INTRODUCTION
Some 200 million people are infected with schistosomes in 74 countries; 120 million of these are symptomatic, with 20 million suffering severe schistosomiasis disease (Ross et al. Reference Ross, Bartley, Sleigh, Olds, Li, Williams and McManus2002). The disease burden in 2010 calculated for schistosomiasis was 3 309 000 disability-adjusted life years (Murray et al. Reference Murray, Vos, Lozano, Naghavi, Flaxman, Michaud, Ezzati, Shibuya, Salomon, Abdalla, Aboyans, Abraham, Ackerman, Aggarwal, Ahn, Ali, Alvarado, Anderson, Anderson, Andrews, Atkinson, Baddour, Bahalim, Barker-Collo, Barrero, Bartels, Basanez, Baxter, Bell and Benjamin2012). A meta-analysis assigned 2–15% disability weight to schistosomasis (King et al. Reference King, Dickman and Tisch2005). Despite the availability of the effective drug praziquantel (PZQ), its relative inactivity against migratory juveniles and developing worms (Gonnert and Andrews, Reference Gonnert and Andrews1977), its inability to prevent re-infection and the possibility of resistant schistosome parasites emerging, due to years of mass administration of the drug, are important shortcomings. Schistosomiasis remains one of the most devastating tropical parasitic diseases (Colley et al. Reference Colley, Bustinduy, Secor and King2014) and imposes a high socioeconomic burden on many developing countries where schistosomiasis is endemic due to their impact on human health, particularly as these blood flukes are commonly found as co-infections with human immunodeficiency virus/acquired immunodeficiency syndrome (Kallestrup et al. Reference Kallestrup, Zinyama, Gomo, Butterworth, van Dam, Gerstoft, Erikstrup and Ullum2006), malaria (Diallo et al. Reference Diallo, Remoue, Schacht, Charrier, Dompnier, Pillet, Garraud, N'Diaye A, Capron, Capron and Riveau2004) and tuberculosis (Elias et al. Reference Elias, Akuffo, Pawlowski, Haile, Schon and Britton2005). Accordingly, the Bill and Melinda Gates Foundation and other agencies, such as the World Health Organization (WHO), have targeted schistosomiasis, along with a number of other neglected infectious diseases, for elimination through investment in strategy evaluation, product development and operational research.
Five schistosome species, Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi, Schistosoma intercalatum and Schistosoma haematobium infect humans. This article focuses mainly on S. japonicum, which is endemic in China, the Philippines and Indonesia and which, in addition to man, infects a range of reservoir mammalian hosts including water buffaloes, cattle, rodents, dogs, sheep and pigs. Human infection is normally acquired through activities such as fishing, bathing, farming, washing clothes and swimming as schistosomes are transmitted via freshwater containing infectious cercariae. After shedding their bifurcated tails, cercariae transform into schistosomula which locate and enter a blood vessel (more rarely a lymphatic vessel) and are carried by the flow of blood to the lungs via the pulmonary artery. After several days, the worms arrive in the hepatic portal system and further develop to adult worms (schistosomes are dioecious). The males and females pair up, mature, migrate downstream, and the female worms of S. japonicum begin egg production when the paired worms reach mucosal branches of the inferior mesenteric and superior haemorrhoidal veins. Many eggs are mainly entrapped in liver and intestines whereas others traverse the intestinal wall and are ejected in the feces. Then the eggs hatch, if they contact with fresh water, to release miracidia, which can infect amphibious Oncomelania hupensis snails. A miracidium forms a sporocyst which asexually produces daughter sporocysts that migrate to the snail hepatopancreas and, following another phase of asexual reproduction, release larval cercariae into fresh water.
Important biological features of S. japonicum are its zoonotic nature and the fact female worms can produce thousands of eggs per day, 10 times more in number than S. mansoni and S. haematobium. It is the schistosome eggs that are responsible for transmission and pathology, the latter due to the granulomas which form around the eggs trapped in the liver and other organs. Unlike the other human schistosome species, the zoonotic transmission of schistosomiasis japonica provides a novel feature that can be utilized for the development of a transmission-blocking veterinary vaccine in domestic animals, especially bovines, to help prevent human S. japonicum infection and resultant disease. Bovines (cattle and water buffaloes [Bubalus bubalis]) are the major reservoirs, contributing about 90% of the S. japonicum infection source in China (Chen and Lin, Reference Chen and Lin2004). A study of bovines in Samar province in the Philippines in 2010 indicated a similar picture to that found in China with more than 90% of bovines infected with S. japonicum (Gordon et al. Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012). It is logical to target bovines for treatment/vaccination in schistosomiasis japonica control programmes because these animals produce considerably more feces on a daily basis than do humans. The first published mathematical model of S. japonicum transmission dynamics predicted that, in the lakes and marshlands of the Yangtze River basin in China, a bovine vaccine with 45% efficacy (the level of many current prototype vaccines) would reduce the endemic prevalence, but would not result in elimination (Williams et al. Reference Williams, Sleigh, Li, Feng, Davis, Chen, Ross, Bergquist and McManus2002). However, if accompanied by an initial period of human treatment and by improvements in human sanitation or a reduction in contaminated water contact by humans, elimination would be possible (Williams et al. Reference Williams, Sleigh, Li, Feng, Davis, Chen, Ross, Bergquist and McManus2002). Owing to the fact that water buffaloes are responsible for much of the schistosomiasis transmission in the marshland areas of China (Guo et al. Reference Guo, Li, Gray, Ning, Hu, Chen, Davis, Sleigh, Feng, McManus and Williams2006), a vaccine with 48–52% efficacy targeting these bovines, used in conjunction with PZQ treatment, could lead to a significant reduction in transmission with the predicted equilibrium prevalence reduced to zero after 5 years (Da'dara et al. Reference Da'dara, Li, Xiong, Zhou, Williams, McManus, Feng, Yu, Gray and Harn2008). Despite a number of research publications, knowledge of schistosome immunology in mammalian hosts is still limited, but this is critical to further understand the mechanism of pathogenesis in schistosomiasis and the processes associated with protective immunity in order to reinforce the rationale for successful vaccine development. Improved diagnostic tests for schistosomiasis are also required that can build on a better understanding of the immune response to schistosomes so that light infections can be identified with high specificity and sensitivity so as to determine the extent of the interruption of transmission and the elimination of schistosomiasis in a particular area. Here we review prospects for the development of vaccines and new diagnostics for zoonotic schistosomiasis.
