Published online by Cambridge University Press: 29 May 2003
Genetically determined variation in host capacity to express resistance to a given parasite plays a major role in determining the outcome of infection. It can be assumed that the same is true of variation in parasites, but very much less is known of its influence on the host–parasite relationship. Phenotypic and genotypic variation within species of intestinal worms is now well documented, detailed studies having been made of parasites such as Ascaris in humans and trichostrongyles in domestic animals. However, the extent to which this variation affects the course of infection or the host immune response in these hosts is limited. Of the nematodes used as experimental models in laboratory rodents, detailed data on phenotypic or genotypic variation are limited to Strongyloides and Trichinella. Parasite variation is known to be subject to host-mediated selection, the emergence of anthelmintic resistance being a good example. Repeated passage has been used to select lines of parasite that survive in abnormal hosts or which show adaptation to host immunity. Experimental studies with Trichinella genotypes in mice have demonstrated the extent to which parasite variation influences the nature and degree of the host's immune and inflammatory responses, the complex interplay between immunogenicity and pathogenicity influencing both partners in the relationship. Recent studies with isolates of Trichuris muris have shown how parasite variation influences the capacity of mice to express the T helper cell responses necessary for resistance. Molecular differences between T. muris isolates have been shown in their excreted/secreted products as well as at the level of their DNA. Knowledge of the functional consequences of parasite variation will add to our understanding of host-parasite evolution as well as providing a rational basis for predicting the outcome of controls strategies that rest on the improvement of host resistance through vaccination or selective breeding.
The outcome of interactions between particular hosts and particular parasites cannot be predicted simply from knowing the species involved. A wide variety of both exogenous and endogenous factors influences the balance of host–parasite relationships and determines the degree to which the fitness of one partner in the relationship is maintained at the expense of the other. At one extreme hosts may regulate or eliminate the parasite, at the other parasites may cause severe pathology or kill the host. All endogenous influences on host–parasite relationships have a genetic component, for example those that arise from responses to altered environmental conditions, to changes in nutritional levels, to reproductive events or to concurrent infections. Particular attention has been focused on genetic factors that influence the capacity of the host to respond protectively to infection through innate and adaptive immune responses. The extensive literature on this subject illustrates the degree to which host variation is now accepted as a major determinant of the outcome of host–parasite relationships and reflects the ease with which host variation can be manipulated as a variable in experimental systems involving laboratory or domestic animals. A common corollary of experimental approaches, however, is the use of parasites that often have had a restricted origin and have been maintained as lines by routine passage, thus potentially showing limited variability. It is particularly the case with experimental studies involving helminths that the emphasis on the influence of host variation has not often been matched by a corresponding interest in the influence attributable to parasite variation. That this is an artificial situation is obvious when one considers the wide, almost global distribution of many species of worms. Such geographical distributions must be accompanied by a very large degree of genotypic and phenotypic variation within parasite populations, but we are still largely ignorant of the impact that such variation has on host protective responses to infection. However, our growing understanding of the nature of immune responses to intestinal worms and of the ways in which these responses are initiated and regulated by the molecular characteristics of the parasites concerned, provides a framework for considering the likely consequences of parasite variation. Knowledge of these consequences could have important theoretical and practical implications for the host's immune response to infection. If genotypic variation within populations is reflected in phenotypic variation in those molecules that elicit immunity (immunogens) or those that reduce or suppress immunity (immunomodulators), then this is likely to impact on the generation of host immune responses and therefore on the outcome of the host-parasite relationship. If genotypic, and consequent phenotypic, variation in worms can be influenced by selection pressures, then there is the possibility that worms may be able to adapt to immunity, whether acquired naturally or by vaccination.
Here we briefly review the evidence for genetic variation within natural populations of intestinal nematodes, describe examples where genotypic and phenotypic characteristics have been altered under selection pressure, and then focus on experimental studies which have provided data on the ways in which the complex interplay between host and parasite variation influences host immune and inflammatory responses. Since individuals vary in the effectiveness or the severity of their immune responses to infection, host immunity can be broadly categorized as weaker or stronger. Equally, as parasites vary in their capacity to elicit or modulate host responses, they can be categorized as being more or less immunogenic. The interactions between these host and parasite categories is discussed in relation to data from work with two genera of intestinal nematodes Trichinella and Trichuris.
