed using repeated measure ANOVA. Data were arcsine square root transformed before statistical analysis.
RESULTS
Impact of viral infection on the perception of host kairomones by females
Infected and uninfected females spent the same amount of time ouside patches (Table 1). Both proved able to perceive host kairomones since they spent more time on the marked patch than on the control one (Table 1). Choice indices of S and NS females were statistically similar. Kinetics of this index (Fig. 1) revealed that the choice remains almost constant over time (F7,271=0·7311, P=0·65) for both S and NS females (interaction: F7,271=0·51, P=0·82). Although in most cases the choice index was higher in S than NS females, this effect was not significant (F1,271=2·12, P=0·14). Kinetics of proportion of time spent outside patches (Fig. 1) was very similar for S and NS females (time: F7,259=10·41, P<0·0001, parasitoid line: F1,37=1·18, P=0·28, interaction F7,259=0·97, P=0·45).

Fig. 1. Kinetics of choice index (time spent on marked patch/time spent on both patches) over an 8-min period (non-connected points) and proportion of time spent outside patches (connected points).
Table 1. Choice index (time spent on marked patch/time spent on both patches) and proportion of time spent outside patches calculated over an 8-min period (Comparison of choice indices with the theoretical value 0·5 (no choice) is significant for both (* P<0·01; ** P<0·001). Mean±S.E., n=number of females tested.)

Impact of viral infection on trajectometric parameters of parasitoid females
Data collected in the previous experiment were also used to determine features of the walking paths of S and NS females. All trajectometric parameters were not significantly different between S and NS females (all P>0·30, Table 2).
Table 2. Comparison of trajectometric parameters of uninfected (NS) and infected (S) females during an 8-min period (Mean±S.E.)

Impact of viral infection on locomotor activity and activity rhythms
Firstly, the activity of both male and female parasitoid was very rhythmical (time effect: females: F23,1081=27·3, P<0·0001, males: F23,1219=66·5, P<0·0001; Fig. 2). Infection resulted in reduced rate of locomotor activity (RLA) for females (F1,46=6·75, P=0·012) whereas males had very similar RLA whatever their strain of origin (F1,52=0·01, P=0·92). Although RLA was reduced in infected females, daily activity pattern was not affected (Table 3, Fig. 2A). For both infected and uninfected females, a peak of activity was observed just before the light phase and another one, less pronounced, at the beginning of the afternoon. Clearly, males had very similar locomotor activity profiles whatever their strain of origin (Table 3, Fig. 2B).

Fig. 2. Mean curves of locomotor activity rhythms of Leptopilina boulardi females (A) and males (B) from infected (S) and uninfected (NS) strains. Shaded and unshaded areas represent the night (from 20.00 to 8.00) and the day (from 8.00 to 20.00) respectively. aci=average confidence interval (see Materials and Methods section).
Table 3. Daily peaks of locomotor activity for males and females from uninfected (NS) and infected (S) strains (Means±S.E. (sample size). F-statistics test the effect of strain origin (NS/S) on the timing of morning and afternoon peaks obtained from a repeated measures ANOVA (sex by sex). The time effect (morning versus afternoon) was, as expected, highly significant for each sex (P<10−15).)

Specificity of horizontal transmission
In these experiments, we investigated the possibility of viral horizontal transmission through food sharing (by feeding on the same food) or hosts sharing (by attacking the same hosts) when the resource (food or hosts) was provided either simultaneously or sequentially to S and NS females. First of all, as expected, S and NS controls showed a clear-cut difference in their superparasitism behaviour (Fig. 3, mean number of eggs/parasitized hosts±S.E.: NS=1·05±0·03, S=2·69±0·20, Wilcoxon test w=26, P<0·0001). From a total of 392 female offspring tested in all modalities (‘simultaneous food sharing’, ‘simultaneous hosts sharing’, ‘sequential food sharing’, ‘sequential hosts sharing’), none of them expressed the superparasitizing phenotype (Fig. 3). However, in the ‘sequential hosts sharing’ experiment, 2 females showed a slight tendency to superparasitize. We thus allowed them to parasitize hosts in order to obtain their offspring. In each female's offspring, all females tested (n=5) had the typical NS phenotype (mean number of eggs/parasitized hosts±S.E.; 1·00±0·00 and 1·04±0·04), suggesting that their respective mothers were in fact not infected. The remaining 390 females tested clearly displayed the typical NS phenotype (Fig. 3). These results suggest that horizontal transmission did not occur in any modality.