THE HOST IMMUNE RESPONSE TO SCHISTOSOMES
The immunobiology of schistosomiasis includes the nature of the host innate and adaptive response to the schistosome parasites, knowledge of which is built on infection and immunization (with schistosome extracts or defined antigens) studies mainly in mice (including wild type and gene knockouts) but also in non-human primates and other mammalian hosts.
As reviewed by Mo et al. (Reference Mo, Agosti, Walson, Hall and Gordon2014), the immune response towards schistosomes, comprises 2 separate components: (1) immunopathogenesis and/or immunoregulation, which results from released antigens from eggs trapped in tissues. This leads to fibrosis and granuloma formation, collagen deposition and, in the case of S. japonicum and S. mansoni, severe hepatic periportal fibrosis, morbidity chronic inflammation and anaemia; and (2) age-dependent concomitant immunity against re-infection resulting over time from repeated natural adult worm death leading to the development of partially protective natural immunity in areas endemic for schistosomiasis. The protective effect of PZQ is thought, in part, to be due to the immunity induced against the drug-killed adult schistosomes (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). This partial protection has been associated with increased eosinophilia, CD23+ B cells, interleukin (IL)-5 and anti-adult worm antigen (AWA) immunoglobulin (Ig)E antibodies together with low levels of IgG4 antibodies against these worm components (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014).
A better understanding of innate immunity to schistosomiasis is necessary in developing strategies to protect hosts from infection or restrict immunopathology. Schistosomiasis results in a range of morbidities, most of which are not caused by the adult worms. Instead they are associated with the T-cell-dependent immune response of the mammalian host induced by schistosome eggs trapped in tissues and granuloma formation and related pathologies in target organs – mainly the liver and intestine with S. japonicum infection. The main immunopathology of schistosomiasis is induced by molecules secreted by the eggs, resulting in a marked CD4+ T-cell-mediated granulomatous inflammation involving monocytes, eosinophils and lymphocytes, likened to a form of delayed type hypersensitivity (McManus and Loukas, Reference McManus and Loukas2008).
Schistosoma blood flukes depend on signals from host CD4+ T cells for their growth and maturation in the mammalian host by inducing T-helper 2 (Th2)-biased inflammatory granulomas (Riner et al. Reference Riner, Ferragine, Maynard and Davies2013). While B cells suppress granulomatous pathology in schistosomiasis, it remains unclear whether these cells effect schistosome maturation, reproduction and granuloma development without the aid of CD4(+) T lymphocytes (Tang et al. Reference Tang, Ming, Liu, Xiong, Grevelding, Dong and Jiang2013). However, it has been shown that T and B cells play a crucial role in both generating protection and exacerbating disease outcomes by orchestrating the immune response during S. japonicum infection in rodent models showing resistance to the parasite (Hu et al. Reference Hu, Lu, Shen, Xu, Yuan, Zhang, Wu, Ni, Liu and Cao2012). Following cercarial infection, the early immune response is predominantly Th1, targeted at the adult worm. After egg deposition in tissues, the Th2 response becomes prominent, suggesting that egg antigens may directly inhibit the Th1 response (Liang et al. Reference Liang, Luo, Lu, Zhou, Wu, Zheng, Ren, Sun, Wang and Zhang2012). In general, recent investigations have demonstrated that T-cell-mediated immunity is necessary to promote acquired resistance to schistosomes in the murine model, a process mediated by activated macrophages (AAMs). Furthermore, cytokine studies also suggest that a schistosome vaccine that can induce AAMs to produce Th1 cytokines [gamma interferon (IFN-γ) and IL-2] may be useful in preventing disease (McManus and Loukas, Reference McManus and Loukas2008). It has been shown that IFN-γ and IgG2 antibodies, characteristic of Th1 responses and cytotoxicity, correlate with the high level of protection induced by an irradiated S. japonicum cercarial vaccine in pigs (Tian et al. Reference Tian, Lin, Wu, Gao, Zhang, Ji and Wu2010).
The CD4+ Th2 cellular response against schistosome egg antigens coordinates the development of granulomas which comprise cells (eosinophils, CD4+ T cells and macrophages) and collagen fibres around individual eggs (Pearce and MacDonald, Reference Pearce and MacDonald2002) (Fig. 1). Dendritic cells (DCs) can activate naive CD4+ T cells during their migration to lymphoid tissues, and acquire egg antigens, thereby inducing a Th2 response. Toll-like receptor 2 (TLR2), present at the DC cell surface, influences their maturation by inducing IL-10-secreting regulatory T cells (Kane et al. Reference Kane, Cervi, Sun, McKee, Masek, Shapira, Hunter and Pearce2004) (Fig. 1). However, exposure of egg antigens to DCs does not stimulate them to synthesize IL-12 or co-stimulatory molecules such as CD80, CD86 or CD40. Generation of the Th2 response depends on IL-4 from an alternative source to the DC. IL-4 limits the Th1 response and acts as a growth factor to increase the Th2 response. IL-10 produced by B cells and DCs induces regulatory T-cell activity, with the potential to suppress the Th1 cell response to helminth worm-derived antigens, thereby ensuring Th2 cell polarization (McKee and Pearce, Reference McKee and Pearce2004). IL-10 may have a role in suppressing IL-12 production and minimizing the progression of the Th1 response.