Genotypic variation within natural populations of intestinal nematode species has been most intensively studied in Ascaris spp. (reviewed Anderson, Blouin & Beech, 1998), in trichostrongyles of domestic ruminants (reviewed Gasser & Newton, 2000) and in Trichinella spp. (see e.g. Zarlenga et al. 1999) using a variety of sequencing and PCR-based techniques. This work, and a number of other studies, has identified very considerable degrees of genotypic variation even within comparatively small population samples. For example, in 265 Ascaris taken from humans and pigs in two Guatemalan villages, Anderson, Romero-Abal & Jaenike (1995) recovered 42 distinct mitochondrial genotypes. Parasites with the same genotype occurred more frequently within particular individuals than chance predicted, suggesting either that these infections arose from ingestion of clustered eggs passed by individual females or that particular worm genotypes were more successful in establishing mature infections. Hawdon et al. (2001) similarly found evidence of considerable genotypic diversity in Necator americanus recovered from individuals living in four villages in China.
The extent of genetic variation within human intestinal nematodes will be influenced by the degree to which the parasites of individual hosts represent isolated populations, and the degree to which individuals exchange parasites. Both of these will be affected by migration, movement and intermixing between human populations in endemic areas. The situation with nematodes of domestic animals may be more complex because of the more extensive mass movement of livestock. Work with Ostertagia ostertagi in cattle showed that more than 98% of nucleotide diversity was found within populations (Blouin et al. 1992), a pattern repeated for Haemonchus contortus and Trichostrongylus circumcincta in sheep, but not found in a trichostrongyle nematode from deer (Blouin et al. 1995). Grant & Whittington (1994), using RFLP analysis, found extensive genetic variation both between and within laboratory and field strains of Trichostrongylus colubriformis. Interestingly, the laboratory strain, which one might have predicted to be less diverse, proved to be as polymorphic as the field strain. Hoekstra et al. (1997), using a PCR-based microsatellite approach, similarly found extensive genetic diversity within populations of Haemonchus contortus taken from four geographical regions.
The genus Trichinella is one of the most widely distributed of all nematodes. Although members of the genus show a remarkable degree of morphological similarity there are good grounds for considering that they fall into ten distinct genotypes, seven of which have been given species status (Murrell et al. 2000). The different genotypes show considerable diversity in terms of host range and in their biological characteristics (Kapel, 2000). Variation within and between the genotypes has been measured by a variety of PCR techniques using both random and specific primers (from internally transcribed spacers, mitochondrial and ribosomal DNA, specific antigens and microsatellites). Variation has been recorded within isolates of particular genotypes (e.g T. spiralis, T. pseudospiralis and T. nelsoni) in several geographically distinct localities (Nagano et al. 1999; Wu et al. 1999; La Rosa & Pozio, 2000; La Rosa et al. 2001).
Extensive intra-specific genetic diversity has also been recorded, using a PCR-RFLP technique, in populations of Strongyloides ratti collected from the UK, the major diversity being found within sub-samples of the total population (Fisher & Viney, 1998). Diversity in Strongyloides is also seen in phenotypic life history characteristics. Chehresa, Beech & Scott (1997) described considerable phenotypic diversity in lines of Heligmosomoides polygyryrus that had been isolated from a starting laboratory population. This variation was reflected in different rates of establishment, development and reproduction. These examples, and many others in the literature, demonstrate that populations of intestinal nematodes do show considerable intraspecific genotypic and phenotypic variation.