Fig. 3. Test of the horizontal transmission of the virus by food or hosts sharing. Superparasitism phenotype was measured on S and NS controls, and in the offspring of NS females that shared food with S females or attacked the same hosts as S females during their adulthood (simultaneously or sequentially). The superparasitism phenotype was individually measured by calculating the mean number of eggs/parasitized larva.
Comparison of the perception of sexual pheromones by males from infected and uninfected lines
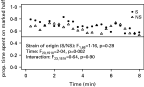
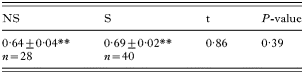
Both males from uninfected and infected lines spent more time in the marked half of the arena (that the virgin female had explored) than in the control half (Table 4), suggesting that they both perceive female pheromones, as shown in other parasitoid species (Delpuech et al. 1998). ‘S’ and ‘NS’ males spent the same proportion of time on the marked half of the arena. Furthermore, for both groups, the proportion of time spent on the marked area decreased with time but in the same way (Fig. 4).

Fig. 4. Dynamics of male preference through an 8-min follow up. The repeated measure anova output is presented on the figure.
Table 4. Proportion of time spent by ‘S’ and ‘NS’ males in the arena half marked with virgin female pheromones over an 8-min period (Comparison with the theoretical value 0·5 (no choice) is highly significant for both (** P<0·001). Mean±S.E. (N).)