Fig. 1. Predicted model of the Th2 immune response induced by schistosome egg antigens. Granulomatous lesions comprise collagen fibres and host cells (macrophages, eosinophils and CD4+ T cells, coloured in green) around the egg. DCs can induce Th2 responses by activating naive CD4+ T cells. TLR2 located at the surface of DCs can be activated by egg-secreted proteins that influence their functional maturation by inducing regulatory T cells to secrete IL-10 (Kane et al. Reference Kane, Cervi, Sun, McKee, Masek, Shapira, Hunter and Pearce2004). IL-10 so generated may suppress IL-12 production, which is a potent inhibitor of Th2 responses, and minimizes the progression of the Th1 response. The interactions of CD40–CD154 and OX40L–OX40 are also important in the induction of Th2 responses to schistosome antigens (de Jong et al. Reference de Jong, Vieira, Kalinski, Schuitemaker, Tanaka, Wierenga, Yazdanbakhsh and Kapsenberg2002). However, the exposure of DCs to egg antigens does not stimulate their expression of the co-stimulatory molecules CD40, CD80 or CD86. Induction of alternatively activated macrophage populations is a dominant characteristic of Th2 immunity. Development of the Th2 response depends on IL-4 from a source other than DCs and the main Th2 cytokine responsible for fibrosis is IL-13 (Fallon et al. Reference Fallon, Richardson, McKenzie and McKenzie2000). A schistosome egg glycoprotein can induce the release of IL-4 and IL-13 from basophils and the fibrogenic role of IL-13 seems to be important, together with IL-4, to induce the expression of arginase in macrophages (Hesse et al. Reference Hesse, Modolell, La Flamme, Schito, Fuentes, Cheever, Pearce and Wynn2001). Arginase uses l-arginine to make proline which is an essential amino acid associated with collagen production and fibrosis. Mediators that are involved in Th1 responses, such as IFN-γ, IL-12, TNF and NO can hamper Th2 response development and also stimulate the expression by macrophages of inducible iNOS which uses arginine for the production of citrulline and NO. During this process, l-hydroxyarginine is produced which inhibits arginase and reduces the level of expressed proline, thereby reducing collagen synthesis. The Th2 response results in an increase in the level of serum IL-5, tissue fibrosis accompanied by massive blood and bone eosinophilia. NKT cells, eosinophils and mast cells are all potential sources of IL-4 (Sabin et al. Reference Sabin, Kopf and Pearce1996). As a signature cytokine of Th17 cells, IL-17, induced by S. japonicum infection in mouse pulmonary lymphocytes, contributes to granuloma formation and fibrosis in the liver (Chen et al. Reference Chen, Luo, Xie, Gao, Fang and Huang2013a ). Th17 cells express more IL-4 and IL-5 than IFN-γ, but less IL-10 (Luo et al. Reference Luo, Chen, Xie, Gao, Fang and Huang2012; Chen et al. Reference Chen, Xie, Luo, Yu, Fu, Gu, Wu, Tang and Huang2013b ).
The roles of TLR2–MHC class II, CD40–CD154 and OX40L–OX40 are important in the generation of Th2 responses to schistosome antigens (de Jong et al. Reference de Jong, Vieira, Kalinski, Schuitemaker, Tanaka, Wierenga, Yazdanbakhsh and Kapsenberg2002). An important feature of this Th2 immunity is the induction of alternatively AAM populations, which is crucial in regulating the pathology and worm expulsion that is essential for the host surviving schistosomiasis (Horsnell and Brombacher, Reference Horsnell and Brombacher2010). IL-13 is the main Th2 cytokine that is responsible for fibrosis (Fallon et al. Reference Fallon, Richardson, McKenzie and McKenzie2000) and a series of recent studies have elucidated that IL-13 is able to promote fibrogenesis (Modolell et al. Reference Modolell, Corraliza, Link, Soler and Eichmann1995; Hesse et al. Reference Hesse, Cheever, Jankovic and Wynn2000, Reference Hesse, Modolell, La Flamme, Schito, Fuentes, Cheever, Pearce and Wynn 2001 ). IL-13 and its receptor complex have been identified as important regulators in controlling the progression of schistosomiasis (Mentink-Kane and Wynn, Reference Mentink-Kane and Wynn2004) suggesting the possibility of IL-13-blocking therapies for the disease. A schistosome egg glycoprotein is able to induce the release from basophils of IL-4 and IL-13 by non-specifically binding and cross-linking cell-surface IgE (Schramm et al. Reference Schramm, Falcone, Gronow, Haisch, Mamat, Doenhoff, Oliveira, Galle, Dahinden and Haas2003). The fibrogenic role of IL-13, together with IL-4, is essential for upregulating the expression of arginase in macrophages (Hesse et al. Reference Hesse, Modolell, La Flamme, Schito, Fuentes, Cheever, Pearce and Wynn2001). Arginase metabolizes l-arginine to produce proline which is necessary for the formation of collagen and fibrosis development. Th1 response mediators [nitric oxide (NO), tumour necrosis factor (TNF), IFN-γ and IL-12] can inhibit the Th2 response and induce macrophages to express, rather than arginase, inducible nitric oxide synthase (iNOS) which uses arginine for the production of citrulline and NO. During this process, l-hydroxyarginine is produced which inhibits arginase and reduces the level of expressed proline, thereby reducing collagen synthesis. The Th2 response stimulates massive blood and bone eosinophilia, increased levels of serum IL-5, and the egg-induced granuloma formation which results in collagen being deposited, tissue fibrosis and the manifestations associated with schistosomiasis (Wilson et al. Reference Wilson, Mentink-Kane, Pesce, Ramalingam, Thompson and Wynn2007). The resolution of any helminth infection is generally correlated with a Th2 immune response by the mammalian host. Recent research has shown that schistosome worms also stimulate functional type 2 responses; for example, a parasite cysteine protease is an inducer of Th2 responses at the early stage of schistosome infection (de Oliveira Fraga et al. Reference de Oliveira Fraga, Lamb, Moreno, Chatterjee, Dvorak, Delcroix, Sajid, Caffrey and Davies2010).
