INTRODUCTION
Environmental pollution has potential to change ecological interactions between species (Brotons et al. Reference Brotons, Magrans, Ferrus and Nadal1998; Eeva et al. Reference Eeva, Ryömä and Riihimäki2005; Lürling and Scheffer, Reference Lürling and Scheffer2007; Weis et al. Reference Weis, Bergey, Reichmuth and Candelmo2011). One such interaction could be the relationship between a parasite and its host. Host–parasite relationships are well known to interact with environmental pollution in plants (Flückiger et al. Reference Flückiger, Braun, Hiltbrunner, Bell and Treshow2002), and there are plenty of examples of such interactions in fish (e.g. Khan and Thulin, Reference Khan and Thulin1991; Williams and Mackenzie, Reference Williams and Mackenzie2003) but much less information is available on terrestrial animals. In aquatic systems parasite numbers have generally decreased in polluted environments and especially so at metal polluted sites and for ectoparasites (Blanar et al. Reference Blanar, Munkittrick, Houlahan, MacLatchy and Marcogliese2009). Avian host–parasite interactions have been intensively studied in free-living terrestrial bird populations during the past decades (Loye and Zuk, Reference Loye and Zuk1991; Clayton and Moore, Reference Clayton and Moore1997) but only rarely in the context of environmental pollution. One of the few case studies in birds reports increased ectoparasite numbers in a tree swallow population foraging at a wetland area contaminated by aromatic hydrocarbons (Gentes et al. Reference Gentes, Whitworth, Waldner, Fenton and Smits2007). Metal mining and smelting sites are typical point sources of heavy metals and sulphuric acid (Kozlov et al. Reference Kozlov, Zvereva and Zverev2009), and they have been found to have detrimental impacts on terrestrial birds at individual, population and community levels (Janssens et al. Reference Janssens, Dauwe, Pinxten and Eens2003; Belskii et al. Reference Belskii, Lugas'kova and Karfidova2005; Berglund et al. Reference Berglund, Ingvarsson, Danielsson and Nyholm2010; Eeva et al. Reference Eeva, Belskii, Gilyazov and Kozlov2012a). Case studies at such sites have shown increased abundance for some ectoparasite taxa while decreased numbers for another taxa, suggesting that responses of parasite populations to pollution are species-specific (Eeva et al. Reference Eeva, Lehikoinen and Nurmi1994; Belskii et al. Reference Belskii, Lugas'kova and Karfidova2005). Pollution–parasite interactions in terrestrial avian populations are currently poorly known and more studies are needed on variable host and parasite taxa to explore the consequences of pollution and to improve the general knowledge of the avian ectoparasites.
The aim of our study was to assess if the prevalence or intensity of avian ectoparasites are affected by long-term environmental pollution and whether such an interaction would have impacts on birds' breeding success. We counted the numbers of ectoparasitic flies in the nests of a small passerine bird, the pied flycatcher (Ficedula hypoleuca), during 7 consecutive years in the polluted sites and unpolluted control sites around a point source of heavy metals and sulphuric oxides (copper smelter). We also wanted to take into account some other potential factors that could explain the prevalence of parasitic flies in F. hypoleuca nests. On the basis of earlier studies we included timing of breeding, brood size, ambient temperature during the nestling period, habitat and population density of F. hypoleuca as possible explanatory factors in our analyses. Note, however, that all of these variables potentially interact with pollution and may partly reflect alternative pathways whereby pollution may affect bird–parasite interactions. For one breeding season we also have data on actual heavy metal exposure of F. hypoleuca nestlings. This enabled us using the fecal metal concentrations to explore direct association between metal levels and parasite prevalence. Finally we analysed the potential effects of parasite intensity on nestling survival. Since some earlier studies have shown that breeding success of F. hypoleuca is inferior in the polluted area due to resource limitations (e.g. food quality) (Eeva et al. Reference Eeva, Lehikoinen and Pohjalainen1997), we expect that further stressors such as ectoparasites may have stronger negative effect on breeding at the copper smelter.