Although there has been a number of studies concerned with phenotypic responses to selection, detailed analysis of the genetic correlates of the selection process is limited to the phenomenon of anthelmintic resistance in trichostrongyle parasites of domestic ruminants, particularly H. contortus (Prichard, 2001). The intensive use of anthelmintics has resulted in widespread resistance, affecting all of the major compounds. This is a textbook example of an external factor selecting a rare gene because it confers significant survival benefits, thus increasing its frequency within the population. Mass movements of ruminants, and their associated worms, within and between countries have contributed to the geographical spread of resistance. The genetic change that confers resistance to benzimidazole-based drugs is a point mutation resulting in the replacement of phenylalanine by tryosine at position 200 in the β-tubulin isotype 1 gene (Grant & Mascord, 1996). This mutation appears to carry no, or only low, fitness costs to the worm and, as a result, once established in populations, the mutation persists. A number of studies have examined the possibility that drug-resistant strains may show additional phenotypic differences, particularly whether they are more or less immunogenic or pathogenic than drug-susceptible lines. Kelly et al. (1978) reported that benzimidazole-resistant H. contortus was more pathogenic in sheep than a drug-susceptible isolate, but contrasting findings were reported by MacLean & Holmes (1987) working with resistant and susceptible isolates of T. colubriformis in gerbils. Maingi, Scott & Pritchard (1990) found that parasitological and pathological parameters of infection with H. contortus were positively correlated with increasing levels of thiabendazole resistance. More recent and well-controlled studies in sheep with a drug-resistant and drug-susceptible isolates of Teladorsagia circumcincta showed no significant differences in faecal egg output (Barrett, Jackson & Huntley, 1998), although infections with the susceptible isolate became patent earlier. Following removal of the worms and a single challenge infection there were no differences in worm burden or numbers of mucosal mast cells between sheep exposed to the two isolates. Mallet & Hoste (1995) reported that a drug-resistant strain of T. colubriformis showed a lower fecundity in rabbits than a susceptible strain, but elicited greater mucosal inflammation and, interestingly, secreted greater quantities of acetylcholinesterase. However, the two strains not only differed in terms of resistance to thiabendazole but also came from different countries. Although it is therefore not possible unambiguously to correlate phenotypic differences affecting host responses with drug resistance, these data do show important levels of differences within the same species that impact on the host response to infection.
Clear phenotypic changes in responses to selection have been established by several workers when adapting nematodes to alternative host species. In the 1960s Haley and colleagues (Haley, 1966; Solomon & Haley, 1966) adapted Nippostrongylus brasiliensis, a natural parasite of the rat, to mice and to hamsters by repeated passage – i.e. selecting those worms able to survive and reproduce in the abnormal host. The nature of this adaptation is unknown, but, as survival and reproduction of N. brasiliensis is determined by the levels of host immunity it may well have involved altered immunogenicity or greater tolerance to immune effector mechanisms. The human hookworm Necator americanus has been successfully adapted to the golden hamster, again by repeated passage (Sen & Seth, 1967; Behnke, Paul & Rajasekariah, 1986).
Although attempts to increase the levels of adaptation of Strongyloides ratti from rats to mice by repeated passage were unsuccessful (Gemmill, Viney & Read, 2000) this species has been used in a variety of other experimental approaches involving selection (reviewed Viney, 2001). Lines established from individual females differ markedly in their propensity to develop via the heterogonic or homogonic route. By using individual lines that show a mixture of both developmental routes it has been shown that selection can convert them into following primarily one or the other routes within a comparatively small number of generations. Switching between developmental routes is influenced by external temperature and by host immunity, and sensitivity to these triggers is again variable between lines. A similar interplay between temperature and immunity is known to affect the propensity of larval Ostertagia ostertagi to undergo arrested development in the mammalian host, although this may be a characteristic only of certain (temperate) isolates (Gibbs, 1986).
Other phenotypic changes that can clearly be related to host immunity as a selection pressure include the altered acetylcholinesterase isoenzyme pattern seen in N. brasiliensis worms as the rat host develops immunity during a primary infection (Edwards, Burt & Ogilvie, 1971). The functional significance of this change is not known, but it has been shown that worms developing in immune rats (Ogilivie, 1972) or worms established through trickle, rather than single pulse, infections (Jenkins & Phillipson, 1972) become adapted to the immune host, elicit a reduced immune response and survive longer. This characteristic of reduced immunogenicity (and presumably that of altered acetylcholinesterase production) represents a phenotypic rather than a genotypic change as infections with the progeny of adapted worms are expelled by host immunity in the normal way.
Genotypic changes arising from adaptation to host immunity were reported by Dobson and colleagues, who carried out a long series of experiments to establish lines of Heligmosomoides polygrus (Nematospiroides dubius) by repeated passage through mice of different status (naïve, immune from single or challenge infections) as well as mice of different genotypes (Dobson & Owen, 1977). These selections resulted in lines of worms that showed a number of heritable phenotypic changes. Lines selected in the most immune mice showed better survival and reproduction in immune hosts (Dobson & Tang, 1991) and elicited lower antibody and inflammatory responses (Su & Dobson, 1997). The precise expression of these changes was quite strongly influenced by the genotype of the host, presumably mediated through variation in levels of genetically-determined resistance.