Natural killer T (NKT) cells are activated to proliferate by glycolipids present in both schistosome eggs and worms (Zaccone et al. Reference Zaccone, Fehervari, Jones, Sidobre, Kronenberg, Dunne and Cooke2003). NKT cells may also play roles in modulating the classical T-cell response, accompanied by the upregulation of CD4 and downregulation of CD94 expression in infected mesenteric lymph node natural killer (NK) cells and enhanced expression of IL-4 and IL-17 in both the NK and NKT cells of infected mice (Luo et al. Reference Luo, Xie, Chen, Yu, Wu, Li, Wu and Huang2013). NKT cells, mast cells and eosinophils are all potential sources of IL-4 (Sabin et al. Reference Sabin, Kopf and Pearce1996). A large amount of IL-17 induced by S. japonicum infection in mouse pulmonary lymphocytes contributes to granulomatous inflammatory and fibrosing reactions in the liver (Chen et al. Reference Chen, Luo, Xie, Gao, Fang and Huang2013a ). However, IL-17 is a signature cytokine of Th17 cells and has been implicated in the induction of chronic inflammatory diseases (Zhang et al. Reference Zhang, Chen, Gao, Hou, Gu, Gui, Huang, Liu, Ren, Wang and Shen2012b ). Severe hepatic granulomatous inflammation is associated with high levels of IL-17 (Zhang et al. Reference Zhang, Chen, Gao, Hou, Gu, Gui, Huang, Liu, Ren, Wang and Shen2012b ) and lower IL-17 levels may result in favourable host-protective responses (Wen et al. Reference Wen, He, Chi, Zhou, Hoellwarth, Zhang, Zhu, Wu, Dhesi, Wang, Liu and Su2011). Th17 cells are able to express more IL-4 and IL-5 than IFN-γ, but not IL-10 (Luo et al. Reference Luo, Chen, Xie, Gao, Fang and Huang2012; Chen et al. Reference Chen, Xie, Luo, Yu, Fu, Gu, Wu, Tang and Huang2013b ). It has been suggested that activated NK cells in the liver can downregulate egg-induced liver fibrosis by producing IFN-γ and killing activated hepatic stellate cells (Hou et al. Reference Hou, Yu, Man, Huang, Zhang, Liu, Ren and Shen2012). It is well established that IFN-γ is essential for the development of acquired resistance against murine schistosomiasis although recent evidence suggests that IFN-γ is not always a positive regulator of immune responses. In IFN-γ knockout mice, the disruption of IFN-γ signalling may upregulate the cytotoxic T-cell-mediated immune responses to S. japonicum infection (Du et al. Reference Du, Wu, Zhang, Gao, Zhang, Hou, Ji and Wu2011). However, studies on cytokine-deficient and B-cell-deficient mice demonstrated that successful anti-schistosome vaccination required the induction of strong Th1 and Th2 responses (McManus et al. Reference McManus, Gray, Li, Feng, Williams, Stewart, Rey-Ladino and Ross2010). Further research has also shown that a balance between Th1, Th17 and Th2 cytokines is required for effective schistosome larval elimination in the mouse model (Tallima et al. Reference Tallima, Salah, Guirguis and El Ridi2009; El Ridi et al. Reference El Ridi, Tallima, Mahana and Dalton2010).
It should be emphasized that the mechanisms of immune responses to schistosome infections in the mice cannot be completely generalized to humans or other natural hosts (Lebens et al. Reference Lebens, Sun, Czerkinsky and Holmgren2004). Clinical vaccine development against schistosome infection has been hampered by a limited understanding of the mechanisms of protective immunity in humans. As reviewed by Siddiqui et al. (Reference Siddiqui, Siddiqui and Ganley-Leal2011), factors predictive of resistance in humans from a number of immune-epidemiological studies include a high concentration of serum parasite-specific IgE, increased circulating CD23+ B cells, eosinophilia and secretion of IL-5 in response to crude worm extracts. However, whether any of these immune responses is directly involved in worm killing has not been elucidated. As arguably the most relevant non-human primate model for human clinical trials, the baboon has similar immune responses, ontogeny, reproductive physiology as a human and develops a human-like schistosomiasis acute syndrome and chronic disease after exposure to schistosome cercariae. The baboon has been used as a ‘protection’ model to determine the efficacy of schistosome vaccine candidates, including S. mansoni 28 glutathione-S-transferase (GST) (Boulanger et al. Reference Boulanger, Reid, Sturrock, Wolowczuk, Balloul, Grezel, Pierce, Otieno, Guerret, Grimaud, Butterworth and Capron1991), S. mansoni calpain (Sm-p80) (Siddiqui et al. Reference Siddiqui, Pinkston, Quinlin, Saeed, White, Shearer and Kennedy2005; Karmakar et al. Reference Karmakar, Zhang, Ahmad, Torben, Alam, Le, Damian, Wolf, White, Carey, Carter, Reed and Siddiqui2014), S. mansoni heat shock protein 70 (HSP70) (Kanamura et al. Reference Kanamura, Hancock, Rodrigues and Damian2002) and attenuated schistosomes including attenuated cercariae (Kariuki et al. Reference Kariuki, Van Dam, Deelder, Farah, Yole, Wilson and Coulson2006) and schistosomula (Reid et al. Reference Reid, Sturrock, Harrison and Tarara1995). Siddiqui and his colleagues showed recently that the Sm-p80 vaccine generates intricately balanced proinflammatory (Th17 and Th1) responses and, to a much smaller extent, anti-inflammatory (Th2) types of immune responses in the baboon model resulting in both prophylactic and therapeutic efficacies (Karmakar et al. Reference Karmakar, Zhang, Ahmad, Torben, Alam, Le, Damian, Wolf, White, Carey, Carter, Reed and Siddiqui2014).
Based on the current approach for the control of S. japonicum in China, studies of protective immunity in bovine schistosomiasis japonica are important when considering the development of a transmission-blocking veterinary vaccine to assist in integrated control efforts (McManus and Dalton, Reference McManus and Dalton2006). However, in contrast to murine schistosomiasis, our understanding of the immunology of schistosome infections in water buffaloes and cattle is also very limited. Recently, it has been reported that, following S. japonicum infection, worm burdens drop over time in water buffaloes. This self-cure phenomenon appears due to parasite clearance by both immune and non-immune factors, with evidence suggesting that most experimental animals susceptible to schistosomes develop some level of acquired immunity following a primary infection (Li et al. Reference Li, McManus, Lin, Williams, Harn, Ross, Feng and Gray2014a ). However, studies of PZQ treatment and re-infection in bovines infected with S. japonicum in China have suggested that age-related resistance likely occurs in water buffaloes but not in cattle (Wang et al. Reference Wang, Zhang, Wu, Zhang, Lu, Ornbjerg and Johansen2006a ). Furthermore, it was shown that worm length, worm recovery rate and the number of paired worms were significantly increased in yellow cattle, another natural host for S. japonicum in China, compared with water buffaloes, in which more serious pathological damage occurs. Immunological analysis suggested that the number of CD4+ T cells, which might constitute an integral component of the immune response, may correlate with worm development in yellow cattle. A shift from Th1- to Th2-type polarized immunity was identified in yellow cattle, but not in water buffaloes infected with schistosomes (Yang et al. Reference Yang, Fu, Feng, Shi, Yuan, Liu, Hong, Li, Lu and Lin2012). Based on the fact that water buffaloes are major reservoirs involved in the transmission of S. japonicum, further studies on the immunology of those bovines are necessary to select effective S. japonicum transmission vaccine candidates (such as the immune response analysis to migrating larvae, which are the likely targets of an anti-schistosome vaccine) and to determine the desirable route of immunization.