MATERIALS AND METHODS
Study area
Our study area was the surroundings of a copper smelter in the town Harjavalta (61°20′N, 22°10′E), SW Finland. Sulphuric oxides and metals (especially As, Cd, Cu, Ni, Pb and Zn) are common pollutants in the area, due to long-term emissions from the smelter and adjoining industry (Kiikkilä, Reference Kiikkilä2003; Kozlov et al. Reference Kozlov, Zvereva and Zverev2009). Study sites (n=17) with nest boxes (20–60 boxes per site) were established since 1991 along the air pollution gradient in three main directions (SW, SE and NW) away from the copper smelter complex. A description of the nest boxes is given in Lambrechts et al. (Reference Lambrechts, Adriaensen, Ardia, Artemyev, Atiénzar, Banbura, Barba, Bouvier, Camprodon and Cooper2010). Metal emissions from the smelter have considerably decreased over the study period (for example, approximately 99% reduction in dust emissions), but still metal levels in bird feces and tissues show increased values in the vicinity of the smelter (Berglund et al. Reference Berglund, Koivula and Eeva2011, Reference Berglund, Rainio and Eeva2012). Sites closer than 2·5 km from the smelter are hereafter referred to as zone 1 (‘polluted area’) and sites over 2·5 km from the smelter as zone 2 (‘unpolluted area’), emission levels approaching the background values beyond the distance of 2·5 km (Berglund et al. Reference Berglund, Rainio and Eeva2012). Daily temperature data were collected by the Finnish Meteorological Institute, the weather station locating within our study area (Peipohja, Kokemäki, 61°16′N, 22°15′E).
Bird species and breeding parameters
Ficedula hypoleuca is a small (12–13 g), insectivorous and migratory passerine bird (Lundberg and Alatalo, Reference Lundberg and Alatalo1992). It breeds in a large range over Europe and Western Siberia and winters in Western Africa. Ficedula hypoleuca is single-brooded and the modal clutch size is 5–6 eggs. It is an abundant species that easily accepts artificial nest boxes, and thus it is a common model species in studies of avian ecology and evolution.
Nest boxes were checked at least once a week to collect basic breeding data, including hatching date, hatchling numbers and fledgling numbers. If the nest was not visited at the day of hatching the date was estimated with the wing length of small nestlings by comparing it with the growth curve drawn from the nests with exact hatching dates (TE: unpublished data). Unlike some other bird species the pied flycatchers do not seem to actively search and remove nest parasites from the nest material (own observation, based on 148 h follow-up of the parents feeding 6–11-day-old chicks; Eeva et al. Reference Eeva, Ryömä and Riihimäki2005). To reduce the among-nest box variation in parasite numbers at the start of each breeding season due to overwintering parasites (especially fleas) we removed old nest material from all the nest boxes after fledging. Nest material was spread on the forest floor near the nest box.
Sampling of parasitic fly pupae
Numbers of fly pupae were counted from 639 nests of F. hypoleuca collected 0–30 days after fledging in summers 2006–2012 (mean±s.d.: 91±16·3 nests per summer). Nests were collected randomly except that we tried to collect samples from all study sites and at different parts of each site to spread the spatial distribution of samples as much as possible. All nest material in the nest box was carefully collected and preserved in plastic bags at −20 °C until thoroughly searched for pupae of parasitic flies in the laboratory. Although some pupae were hatched before the nest collection the empty fly cocoons could be found in the nest material and were included in the total numbers. We counted the numbers of pupae in nest material for two ectoparasitic fly genera, a calliphorid fly Protocalliphora sp. and a hippoboscid fly Ornithomyia sp. (Fig. 1). Note that our data only include the nests that produced at least one fledgling, so the possible effects on nesting failure cannot be estimated. Comparing only successful nests, however, was reasonable because early failed nests may not be parasitized and later failed nests would vary in periods where infection by flies would be possible, and this would again affect the number of fly pupae in the nest.

Fig. 1. Examples of pupae of Protocalliphora sp. (a) and Ornithomyia sp. (b) collected from the nests of Ficedula hypoleuca.
The calliphorid pupae most likely consist of one species, Protocalliphora azurea (Fallén), which is the most common and widely distributed Protocalliphora (Diptera, Calliphoridae) species in Fennoscandia (Rognes, Reference Rognes1991). In a sample of pupae grown to adulthood (n=107 individuals), collected from our study sites in the beginning of the 1990s, no other species were found (Eeva et al. Reference Eeva, Lehikoinen and Nurmi1994). Adult flies are not parasitic but feed on flowers (Bennett and Whitworth, Reference Bennett and Whitworth1991). They lay their eggs in bird nests and the larvae are nest-dwelling ectoparasites that intermittently feed on nestling blood before burrowing into the nest material to pupate (Sabrosky et al. Reference Sabrosky, Bennett and Whitworth1989; Bennett and Whitworth, Reference Bennett and Whitworth1991). The adult flies eclose some weeks later and they overwinter (Bennett and Whitworth, Reference Bennett and Whitworth1991; Rognes, Reference Rognes1991).