Infections with intestinal nematodes stimulate strong immune responses in all hosts, but the relation of these responses to protection against infection remains unclear in all but a few cases. In general, infections appear to elicit weaker protective responses in humans than in other host groups, although whilst this may be true for populations it probably does not apply at the level of individuals (Maizels et al. 1993). It is true of all intestinal nematode infections that worms are distributed very unevenly within a population. Distributions are aggregated, which implies that certainly some individuals in populations living in endemic areas remain worm free, or sustain only small infections, despite frequent exposure to infection. This pattern of infection makes it difficult to draw conclusions about the possible influences of worm population variation on host immunity. However, there are data from work with Ascaris and Trichuris that suggest both that worm variation exists and that it may be important in terms of the host–parasite relationship. Fraser & Kennedy (1991) found variation in expression of surface antigens of A. lumbricoides infective larvae when these were exposed to antibody from the host population from which the larvae originated. Binding of antibody from an individual donor to surface antigens showed heterogeneity between larvae, suggesting either polymorphism in these antigens or differences in their expression. The antibodies concerned would have been elicited by the somatic rather than the intestinal stages of the worms, but the target antigens would presumably represent the repertoire determined by the worm's genome. It has been reported that adult A. lumbricoides from different geographical regions show striking differences in female worm fecundity (Hall & Holland, 2000). The mean worm burden in children from Bangladesh was 20.2 compared with 12.6 from Nigeria. However, the mean faecal egg counts were 2473 and 13609, respectively. It is tempting to implicate host immunity and differences in worm immunogenicity as casual factors, although there are no data to support this. Antigenic variation, detectable by immunoblotting with plasma from individual hosts, was found at the level of individual T. trichiura worms taken from the population present within the plasma donor (Currie et al. 1998).
The greater control over experimental variables that is possible with the use of domestic livestock or experimental rodents makes it easier to devise experiments that look for evidence of parasite variation in the induction and expression of host immunity. Despite this, relatively few workers have exploited these approaches. The defined antigens developed as a vaccine candidate for H. contortus have been used in a comparative assay of the level of protection achieved in Australian lambs vaccinated with antigen (H11 – a gut membrane-derived molecule) prepared from British and Australian sources (Newton et al. 1995). Both antigen preparations protected well, but that from Australian worms gave rather better protection than antigen from British worms, protection from intra-muscular or subcutaneous administration being 75.5 and 87.7% (Australian), 60 and 55.9% (British). No major antigenic differences were detected by SDS-PAGE analysis.
The ability to H. polygyrus lines selected by passage through resistant mice to survive better in more resistant hosts could reflect reduced immunogenicity (as suggested for N. brasiliensis – see above), increased immunomodulation (it is known that H. polygyrus promotes its survival in this way – Behnke, Hannah & Pritchard, 1983; Telford et al., 1998) or an enhanced ability to resist the harmful actions of effector mechanisms (H. polygyrus produces significant levels of antoxidants – Smith & Bryant, 1986). Tang, Dobson & McManus (1995) attempted to correlate phenotypic differences with molecular characteristics of worms selected by passage in a variety of mice, but although protein and antigen profiles showed differences between worms selected by passage through resistant as compared with naïve mice, no clear correlations were apparent between the profiles and worm phenotype.
The H. polygyrus used in almost all experimental work, derived initially from Peromyscus maniculatus, is one of four closely related sub-species and is correctly designated as H. polygyrus bakeri. Comparative studies using laboratory mice and the field mouse A. sylvaticus as hosts for this sub-species and H. p. polygyrus from A. sylvaticus revealed strongly contrasting patterns of parasite development and survival (Quinnell, Behnke & Keymer, 1991). H. p. bakeri survived well in laboratory mice but poorly in field mice, despite establishing well initially. In contrast H. p. polygyrus, survived in field mice but established very poorly in laboratory mice. Immunosuppressive treatment with corticosteroid allowed survival in all cases, suggesting that the failure of a given sub species to survive in the ‘wrong’ host was immune-mediated and therefore a reflection of differential immunogenicity compared with the native sub species.
The taxonomy of the genus Trichinella is based on both genotypic and phenotypic characteristics. Among the latter is the capacity to induce cyst formation in the muscles, which quite clearly delineates the majority of genotypes from T. pseudospiralis and T. papuae, neither of which forms cysts. Phenotypic characteristics relevant to the intestinal phase include its duration, the duration and level of female worm fecundity, the region of the intestine occupied by the worms and the degree of pathology associated with infection. All of these are known to be influenced by the host immune response and are therefore likely to be variable if there is variation within genotypes in the expression of antigens or immunomodulators.