CURRENT STATUS OF VACCINE DEVELOPMENT FOR S. JAPONICUM
Highly effective vaccines have been developed against several tapeworm species (Vercruysse et al. Reference Vercruysse, Schetters, Knox, Willadsen and Claerebout2007) indicating it is possible to generate a reliable, high level of protection against complex metazoan parasites, using defined recombinant antigens. A schistosomiasis vaccine is not yet available and substantial development efforts will be required to produce a viable product. Nevertheless, there is evidence indicating that at least partial natural human immunity can develop in schistosomiasis-endemic areas, and that part of this protective effect can be attributed to the immunity that is generated after adult schistosome worms are killed by PZQ. Furthermore, irradiated schistosome cercariae or schistosomula can confer considerable levels of protection against infection in experimental animal challenge models. For example, high levels of protective efficacy (77·62, 88·8 and 99·78% reduction in worm burden, liver eggs and fecal eggs, respectively) against S. japonicum challenge were obtained with a ultraviolet-attenuated cercarial vaccine in pigs, with vaccination evoking an effective IFN-γ response and a strong antibody-mediated response, especially in increased levels of IgG2 antibodies (Lin et al. Reference Lin, Tian, Wu, Gao, Wu, Zhang, Ji, McManus, Driguez and Wu2011a ). Earlier research in the 1990s showed that water buffaloes vaccinated with irradiation-attenuated S. japonicum cercariae gained weight after unattenuated cercarial challenge and developed 89% resistance to S. japonicum re-infection (Shi et al. Reference Shi, Jiang, Han, Li and Ruppel1990). In addition, Chinese bovines (including cattle and buffaloes) immunized with 36 kR γ-irradiated schistosomula reduced the worm burden by 65–76% following a normal cercarial re-challenge (Hsu et al. Reference Hsu, Xu, He, Shi, Shen, Hsu, Osborne and Clarke1984). A major challenge is our limited knowledge of the immunology of schistosome infections in cattle and, especially water buffaloes – due in part to the scarcity of immunological reagents for studying immune responses (McManus and Loukas, Reference McManus and Loukas2008). PZQ-treatment and re-infection studies of bovines infected with S. japonicum in China have indicated that age-related resistance may occur in water buffaloes but not cattle but whether this self-cure phenomenon has an immunological basis has yet to be determined (Li et al. Reference Li, McManus, Lin, Williams, Harn, Ross, Feng and Gray2014b ). Additional studies on the immunology of buffaloes and cattle represent an important research area and these will be vital to aid in the process of selecting S. japonicum vaccine antigens and in defining optimum routes of immunization. In this respect, a recent study described immunological profiles in previously exposed and re-challenged water buffaloes in China and showed that the intense type-2 immune response at the site of cercarial penetration was significantly different from that seen in naive and permissive animal models, such as mice, suggesting a possible immune mechanism (McWilliam et al. Reference McWilliam, Piedrafita, Li, Zheng, He, Yu, McManus and Meeusen2013). This study also revealed a reduced and delayed immune response in water buffaloes given a high cercarial challenge infection compared with a moderate infection, particularly in the skin and, overall, the study provided new insights of the immunobiology of schistosomiasis in a natural host (McWilliam et al. Reference McWilliam, Piedrafita, Li, Zheng, He, Yu, McManus and Meeusen2013).
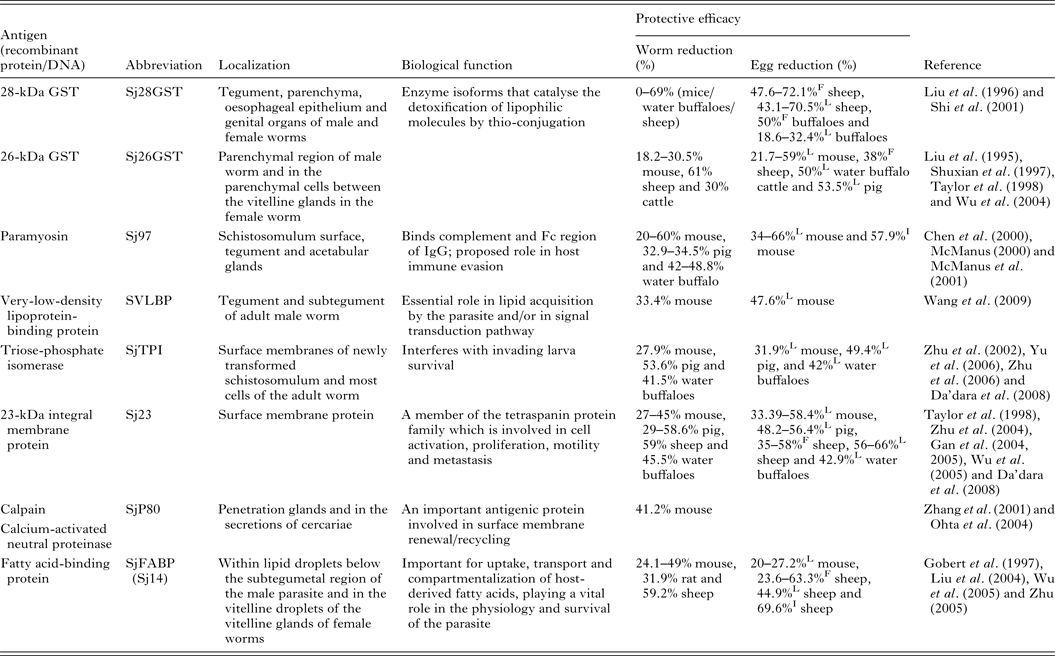
Of the current leading S. japonicum vaccine candidates, a number include membrane proteins, enzymes and muscle components (Table 1). Detailed information of the characteristics and vaccine efficacy of these and other vaccine candidates can be found elsewhere (McManus and Loukas, Reference McManus and Loukas2008). One of the encouraging vaccine targets is paramyosin, a 97-kDa protein (Sj97), which can induce a reduction in worm numbers of 52% in mice and 50% in water buffaloes against S. japonicum infection. Sj97 is expressed on the schistosomular tegument and in the acetabular glands. It appears to have similar function as an Fc receptor (Loukas et al. Reference Loukas, Jones, King, Brindley and McManus2001) and an exogenous form inhibits activation of the terminal pathway of complement, suggesting a key involvement in host immune evasion (Gobert and McManus, Reference Gobert and McManus2005). Currently, Sj97 is in early preclinical process development and in further proof-of-concept studies in mice and water buffaloes (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). Other important vaccine candidates are S. japonicum 26GST (Sj26GST) and S. japonicum 28GST (Sj28GST) which have shown encouraging protective efficacy against S. japonicum in different mammalian hosts (Table 1). A phase II clinical trial of S. mansoni 28GST (Sm28GST) has been carried out (Li et al. Reference Li, Wang, Zhang, Wang, Ji, Zhu, Liu, Cai, Wu and Wu2005) and phase I and II trials with S. haematobium 28GST (Sh28GST) were completed recently (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). Three of the leading vaccine candidates against S. mansoni – fatty acid-binding protein (Sm14), tetraspanin (Sm-TSP-2) and calpain (Sm-p80) – have been subjected to animal proof-of-concept studies and preclinical process development (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). Notably, none of these molecules from S. japonicum were able to generate effective protection against challenge infection (Liu et al. Reference Liu, Cai, Lin, Fu, Yang, Shi, Cai, Shen, Taylor and Wu2004; Wu et al. Reference Wu, Lu and Yu2005; Zhu, Reference Zhu2005).