We could not identify the hippoboscid species, but Ornithomyia fringillina (Curtis) and Ornithomyia chloropus (Bergroth) remain as most likely species on the basis of their size, distribution and ecology (Maa, Reference Maa1969; Grunin, Reference Grunin, Bei-Bienko and Steyskal1989). See Petersen et al. (Reference Petersen, Damgaard and Meier2007) for their taxonomic status. In Ornithomyia the larval development takes place within the mother's body (vivipary). The females feed on bird blood and deposit single, fully grown and immobile larvae, which pupate immediately and overwinter as the pupal stage (Corbet, Reference Corbet1956). Larvae are deposited on the bodies of nestlings and parent birds, where they fall on the nest (from nestlings and parents) or elsewhere outside the nest (from parents) (Corbet, Reference Corbet1956).
Habitat and forest floor vegetation
The forests in the area are dominated by Scots pine Pinus sylvestris L., which forms mixed stands with Norway spruce Picea abies (L.) Karsten and birches (Betula spp.). To avoid extra variation in the data due to varying habitat quality, we selected study sites of the same habitat type, i.e. relatively barren, pine-dominated forests typical of the study area. Shrubs like Vaccinium myrtillus L. and Vaccinium vitis-idaea L. dominate in the field layer, but in the polluted area ground-layer vegetation is more scanty due to long-term pollution effects (Salemaa et al. Reference Salemaa, Vanha-Majamaa and Derome2001). Since earlier studies have found associations between forest floor vegetation type and occurrence of P. azurea (Eeva et al. Reference Eeva, Lehikoinen and Nurmi1994), we classified all F. hypoleuca territories (in 50 m radius from the nest box) as barren or luxuriant by following the forest type classification of Kalliola (Reference Kalliola1973) that is based on indicator plant species. In short, territories characterized by Cladonia sp., Empetrum sp., Calluna sp. and V. vitis-idaea were classified as barren and those characterized by V. myrtillus, Oxalis sp., Maianthemum sp. and Ledum sp. were classified as luxuriant. Territories with no or scanty forest floor vegetation were included in the barren category. Therefore, it needs to be recalled that variation in habitat quality partly reflects the effects of long-term pollution.
Heavy metal analyses
In 2008 we collected fecal samples from some F. hypoleuca broods (n=48) to assess the levels of metal pollution in birds. Fecal samples have been proven to be a good tool to monitor environmental metal levels in birds (Dauwe et al. Reference Dauwe, Bervoets, Blust, Pinxten and Eens2000; Berglund et al. Reference Berglund, Rainio and Eeva2012). Fresh feces were collected from defecating nestlings at the age of 7 days directly to plastic Eppendorf tubes and dried in the laboratory at 50 °C for 72 h. Metal levels (As, Cd, Cu, Ni and Pb) were determined with ICP-MS (Elan 6100 DRC+ from PerkinElmer-Sciex) together with certified reference material (mussel tissue ERM-CE278). A more detailed description of the method and quality control is given in Eeva et al. (Reference Eeva, Rainio, Kanerva and Salminen2012b). To provide a single measure describing the variation in pollution levels, we calculated the first principal component (PC1) of the concentrations of the five heavy metals. The PC1 explained 73% of the variation in data (eigenvalue: 3·66), describing well the general level of heavy metal exposure. PC1 correlated strongly negatively with logarithmic distance to the copper smelter (r=−0·81, P<0·0001, n=48) and was used to explain ectoparasite prevalence in further analyses.