There have been relatively few studies of immune responses to Trichinella genotypes in hosts other than laboratory mice. Differences in the antibody responses of pigs infected with Spanish origin T. spiralis and T. britovi were described by Bolas-Fernandez et al. (1993). Kapel & Gamble (2000) made a more detailed study in pigs infected with eight genotypes. T. spiralis was found to be the most infective genotype, giving a mean larval burden of 427 larvae/g body weight, T. murrelli and the genotype T6 being minimally infective producing a maximum burden of 5 larvae/g. The level and time course of antibody responses against ES antigens varied significantly between the genotypes, being, overall, highest against antigens from the homologous parasite. A similar study was made using nine genotypes in wild boars (Kapel, 2001) and again marked differences were found in antibody response, particularly during the post-intestinal phase of infection. A comparison of responses to nine genotypes carried out in rats by Malakauskas, Kapel & Webster (2000) showed that, although all became established, infectivity varied considerably. T. spiralis and three genotypes of T. pseudospiralis had the greatest infectivity, the others (T. nativa, T. britovi, T. murrelli, T. nelsoni and T6) showed low or negligible infectivity. The three genotypes of T. pseudospiralis showed differences in infectivity, particularly when this was measured in terms of larval survival over a 40-week period.
The ease with which Trichinella infections can be established in mice has resulted in a relatively large literature dealing with the influence of inter- and intra-genotypic variation on host responses (reviewed Wakelin & Goyal, 1996). Among more recent studies are those of Goyal & Wakelin (1993a, b) who described variations in infectivity and immunogenicity of different geographical isolates of T. spiralis when used to infect a single mouse strain. Although the isolates cross-immunized there were isolate-specific differences in the ability to elicit levels of immunity to challenge. In mice given single primary infections there were differences in levels of serum parasite-specific antibodies (IgG1, IgG2a, IgE) and inflammatory responses (mucosal mastocytosis, peripheral eosinophilia). In a subsequent paper, Goyal, Hermanek & Wakelin (1944) followed cytokine responses in mesenteric lymphocytes from infected mice and found that the isolates generating the greatest immune and inflammatory responses showed the earliest switch from a type 1 to a type 2 cytokine profile.
One of the most striking genotype-dependent differences in infectivity was observed when mice were infected with T. spiralis or T. nativa (a species found in wild animals in northern latitudes). T. nativa was expelled very rapidly from mice and reproduced poorly, but survival and reproduction were considerably improved when mice were immunesuppressed by corticosteroid treatment (Fig. 1) indicating that the genotypes differed in their immunogenicity. These two genotypes can be differentiated by SDS-PAGE and immunoblot analysis of larval homogenates, but no direct correlation with the greater immunogenicity in T. nativa has been established (Bolas-Fernandez & Wakelin, 1989). Differences in the IgG3 (anti-carbohydrate) responses of mice infected with genotypes including T. spiralis and T. nativa, have been described by Dea-Ayueal et al. (2000).

Fig. 1. Differential survival of Trichinella genotypes in NIH mice reflects differential immunogenicity. Two groups of mice were infected with 300 larvae of T. spiralis ([bull ]) or T. nativa ([squf ]). An additional group of T. nativa-infected mice was immunosuppressed with cortisone acetate ([utrif ]). Worm recoveries are shown as a percentage of the numbers present at day 2. (Data from Bolas-Fernandez & Wakelin, 1989).
Although there has been, and continues to be, some debate about species identity in the cyst- forming genotypes of Trichinella, the species status of T. pseudospiralis has been readily accepted because of the distinctive characteristics of its muscle phase. As with the other Trichinella species there is evidence of molecular and genetic diversity between isolates of T. pseudospiralis taken from different regions (Finland, France, Kazakhstan, Russia, Tasmania, USA – La Rosa et al. 2001). Differences have also been recorded in aspects of the host response to infection. T. pseudospiralis has a marked immunosuppressive influence on the host, and this down regulates inflammatory responses to both the intestinal and muscle phases of infection (Stewart, 1989). A comparison of inflammatory responses induced in mice by a long-established American and a recent Australian isolate showed that the latter induced less inflammation in the intestine but more in the muscle; both isolates produced considerably less inflammation than T. spiralis (Alford et al. 1998). When concurrent infections were established between T. spiralis and each isolate, muscle inflammation was reduced to a much greater degree by the American isolate. It is not clear whether the biological characteristics of the American isolate may reflect its long-term passage through mice (>20 years), and the nature of the differences in anti-inflammatory capacity is not known. There is evidence that the immune suppression associated with infection of the American T. pseudospiralis reflects the induction of elevated plasma corticosterone levels (Stewart et al. 1988) and it is therefore possible that the two isolates differ in this property.