Table 1. Some leading vaccine candidates against S. japonicum
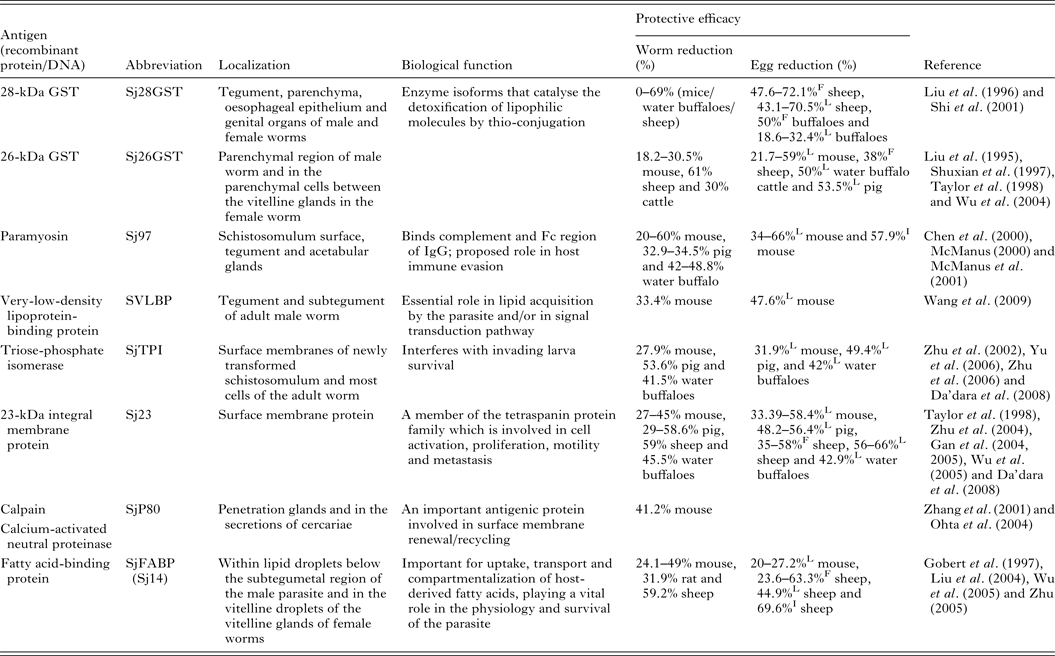
L, liver egg reduction; F, fecal egg reduction; I, intestine egg reduction.
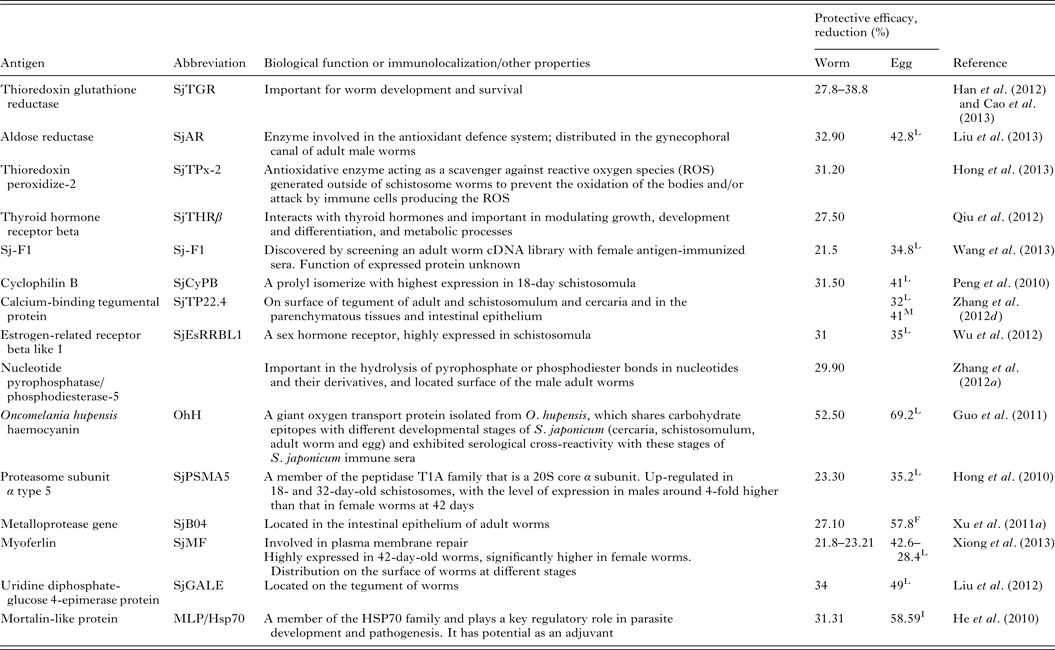
Renewed efforts have been made recently to characterize molecules that control schistosome survival, growth and reproduction in order to identify new targets for vaccine development. Accordingly, a series of recently discovered and tested components (including Sj23LHD-GST, Sj-F1, SjCYPB, SjCE-2b, SjTGR, SjAR, SjTPx-2, SjTP22·4, SjEsRRBL1, SjPSMA5, SjB04, SjMF, SjGALE and MLP/HsP70) have been tested as vaccines, details for which are shown in Table 2.