Statistics
All analyses were performed with SAS statistical software (SAS, 2008). We first analysed the annual and pollution-related variation in prevalence (0=not parasitized, 1=parasitized) and intensity (numbers/infected nests) with generalized linear models (GLM), by using year, zone and their interaction as fixed effects. In these models we used binomial error distribution for prevalence and negative binomial distribution for intensity. Second, we wanted to find out which factors could explain the prevalence of flies in F. hypoleuca nests. In these generalized linear mixed models (GLMM) with binomial error distributions we used yearly standardized hatching date (yearly mean=0 and standard deviation=1; separately for the two zones), brood size (at hatching), average daily temperature during the nestling period (14 day period, including the hatching day), field layer vegetation type (barren vs. luxuriant), and the relative numbers of breeding F. hypoleuca pairs (% nest boxes occupied by F. hypoleuca at each site) as explanatory factors. Standardization of hatching date makes the years more comparable and reduces collinearity between hatching date and temperature. Since the distances of nest boxes are relatively constant in our study sites (mean±s.d.: 34±5 m) the occupation rates correlate strongly with actual breeding density per unit area (T. Eeva, unpublished) and we use here occupation rates as a surrogate of population density. Year was included in the model as a random factor to control for the non-independence of the observations within years. Missing values in some of the explanatory variables decrease the sample size, depending on the model. Finally, we analysed the possible effects of parasite numbers (including nests with no parasites) on nestling survival with a binomial model where we used events/trials type dependent variable, events denoting fledgling number and trials denoting hatchling numbers. Year was used here as a random factor as well. Non-significant terms were dropped from all the models one-by-one, starting from interactions. They were added again in the final model one-by-one and kept if significant. For the models containing random factors the denominator degrees of freedom were calculated with Kenward–Rogers method. The significance level was set at P<0·05 in all analyses.
RESULTS
Prevalence and intensity
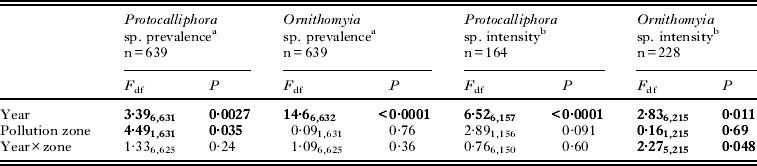
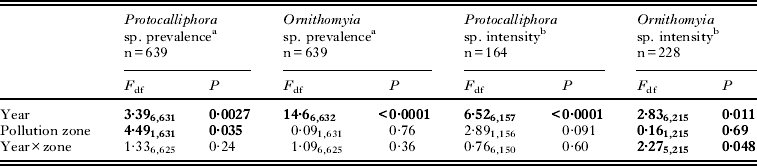
The sample sizes, annual prevalences (% infested) and intensities (numbers of fly pupae/infested nests) are shown for both ectoparasites and study zones in Fig. 2. The mean (with 95% CLs) prevalence of Protocalliphora was smaller in the polluted zone (21·4%; 16·7–26·9) than in the unpolluted zone (29·1%; 24·6–34·1) (Table 1). The prevalence of Protocalliphora pupae also varied among years (range 18–43%), but there was no interaction between zone and year (Table 1). The mean prevalence of Ornithomyia did not differ between the polluted (30·8%; 24·8–37·5) and unpolluted (32·0%; 26·7–37·8) zone (Table 1). Their prevalence still varied considerably among years (range 7·6–61%), again with no interaction between zone and year (Table 1; Fig. 2).

Fig. 2. Annual prevalences (proportion of nests parasitized±95% CL) and intensities (numbers/infected nests; means±95% CL) of pupae of ectoparasitic flies, Protocalliphora and Ornithomyia in the nests of a passerine bird, Ficedula hypoleuca, breeding in metal-polluted (near copper smelter) and unpolluted sites. Confidence limits come from the full factorial GLM models (including year and zone). The numbers above the bars indicate sample sizes.
Table 1. Generalized linear models (GLM) for the prevalence (% nests infested) and intensity (numbers/infected nests) of pupae of two parasitic fly species in the nests of F. hypoleuca during 2006–2012. Terms left in the final model are shown in bold
a GLM with binomial error distribution and logit link function.
b GLM with negative binomial error distribution and log link function.
The intensity of Protocalliphora infestation was slightly lower in the polluted (3·7 pupae/nests; 2·8–5·0) than in the unpolluted (5·1 pupae/nests; 4·2–6·2) zone, but this difference was not statistically significant (Table 1). Protocalliphora intensity varied among years (range 3·2–10·4 pupae/nests), with no interaction between zone and year (Table 1; Fig. 2). The intensity of Ornithomyia varied annually as well but their intensity further showed an annually varying pattern between the two study zones, being significantly higher in the unpolluted area in 2010 but not in the other years (Table 1; Fig. 2). The mean Ornithomyia intensity in our study area was 2·5 pupae/nest (2·3–2·8).