Species of Trichuris occur in many mammalian hosts but, unlike Trichinella, show high host specificity. Apart from evidence for intraspecific antigen variation within the human Trichuris (T. trichiura – Currie et al. 1998) nothing is known about species other than T. muris, a natural parasite of murine hosts that has been widely used as an laboratory model.
T. muris elicits strong protective immune responses in the majority of laboratory mouse strains, and these result in the elimination of worms before they reach patency. Certain inbred strains, however (e.g. B10.BR, AKR) are permissive – i.e. they remain susceptible to infection and allow the worms to become sexually mature (Else & Wakelin, 1986). Recent work has shown that mouse strains that express resistance to T. muris infections characteristically develop immune responses mediated by T lymphocytes of the T helper 2 (Th2) subset, whereas permissive mice express responses mediated by Th1 cells (Grencis, 1996). The mechanisms underlying resistance are still undefined, but under the appropriate circumstances it can be shown that immunity is transferable with both T cells and antibodies separately (Else & Grencis, 1999; Blackwell & Else, 2001). Resistance and susceptibility are under strong genetic control, involving both MHC-linked and non-MHC (background) genes (Else & Wakelin, 1988). Some resistant mice expel worms early in infection (e.g. NIH mice within two weeks) others take one to two weeks longer (e.g. BALB/c and CBA). Expulsion of worms before week four of infection appears to be critical – mice unable to do this not only fail to expel a primary infection, but fail also to eliminate a subsequent challenge. Such permissive mice appear anergic to T. muris antigens (Soltys, Goyal & Wakelin, 1999), and there is evidence that the switch from resistance to permissiveness, and the corresponding alteration in cellular immunity, may be induced by factors released from the maturing worms (Grencis & Entwistle, 1997).
The stimulation of protective immunity requires exposure of the mouse to infections that are above a threshold level (approximately 10 eggs – Wakelin, 1973). Below this level worms survive to sexual maturity. There is a strong correlation between the level of infection experienced and the Th response elicited in the mouse, low level infection up-regulating Th1 responses, higher levels a Th2 response (Bancroft, Else & Grencis, 1994).
The data summarized above have come from work with one particular laboratory-maintained isolate, obtained initially in 1954 from wild Mus musculus in Edinburgh Zoo (the E isolate) and maintained subsequently in immunesuppressed or immunodeficient laboratory mice. Two other isolates are also now available. The J isolate is derived from a batch of the E isolate sent in the 1960s to the USA and then, in 1971, made available to Y. Ito in Japan and passaged in immunosuppressed mice ever since. These isolates have therefore been maintained separately for some 60–100 generations. The third (S) isolate was recovered from Mus spretus in Portugal by J. M. Behnke in 1992 and has since been maintained in Nottingham in immunosuppressed mice.
The three isolates of T. muris show quite different patterns of infection in laboratory mouse strains and elicit different immune responses in the host (Bellaby et al. 1995; Bellaby, Robinson & Wakelin, 1996; Koyama & Ito, 1996, 2001). Key features of infections with the J and S isolates are illustrated in Fig. 2a. The J isolate is the most immunogenic of the three isolates and as a consequence is expelled most rapidly by mice that are genetically resistant, e.g. CBA. In these mice infection generates an IgG1 antibody response, a Th2-dependent isotype, with little or no IgG2a. J isolate worms are also expelled before patency in genetically permissive B10.BR, again with a predominantly IgG1 response. The S isolate appears to be the least immunogenic (or the most immunosuppressive), sexually mature infections developing even in genetically resistant mice. With this infection CBA mice develop similar IgG1 and IgG2a (Th1-dependent) responses. In B10.BR mice infections are chronic and the antibody response is dominated by IgG2a. The E isolate occupies a position that is intermediate between the other two, being expelled more slowly than J from resistant mice and surviving less well than S in permissive mice. The difference between the two isolates is clearly seen when they are used to infect C57BL/10 (B10) mice, which themselves are intermediate in resistance between CBA and B10.BR (Fig. 2b). The threshold level of infection necessary to elicit immunity differs between isolates. Thus with the J isolate, infections as low as 25 or 12.5 eggs trigger protective responses that result in the loss of worms from resistant CBA mice (mean recoveries day 35 were 0.45 and 0.73, respectively) in contrast similar infections with the S isolate result in the establishment of mature worm infections (mean recoveries 8.2 and 3.6).