Table 2. Recently tested vaccine candidates against S. japonicum infection
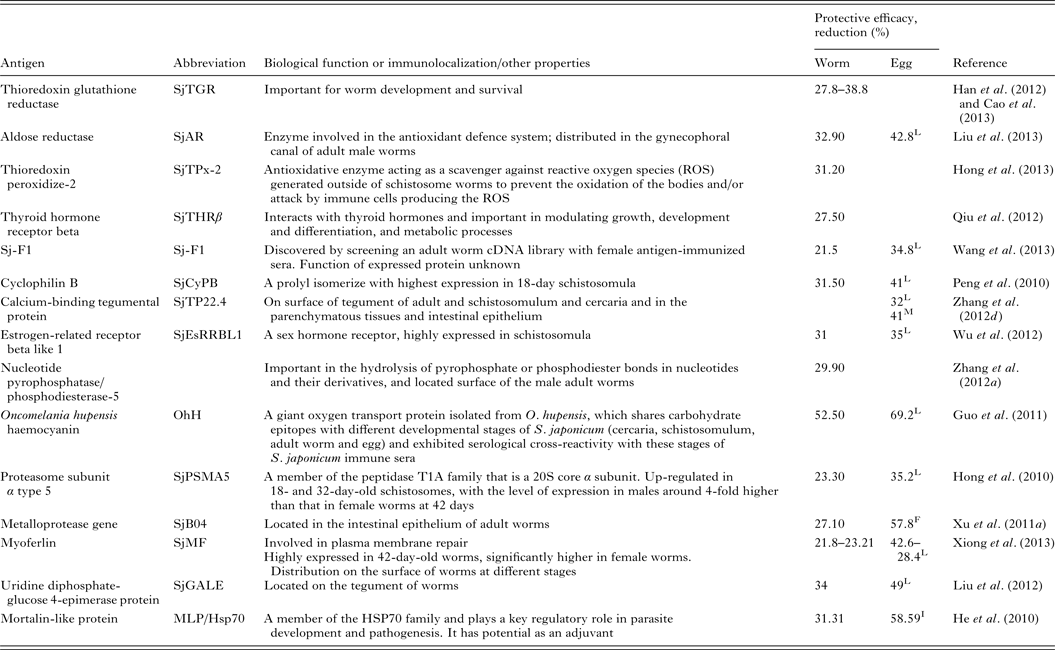
L, liver egg reduction; F, fecal egg reduction; I, intestine egg reduction; M, egg mature rate.
The recent availability of genomic, transcriptomic and proteomic information has allowed the research community to gain new insights into the highly adapted relationship between schistosomes and their mammalian hosts and for identifying novel therapeutic and vaccine targets (Zhou et al. Reference Zhou, Zheng, Chen, Zhang, Wang, Guo, Huang, Zhang, Huang, Jin, Dou, Hasegawa, Wang, Zhang, Zhou, Tao, Cao, Li, Vinar, Brejova, Brown, Li, Miller, Blair, Zhong, Chen, Liu, Hu, Wang and Zhang2009; Berriman et al. Reference Berriman, Haas, LoVerde, Wilson, Dillon, Cerqueira, Mashiyama, Al-Lazikani, Andrade, Ashton, Aslett, Bartholomeu, Blandin, Caffrey, Coghlan, Coulson, Day, Delcher, DeMarco, Djikeng, Eyre, Gamble, Ghedin, Gu, Hertz-Fowler, Hirai, Hirai, Houston, Ivens and Johnston2009; Young et al. Reference Young, Jex, Li, Liu, Yang, Xiong, Li, Cantacessi, Hall, Xu, Chen, Wu, Zerlotini, Oliveira, Hofmann, Zhang, Fang, Kang, Campbell, Loukas, Ranganathan, Rollinson, Rinaldi, Brindley, Yang, Wang, Wang and Gasser2012). As a result, an insulin receptor was first shown present in S. japonicum (Zhou et al. Reference Zhou, Zheng, Chen, Zhang, Wang, Guo, Huang, Zhang, Huang, Jin, Dou, Hasegawa, Wang, Zhang, Zhou, Tao, Cao, Li, Vinar, Brejova, Brown, Li, Miller, Blair, Zhong, Chen, Liu, Hu, Wang and Zhang2009). It is striking that schistosomes absorb their dry weight of glucose from their hosts every 5 h (Bueding, Reference Bueding1950), but as they are unable to synthesize insulin (Hu et al. Reference Hu, Yan, Shen, Liu, Zhu, Song, Xu, Wang, Rong, Zeng, Wu, Zhang, Wang, Xu, Wang, Fu, Zhang, Wang, Brindley, McManus, Xue, Feng, Chen and Han2003), they thus depend on host insulin to facilitate glucose uptake for metabolism, growth and fecundity (You et al. Reference You, Zhang, Moertel, McManus and Gobert2009). Accordingly, we have isolated 2 types of insulin receptors from S. japonicum (SjIRs) that can bind human insulin and which may be involved in modulating the process of glucose uptake in a manner similar to that in Caenorhabditis elegans and mammalian cells [56]. Most recently, we showed in a murine vaccine/challenge model that immunization with the L1 subdomain (major insulin-binding domain) of the SjIR (SjLDs) fusion proteins expressed in Escherichia coli, conferred highly significant reductions in fecal eggs (56–67%), in liver granuloma density (45–55%) and stunting of adult worms (12–42%), and a reduction in the numbers of mature intestinal eggs (75%) (You et al. Reference You, Gobert, Duke, Zhang, Li, Jones and McManus2012). Although we did not find a reduction in adult worm burden, our results strongly suggested that the SjLD vaccines were able to induce a significant retardation in the growth of worms and depress fecundity (egg production), emphasizing their potential as encouraging transmission-blocking vaccine candidates. Furthermore, we also found the SjLD vaccines could depress long-term female growth and egg production (unpublished data), thereby inducing long-lived protection against S. japonicum infection in the murine model, reinforcing their potential as encouraging transmission-blocking vaccines. Vaccination against schistosomes can be targeted towards the prevention of infection and/or reduced parasite fecundity. A significant reduction in worm burden is regarded as the ‘gold standard’ for development of anti-schistosome vaccines. However, as schistosome eggs are responsible for pathology and transmission, and the alteration of immune responses in disease progression in schistosomiasis, a vaccine targeting parasite fecundity and/or egg viability may represent a more realistic strategy for vaccine development (McManus and Loukas, Reference McManus and Loukas2008). In order to obtain optimum vaccine efficacy, one logical approach is to design multivalent vaccines targeting 2 or more antigens which promote the depression of adult parasite growth and egg production and a reduction in worm numbers.