Factors explaining prevalence
After including the other explanatory factors (standardized hatching date, temperature during nestling period, field layer vegetation and population density of F. hypoleuca) in the model the Protocalliphora prevalence indicated a pollution-related density effect, prevalence decreasing with decreasing host population density in the polluted area while such an effect was not found in the unpolluted area (Table 2; Fig. 3a). The prevalence of Ornithomyia decreased along with decreasing host density, independent of pollution level (Table 2; Fig. 3a). Late F. hypoleuca nests (relative to the yearly mean of the population) showed much higher prevalence of Ornithomyia than the early ones (probabilities ranging from 8% to 71%) while Protocalliphora prevalence was not dependent on hatching date (Table 2). There were no significant associations between brood size and prevalence, though Ornithomyia showed a weak positive association with hatchling numbers (Table 2). Instead, the prevalence of both parasites was positively related to nestling time temperature, the probability of occurrence increasing from 16% to 42% in Protocalliphora and from 15% to 65% in Ornithomyia in the range of mean temperatures from 12 to 18 °C (Table 2; Fig. 3b). In Ornithomyia this association was further pollution-dependent, temperature increasing prevalence more in the polluted area (Table 2; Fig. 3b). The prevalence of Protocalliphora was also associated with the quality of field layer vegetation, the probability of occurrence being significantly higher in territories with luxuriant vegetation (33%; 23·9–44·2) than in less productive territories (21%; 14·3–29·2) (Table 2). This effect was not found for Ornithomyia.

Fig. 3. The prevalence (probability±95% CL) of parasitic fly pupae in the nests of Ficedula hypoleuca (n=634) as a function of (a) host density (occupation rate of nest boxes) and (b) nestling time mean temperatures. Circles: Protocalliphora; triangles: Ornithomyia. Data points are predicted values from logistic regression. For the sake of clarity the other explanatory factors (see Table 2) were omitted from the logistic regression model to plot the predicted prevalence values against density or temperature.
Table 2. Generalized linear mixed models (GLMM)a for explaining the prevalence of pupae of two parasitic flies, Protocalliphora sp. and Ornithomyia sp. in the nests of Ficedula hypoleuca (n=634) during 2006–2012. Terms left in the final model are shown in bold

a GLMM with binomial error distribution and logit link function. Year was included in the model as a random factor.
Prevalence and heavy metal levels
In the subsample of nests from 2008, we found no association between fecal metal levels (PC1) of 7-day-old F. hypoleuca nestlings and prevalence of Protocalliphora sp. (F 1,46=0·06, P = 0·81) or Ornithomyia sp. (F 1,46=1·24, P = 0·27).
Effects on breeding success
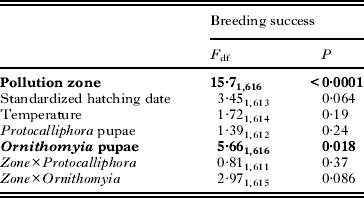
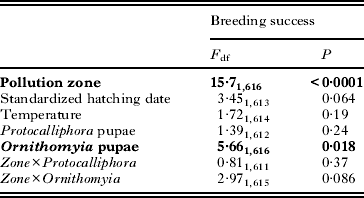
Nestling survival was slightly higher in the unpolluted zone (94%; 87·8–97·1) as compared with the polluted one (91%; 81·7–95·5) (Table 3). The number of Ornithomyia pupae had a significant, albeit weak negative effect on nestling survival, the probability of surviving decreasing 5·6% in the range of infestation from 0 to 16 pupae/nest (Table 3; Fig. 4). This association was marginally stronger in the polluted zone (Table 3). However, the variation in the numbers of Protocalliphora pupae was not associated with nestling survival (Table 3), despite a much higher range in intensity, the numbers varying from 0 to 50 pupae/nest. Mean temperature during nestling period or yearly standardized hatching dates did not significantly explain nestling survival, though the latter showed a marginally non-significant negative association with nestling survival (Table 3).