Fig. 2. Time course of infection (histograms) and IgG isotype responses (line graphs) of Trichuris muris isolates in mice of different genotypes. Worm numbers are given as a% of the day 14 recovery. ELISA values are given as optical density (O.D.). (Data from Bellaby et al. 1996). A,B. Resistant CBA and permissive B10.BR mice infected with the Japanese (J) or Sobreda (S) isolates. C. C57B/10 mice infected with the Japanese (J) or Edinburgh (E) isolates.
These striking isolate-dependent differences in host–parasite relationship must reflect molecular differences associated with the induction or suppression of immune responses. Our laboratory at Nottingham has begun to look for evidence of such differences and current findings are summarized below.
Small differences between isolates can be detected when worm homogenates and excretory-secretory material are analysed by SDS-PAGE. That some of these differences are functionally significant is supported by the fact that differences in antibody recognition are apparent when separated proteins from each isolate are blotted with homologous or heterologous sera from infected mice. In addition there are differences in patterns of serine protease activity when homogenate and ES material are run on substrate gels (Fig. 3). On SDS-PAGE all of the isolates show a major band at 43 kDA, a component known from previous studies to be an important immunogen. However, N-terminal sequencing of this component has failed to show any sequence difference between the isolates, suggesting the possibility that functionally important differences may exist in other molecules. A number of the components that separate on SDS-PAGE are glycosylated and some of these carry phosphorylcholine (PC), a molecule with potent immunomodulatory properties (Harnett et al. 1999). Resistant CBA and permissive B10.BR mice infected with each of the three isolates show very different patterns of anti-PC antibody responses, and in B10.BR these patterns differ strikingly between the isolates (Fig. 4), suggesting significant differences in PC presentation between them.

Fig. 3. Proteolytic activity of excretory/secretory material from the Edinburgh (lanes 1, 2) Japanese (lanes 3, 4), or Sobreda (lanes 5, 6) isolates of Trichuris muris run on a 12% SDS-polyacrylamide resolving gel containing 0.1% gelatin. After separation the proteins were renatured and incubated at 37 °C at pH 7.0 for 48 hours.

Fig. 4. IgG antibody responses of B10.BR mice infected with the Japanese (J), Edinburgh (E) or Sobreda (S) isolates of Trichuris muris. Sera were tested in ELISA against a purified preparation of the major immunogen from each isolate (43 kDa molecule from excretory/secretory (ES) material) and against a BSA-Phosphorylcholine (PC) conjugate. ELISA values are given in terms of optical density (O.D.).
The Random Amplified Polymorphic DNA (RAPD) PCR technique has been used to look for genetic differences between the three isolates. A number of primers have revealed major band differences both with DNA from batches of small numbers (10) of worms and with DNA from individual worms (Fig. 5). These will provide markers for more detailed studies on the genetic basis of the phenotypic differences between the isolates. One approach will be to produce hybrids between the isolates and then monitor the segregation of phenotypic and genetic markers.

Fig. 5. Gel of RAPD-PCR products using four random primers and DNA from the Edinburgh (E), Japanese (J) and Sobreda (S) isolates of Trichuris muris. The results from each primer show major band differences between the isolates. The marker indicates MW in base pairs. Primer 1 – GACCGCTTGT; Primer 2 – CAGCACCCAC; Primer 3 – ACCCCCACAC; Primer 4 – GTCACGTCTC.
Intestinal nematodes, like all organisms, show considerable intraspecific genetic diversity. We now know that at least some of this diversity is reflected in phenotypic differences that affect the host–parasite relationship and that these differences can be subject to selection pressures originating from the host. The best studied example concerns the genetic and molecular basis of anthelmintic resistance in trichostrongyle nematodes, and this provides a model for the capacity of these parasites to respond to intense selection pressure. The evidence from this example is that nematodes can respond rapidly to selection, and this is confirmed by the data from work with the isolates of T. muris described above. In the absence of externally applied drug treatment the other major selection pressure acting on intestinal worms is the host's immune response. Although the evidence for protective immunity remains weak in humans, the evidence from experimental studies, and the principle of biological economy, suggest that host-protective responses act as a major environmental constraint on all intestinal nematodes. It can therefore by expected that there will be selection for those genotypes that survive and reproduce best in hosts of a given response phenotype. Selection may increase or decrease immunogenicity, or increase or decrease immunomodulatory ability, depending on which is most likely to optimize parasite fitness.