DISCUSSION
The main aims of the present study were to test whether superparasitism behaviour is the only behavioural component modified by LbFV infection of L. boulardi and whether the already demonstrated contamination between wasp immatures within Drosophila hosts (Varaldi et al. 2003) is the only route for horizontal transmission of LbFV.
In the field, most parasitoid species exploit patchily distributed hosts (Van Alphen, Bernstein and Driessen, 2003; Varaldi et al. 2005b). In such environments, females are likely to be in close vicinity with competitors on the same patch of hosts, and this situation is frequent in L. boulardi (personal observation). We tested whether uninfected females can get contaminated by consuming the same food as infected females or by stinging the same hosts as infected females. We did not find any evidence of horizontal transmission in spite of the relatively high sample size (a total of 392 potential contamination was tested). Although this does not completely exclude the possibility of adult contamination, it clearly indicates that it is highly infrequent, if present. This result should be in contrast to the high infectious power of the virus under superparasitism conditions (competition between S and NS immature stages within a Drosophila host, probability that NS becomes infected±S.E.=0·55±0·16, Varaldi et al. 2006) which remains the unique documented way of horizontal transmission (Varaldi et al. 2003). This result is a key point in the evolutionary interpretation of the behavioural alteration (superparasitism). Indeed, an adaptive interpretation of viral modification of superparasitism behaviour would have been weakened if ovipositor contamination had occurred. In this case, selective pressures that act on the virus for inducing superparasitism behaviour would probably be reduced (depending on the efficiency of ovipositor contamination). This absence of ovipositor contamination is opposed to other studies which showed that viral particles infecting some lepidopteran larvae can be mechanically transmitted within lepidopteran populations through contamination of ovipositors of their parasitoids (Hochberg, 1991; Lopez et al. 2002; Stasiak et al. 2000).
On the other hand, we found that viral-induced changes in behaviour were restricted to superparasitism acceptance. What proximal mechanism allows viral particles to specifically change this behaviour? Little attention has been paid to the factors that mediate behavioural changes and most of the existing investigations concern mammals-macroparasites (Kavaliers, Colwell and Choleris, 1999) or arthropods-macroparasite systems (Helluy and Thomas, 2003; Thomas et al. 2003, 2005). Although our data do not provide any insight on this aspect, they tell us that this mediation is rather specific in the L. boulardi/virus system. Precise localization of viral particles in sensory receptors involved in host dicrimination could be an efficient way to obtain such specificity. Indeed, in most parasitoids, females mark the host they have parasitized through injection of chemicals produced in reproductive accessory glands (Van Lenteren, 1981; Quicke, 1997). Upon a new encounter with a parasitized host, the female must pierce the host wall with its ovipositor to be able to perceive the sanitary status of the host. Thus, this recognition process probably involves chemoreceptors located on the ovipositor. In L. boulardi, 2 types of sensillae have been reported of which one is a chemoreceptor innervated by 5 dendrites (Le Ralec, 1991). Viral particles may interfere with the normal functioning of these sensillae. An alternative hypothesis is that infected females would be unable to properly integrate such a signal into their central nervous system, or that infected females properly integrate the signal, but the virus modifies their subsequent decision (reduced threshold for accepting superparasitism, for instance).
In this study, we choose to incorporate behavioural components that are relevant to wasp fitness (activity rhythms, orientation to kairomones and searching path). First, behavioural rhythms can be ecologically and evolutionary significant, as already reported in the Drosophila/parasitoid community. In this community, temporal segregation of activities between parasitoid species appears to be driven by competitive ability. Indeed, inferior competitors show earlier activity, thus reducing their disadvantage in cases of multiparasitism (Fleury et al. 2000). Second, it is well known that females locate their hosts through the use of chemical cues released by host habitat (Ignacimuthu, Wackers and Dorn, 2000; Kester and Barbosa, 1994; Van Alphen and Vet, 1986; Vet et al. 1998) or by hosts themselves (immatures; Shaltiel and Ayal, 1998; Vet et al. 1993; or adults; Hedlund et al. 1996). Obviously, the ability to locate hosts through olfactory cues is an important trait for parasitoid fitness. Lastly, the path followed by a foraging female is also directly linked to its fitness, as it will determine the size of the area searched and consequently the number of hosts encountered per unit of time (Wajnberg and Colazza, 1998). None of these behavioural components appear to be modified by viral infection, with the exception of the rate of locomotor activity, which is probably reduced due to the energetic cost of infection. However, temporal organization of activity is unaffected. This overall low level of ‘pathogeny’ (on other behavioural components than superparasitism) appears to be well suited to viral transmission. Indeed, both vertical and horizontal transmissions require that females have efficient foraging behaviours to locate hosts (unparasitized or parasitized). Morevover, it is accepted that superparasitism behaviour can be an adaptive strategy when competition for hosts is strong (Van Alphen and Visser, 1990). Consequently, since only superparasitism behaviour is affected by viral infection, conditions where superparasitism is advantageous can lead to higher fitness of infected females. Hence, the evolutionary outcome of the association can probably change according to local competition for hosts (Bronstein, 1994; Thomas et al. 2000).
Conclusion
Our data suggest that LbFV cannot contaminate new parasitoid lines through food sharing of infected and uninfected females or through contamination of female ovipositors. Superparasitism thus remains the unique way for horizontal transmission of LbFV. In addition, we show that LbFV increases the superparasitism behaviour of females while it does not influence several other behavioural components of females. Importantly, a theoretical model allowing derivation of the optimal superparasitism strategy for both the parasitoid and the virus has shown that increasing superparasitism tendency of infected females enhances viral fitness (Gandon et al. 2006). Taken together, these results strongly suggest that the modification of superparasitism behaviour has been selected in the virus genome, thus constituting an excellent example of extended phenotype (Dawkins, 1982). We believe that this double approach (exploration of all possible transmission routes/of all behavioural modification induced) can be helpful in elucidating the significance of parasite-induced behaviour change.
We thank D. McKey, K. Parker and B. Pannebakker for English corrections, J. M. Delpuech for assistance with the automatic video system, D. Charif for activity figures and 2 anonymous referees for helpful comments. This work was financially supported by the Centre National de la Recherche Scientifique (UMR CNRS 5558 and GDR 2153) and the French Ministery ‘Education Nationale, de l'Enseignement Supérieur et de la Recherche, Fond National de la Science, ACI Jeunes Chercheurs 2004’.