Fig. 4. The association between fledging probability (±95% CL) and number of Ornithomyia pupae in the nests of Ficedula hypoleuca (n=619). Open circles: unpolluted zone; filled circles: polluted zone. Probabilities are based on generalized linear model with zone, number of pupae and zone×n of pupae as explanatory factors (year is included in the model as a random factor, but common predicted values and confidence limits are presented for all years).
Table 3. Generalized linear mixed model (GLMM)a for explaining the breeding success (fledglings/hatchlings) of Ficedula hypoleuca (n=619) during 2006–2012. Terms left in the final model are shown in bold
a GLMM with binomial error distribution and logit link function, with events/trials type dependent variable (events=fledgling number; trials=hatchling numbers). Year was included in the model as a random factor.
DISCUSSION
The prevalence and intensity of both ectoparasites varied among years, the annual variation in prevalence being more prominent in the hippoboscid fly, Ornithomyia. The prevalence of the calliphorid fly, Protocalliphora, was further dependent on location, the nests in the polluted zone being less frequently parasitized. A similar pattern was noted for Protocalliphora in the same study area in the beginning of the 1990s (Eeva et al. Reference Eeva, Lehikoinen and Nurmi1994). Although emission levels of metals and sulphuric oxides have been considerably reduced during the past decades (Berglund et al. Reference Berglund, Rainio and Eeva2012), the difference in prevalence seems to persist, aside of yearly variation. This is not surprising because the Protocalliphora prevalence seems to be rather associated with habitat disruption than the metal levels per se. More luxuriant vegetation seems to support more ample fly populations (this study; Eeva and Lehikoinen, Reference Eeva and Lehikoinen1994), but despite reduced emissions the recovery of forest floor vegetation is a slow process (Salemaa et al. Reference Salemaa, Vanha-Majamaa and Derome2001). Our study therefore suggests that lower Protocalliphora prevalence in the polluted environment is not likely due to flies suffering from metal toxicity or due to birds suffering from metal-related deterioration of parasite defence but rather because of the suboptimal habitat for the parasite.
Adult Protocalliphora are known to feed on flowers of Vaccinium species (Bennett and Whitworth, Reference Bennett and Whitworth1991; Rognes, Reference Rognes1991). The abundance of some common dwarf shrubs in our study area, such as V. vitis-idaea, decrease towards the copper smelter together with a general decrease in vegetation coverage and number of plant species (Salemaa et al. Reference Salemaa, Vanha-Majamaa and Derome2001). Less abundant food sources might therefore decrease Protocalliphora abundance in the polluted environment. Instead, adult Ornithomyia only feed on blood (Corbet, Reference Corbet1956; Grunin, Reference Grunin, Bei-Bienko and Steyskal1989) and therefore do not show a corresponding association to forest floor vegetation quality. Our result on smaller prevalence of Protocalliphora in the polluted area is an opposite to the one observed around a Russian copper smelter (Revda), where proportion of infested F. hypoleuca chicks and intensity of infestation were higher in the polluted areas than in the unpolluted reference areas (Belskii et al. Reference Belskii, Lugas'kova and Karfidova2005). Belskii et al. (Reference Belskii, Lugas'kova and Karfidova2005), however, report that the fly larvae were found under the skin of nestlings, which is not typical for Protocalliphora, and their result likely applies to a closely related fly, Trypocalliphora sp., as was later identified from a sample collected from F. hypoleuca nestling at their study site (E. Belskii, personal communication). Different parasite species show variable trends in abundance also within our study area, since while Protocalliphora decreased and Ornithomyia generally showed no change, the numbers of ectoparasitic fleas (Ceratophyllus gallinae) increased in F. hypoleuca nests towards the pollution source (Eeva et al. Reference Eeva, Lehikoinen and Nurmi1994). Taken together, pollution-related trends are species-specific and there seems to be no general abundance pattern among avian ectoparasites in relation to environmental pollution.