At present there are remarkably few data to provide a picture of the relationship between variation and immune responses as these relate to intestinal nematodes. The bulk of the data comes from experimental studies in mice with a handful of parasite species. Nevertheless these studies have provided a framework for future work that will have both theoretical and practical value. It is important, given the limitation on chemotherapeutic control, to quantify the ability of worm populations to modify their immunogenicity (or their immunmodulatory ability) from the standpoint of potential vaccine usage. Equally, it is important from the point of view of parasite population studies to understand how evolution acts on both host and parasite to optimize the fitness of each. This is not necessarily achieved by simply maximizing host resistance and minimizing parasite immunogenicity. All parasitism causes a loss of host resources. These arise from the costs of mounting immune and inflammatory responses, from the effects these may have on digestion, absorption and utilization of nutrients (a factor of major importance with intestinal infections) and from the loss of resources directly to the parasites themselves. Hosts must establish a trade-off between all of these factors to achieve maximum fitness in the face of continual exposure to infection. In turn, parasites must trade off the benefits of increased survival from reduced immunogenicity (or greater immunomodulation) against the possibility that the host may succumb to over infection. As seen very clearly with Trichinella, moderate immunogenicity may prevent over-infection (and keep out competing genotypes) but it carries the risk of eliciting immunopathological responses in the very environment that the parasites occupy. Knowledge of how specific genes in intestinal nematodes contribute to these crucial questions of host and parasite adaptation will lead to a much greater understanding of the population biology of this important group of parasites, as well as of the evolutionary pressures acting on their host–parasite relationships.
Dr S. E. Farias is a recipient of a CAPES postdoctoral fellowship.

Fig. 1. Differential survival of Trichinella genotypes in NIH mice reflects differential immunogenicity. Two groups of mice were infected with 300 larvae of T. spiralis ([bull ]) or T. nativa ([squf ]). An additional group of T. nativa-infected mice was immunosuppressed with cortisone acetate ([utrif ]). Worm recoveries are shown as a percentage of the numbers present at day 2. (Data from Bolas-Fernandez & Wakelin, 1989).

Fig. 2. Time course of infection (histograms) and IgG isotype responses (line graphs) of Trichuris muris isolates in mice of different genotypes. Worm numbers are given as a% of the day 14 recovery. ELISA values are given as optical density (O.D.). (Data from Bellaby et al. 1996). A,B. Resistant CBA and permissive B10.BR mice infected with the Japanese (J) or Sobreda (S) isolates. C. C57B/10 mice infected with the Japanese (J) or Edinburgh (E) isolates.
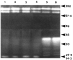
Fig. 3. Proteolytic activity of excretory/secretory material from the Edinburgh (lanes 1, 2) Japanese (lanes 3, 4), or Sobreda (lanes 5, 6) isolates of Trichuris muris run on a 12% SDS-polyacrylamide resolving gel containing 0.1% gelatin. After separation the proteins were renatured and incubated at 37 °C at pH 7.0 for 48 hours.

Fig. 4. IgG antibody responses of B10.BR mice infected with the Japanese (J), Edinburgh (E) or Sobreda (S) isolates of Trichuris muris. Sera were tested in ELISA against a purified preparation of the major immunogen from each isolate (43 kDa molecule from excretory/secretory (ES) material) and against a BSA-Phosphorylcholine (PC) conjugate. ELISA values are given in terms of optical density (O.D.).
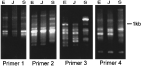
Fig. 5. Gel of RAPD-PCR products using four random primers and DNA from the Edinburgh (E), Japanese (J) and Sobreda (S) isolates of Trichuris muris. The results from each primer show major band differences between the isolates. The marker indicates MW in base pairs. Primer 1 – GACCGCTTGT; Primer 2 – CAGCACCCAC; Primer 3 – ACCCCCACAC; Primer 4 – GTCACGTCTC.