Both ectoparasitic flies showed higher prevalence when nestling time temperatures were high. Being poikilothermic animals the emergence time and flying activity of these insects can be expected to be temperature-related. Senar et al. (Reference Senar, Copete, Domenech and Vonwalter1994) showed that prevalence of bird louse flies (Ornithoica sp. and Ornithomyia sp.) in adult serins (Serinus sp.) was positively correlated with preceding temperatures and they considered weather variables (temperature and rainfall) as important determinants of annual variations in prevalence. For a moose-dwelling hippoboscid species, Lipoptena cervi, it was found that low summer temperatures prolonged the developmental periods and led to reduced emergence probabilities and reduced host search times (Härkönen et al. Reference Härkönen, Härkönen, Kaitala, Kaunisto, Kortet, Laaksonen and Ylönen2010). Although bird-dwelling hippoboscids, such as Ornithomyia, supposedly experience relatively steady ambient temperatures during their on-host period we may expect that cold and perhaps rainy periods reduce their flight activity and dispersal, and may produce such seasonal and yearly variation in their prevalence that was apparent in our data. The response of Ornithomyia number to temperature was further slightly stronger in the polluted area. This may be because of slightly higher daily temperatures at polluted sites (daily maximum in June being 1·3 °C higher; T. Eeva, unpublished data) due to the relatively sparse forests enabling the ground to be more exposed to sunlight. The same explanation might apply to Protocalliphora, though for them the relationship between prevalence and temperature was much weaker (see also Rogers et al. Reference Rogers, Robertson, Stutchbury and Loye1991; Dawson et al. Reference Dawson, Hillen and Whitworth2005). Temperature-dependent annual prevalence of Protocalliphora was also noted in a F. hypoleuca population by Merino and Potti (Reference Merino and Potti1996). A positive association between prevalence and summer temperature was further observed for another parasitic calliphorid, Trypocalliphora braueri (Pavel et al. Reference Pavel, Chutný, Petrusková and Petrusek2008). Temperature dependence of ectoparasite prevalence may change host–parasite relationships along changing climate. Advanced phenology and increased prevalence and intensity of Ornithomyia were indeed recently reported in a long-term study on barn swallows (Hirundo rustica) (Møller, Reference Møller2010).
The prevalence of Ornithomyia further increased in the course of the breeding season, late broods getting parasitized more heavily. This association might partly be explained by increasing average temperatures in the course of the breeding season. However, in our data there was a great deal of independent variation between breeding times and temperatures and we believe that both factors are involved. Late summer peak prevalence seems to be typical of Ornithomyia and some other hippoboscids (Corbet, Reference Corbet1956; McClure, Reference McClure1984; Senar et al. Reference Senar, Copete, Domenech and Vonwalter1994; but see Walker and Rotherham, Reference Walker and Rotherham2010a) and higher prevalence among late breeding birds may just reflect the seasonal phenology and abundance pattern of flies but phenology of the birds may also play a role. Ornithomyia are not host specific but thrive on many bird species and also change host species (Corbet, Reference Corbet1956). For example, they parasitize the Parids (Ash and Monk, Reference Ash and Monk1959; Trilar and Krčmar, Reference Trilar and Krčmar2005; own observations) that frequently breed at our nest box sites (e.g. Parus major and Cyanistes caeruleus). Nestlings of the Parids fledge c. 2 weeks earlier than those of F. hypoleuca, and while many of the Parid broods are still in their nests during the early F. hypoleuca broods, very few remain during the late F. hypoleuca broods. Since outside the nest birds can more easily remove ectoparasites from their plumage we consider it possible that a part of the Ornithomyia flies leave their original host bird after fledging and search for other broods still in the nestling phase, such as those of later breeding F. hypoleuca. This would increase prevalence in late broods. Late broods might also show lower immune defence against parasites and consequently suffer from higher parasite load (Dubiec and Cichon, Reference Dubiec and Cichon2005).
We found that the Ornithomyia prevalence was higher when F. hypoleuca breeding density was high. When parasite transmission is a function of direct contacts, the prevalence is expected to increase with population density (Arneberg, Reference Arneberg2001; McCallum et al. Reference McCallum, Barlow and Hone2001). The host switch behaviour of Ornithomyia is poorly known and we are not aware of any other studies relating host population density and Ornithomyia prevalence. We, however, believe that dense F. hypoleuca populations could promote the transmission of flies between individuals. Ficedula hypoleuca males typically check and visit several closely locating nest boxes, a part of which are occupied by the same or different bird species. Such behaviour could increase parasite transmission in a density-dependent manner. Instead, Protocalliphora flies are not carried by birds and we did not find a general association between their prevalence and host density but the association differed between polluted and unpolluted sites, numbers decreasing with decreasing bird population density only in the polluted area. This might be due to the fact that the sites where long-term pollution has resulted in most scanty ground layer vegetation and relatively low Protocalliphora populations also tend to be less attractive for F. hypoleuca and many other forest passerines (Eeva et al. Reference Eeva, Belskii, Gilyazov and Kozlov2012a). The lack of direct association between Protocalliphora prevalence and host density was apparent also among colonial and more dispersed populations of tree swallows, Tachycineta bicolor (Rogers et al. Reference Rogers, Robertson, Stutchbury and Loye1991).
Breeding success was slightly higher in those F. hypoleuca nests that were not parasitized by Ornithomyia or only contained a few pupae. However, the numbers of pupae in infested broods were generally low in our study population, only 9% of nests having more than five pupae (or more than one pupa/nestling), though one should recall that some pupae may have been laid outside the nest. Several studies have reported negative effects of Protocalliphora infestation on blood parameters (reduced haematocrit and haemoglobin levels, increased leukocyte concentration) but generally they increased nestling mortality only when occurring in very high numbers (Pinkowski, Reference Pinkowski1977; Gold and Dahlsten, Reference Gold and Dahlsten1983; Sabrosky et al. Reference Sabrosky, Bennett and Whitworth1989; Whitworth and Bennett, Reference Whitworth and Bennett1992; Merino and Potti, Reference Merino and Potti1995; Saino et al. Reference Saino, Calza and Møller1998; Puchala, Reference Puchala2004; Hannam, Reference Hannam2006). Although Protocalliphora larvae are larger than those of Ornithomyia, acute detrimental effects have been observed only when their numbers have been much higher than in our study. Therefore we consider it unlikely that relatively low numbers of smaller Ornithomyia larvae could have had direct effects on nestling mortality via the amount of blood consumed. Instead, the association between Ornithomyia intensity and breeding success might be due to broods in poor condition being parasitized more heavily. Negative correlation between parasite numbers and offspring survival might also arise if lower-quality parents (e.g. in terms of parental care) are more heavily parasitized than good-quality parents (Gallizzi et al. Reference Gallizzi, Alloitteau, Harrang and Richner2008; Heylen et al. Reference Heylen, Adriaensen, Dauwe, Eens and Matthysen2009). Correlational and experimental studies have not found any association between the reproductive success of swifts (Apus sp.) and the numbers of parasitic hippoboscid flies, Crataerina sp., suggesting generally low virulence (Lee and Clayton, Reference Lee and Clayton1995; Tompkins et al. Reference Tompkins, Jones and Clayton1996; Walker and Rotherham, Reference Walker and Rotherham2010b). Hippoboscids, however, serve as intermediate hosts for some avian blood protozoans (e.g. Haemoproteus sp. and Trypanosoma sp.), and this way they could have indirect/delayed effects on nestling condition or mortality (Baker, Reference Baker1967; Martinez-De La Puente et al. Reference Martinez-De La Puente, Merino, Tomas, Moreno, Morales, Lobato, Garcia-Fraile and Belda2010).
In conclusion, our study shows that environmental pollution decreased the prevalence of ectoparasitic Protocalliphora via changed habitat quality but did not affect the prevalence of another ectoparasitic fly, Ornithomyia. Both ectoparasites were more common in warm breeding seasons, emphasizing the potential of climate change to modify host–parasite relationships. The prevalence of Ornithomyia was further dependent on host population density and showed a marked seasonal abundance pattern, the prevalence being highest in dense F. hypoleuca populations and late broods. We found a negative relationship between Ornithomyia number and nestling survival and this association was somewhat stronger in the polluted area, though the correlative nature of our study does not allow confirming causality of this association. In general, the numbers of ectoparasitic flies in our study population are rather low. However, despite overall weak effect of parasites on survival, the possible delayed and/or sublethal effects of these ectoparasites call for further studies. Our results suggest that pollution-related effects on avian ectoparasite numbers are species-specific and reflect habitat changes rather than direct toxic effects of heavy metals.
ACKNOWLEDGEMENTS
We thank Jorma Nurmi, Miia Rainio and all the other persons involved in the fieldwork over the past years. Veikko Rinne (Zoological Museum, University of Turku) is acknowledged for the images of fly pupae. Biology students in the University of Turku are warmly acknowledged for their contribution in sorting out the nest material. Three anonymous referees gave valuable comments on our manuscript.