Another lead vaccine candidate against S. japonicum infection that is involved in glucose metabolism is triose-phosphate isomerase (SjCTPI), which reduces worm burdens in mice (27·9%) (Zhu et al. Reference Zhu, Si, Ham, Yu, He, Hua, Yin, Liang, Xu and Xu2002), pigs (48%) (Zhu et al. Reference Zhu, Si, Harn, Xu, Ren, Yu, Liang, Yin, He and Cao2006) and water buffaloes (48–52%) (Yu et al. Reference Yu, He, Xiong, Zhao, Shi, Zhou, Liu, Luo, Fu, He, Harn and Li2006; Da'dara et al. Reference Da'dara, Li, Xiong, Zhou, Williams, McManus, Feng, Yu, Gray and Harn2008). This enzyme is able to convert glyceraldehyde-3-phosphate to dehydroxyacetone phosphate, which is a key step in the glycolytic pathway, whereby a cell breaks down glucose into energy. TPI is located in most cells of schistosome worms and on the surface membranes of newly transformed schistosomula, the stage most likely to be the target of an anti-schistosome vaccine. Vaccination with a SjCTPI DNA vaccine had a significant effect in reducing female worm burdens (53·6–59·6%) and liver egg numbers (49·4–65·8%) in the pig model of schistosomiasis japonica; in addition, the granuloma sizes in vaccinated animals were reduced by 42% (Zhu et al. Reference Zhu, Fu, Zhang, Han, Hong, Li, Zhao, Shi, Li and Lin2012). The efficacy of SjCTPI and the tetraspanin, SjC23, on their own, and as fusions with the heat-shock protein 70 (Hsp70) were assessed as DNA vaccines in water buffaloes in China against S. japonicum challenge (Da'dara et al. Reference Da'dara, Li, Xiong, Zhou, Williams, McManus, Feng, Yu, Gray and Harn2008). The most encouraging vaccine was the SjCTPI-Hsp70 construct, that generated 51·2, 61·5 and 52·1% reduction in worm burden, hepatic eggs and fecal eggs, respectively (Da'dara et al. Reference Da'dara, Li, Xiong, Zhou, Williams, McManus, Feng, Yu, Gray and Harn2008). The SjCTPI-Hsp70 vaccine is currently undergoing field testing in bovines in China and the Philippines.
It is now clear that several S. japonicum vaccine candidates are able to induce levels of between 50 and 70% protective efficacy in vaccination/challenge experiments with mice and larger mammalian species, with further immunization boosts increasing these levels so that their further development for veterinary use is a realistic proposition (McManus and Loukas, Reference McManus and Loukas2008). However, further study is necessary on the development of multivalent vaccines and novel adjuvants to improve on these levels of protection (Siddiqui et al. Reference Siddiqui, Siddiqui and Ganley-Leal2011; You et al. Reference You, Stephenson, Gobert and McManus2014). Furthermore, there are some challenges that will need to be overcome, including the possible risk of atopic IgE responses generated by the various vaccines, the use of appropriate adjuvant/vaccine formulations, whether vaccine efficacy is reduced due to co-infection with other pathogens in schistosomiasis-endemic areas, and the practical requirement of developing a vaccine that can be given as a single dose, ideally orally, without the requirement of boosting.
DEVELOPMENT AND TESTING OF NEW DIAGNOSTICS FOR SCHISTOSOMIASIS
To date, selective chemotherapy with PZQ is one of the components of schistosomiasis control programmes so that correct diagnosis of infected individuals is important. However, a sensitive and specific assay for field diagnosis of schistosomiasis japonica that is simple and affordable is not currently available. This poses a barrier to control efforts leading to schistosomiasis elimination, especially when the schistosome prevalence drops to low levels, as is now occurring in China [41]. Hence, the search for novel diagnostic tools for schistosomiasis is recognized as an urgent priority. Generally, S. japonicum infections can be detected by 3 approaches: parasitological, immunological and molecular.
Parasitological methods
Detection of eggs in stool samples is the customary method for diagnosing schistosome infections. The Kato-Katz (KK) thick smear technique is the most widely used procedure, being the standard method recommended by the WHO for both qualitative and quantitative diagnoses of intestinal schistosomiasis. However, the sensitivity of the KK method can vary from 40 to 100%, with the negative predictive values ranging from 52·5 to 100% (Zhou et al. Reference Zhou, Yang, Wang, Zhao, Wei, Peng and Jiang2007). Furthermore, if only a single KK slide is prepared from a single stool specimen, sensitivity is low (Lin et al. Reference Lin, Liu, Hu, Li, Tao, Yuan, Xie, Huang, Jiang, Li, Gao and Wang2011b ). This leads to a marked underestimation of the prevalence and intensity of infection, particularly in low prevalence areas (Lier et al. Reference Lier, Johansen, Hjelmevoll, Vennervald and Simonsen2008), and this can confound confirmation of cure after PZQ treatment (Berhe et al. Reference Berhe, Medhin, Erko, Smith, Gedamu, Bereded, Moore, Habte, Redda, Gebre-Michael and Gundersen2004). Ideally, multiple fecal examinations (typically 2 fecal samples per individual; 3 KK slides each sample) should be undertaken to reduce the false-negative results, but this is time and labour intensive and compliance drops with the number of stool samples requested. If an endemic population is first screened serologically by indirect haemagglutination assay (IHA) or enzyme-linked immunosorbent assay (ELISA) and seropositives confirmed by the KK method, correlation analysis indicates that the positivity rate with KK rises with the number of fecal specimens and slides used. Those individuals that were egg-positive but negative by IHA or ELISA were mainly cases with low infection intensity (Zhang et al. Reference Zhang, Hu, Xie, Tao, Yuan, Liu, Li, Li and Lin2012a ).
It is important to obtain purified eggs free from fecal components in order to increase diagnostic sensitivity and improve microscopic visualization of S. japonicum eggs. Two novel egg purification methods were recently described, which have potential for use in areas with low infection intensity, or where there is suspected elimination of schistosomiasis japonica following control efforts. The formalin-ethyl acetate sedimentation-digestion (FEA-SD) technique (Xu et al. Reference Xu, Gordon, Hu, McManus, Chen, Gray, Ju, Zeng, Gobert, Ge, Lan, Xie, Jiang, Ross, Acosta, Olveda and Feng2012) is effective for identifying and quantifying S. japonicum eggs in fecal samples from infected bovines in endemic areas. FEA-SD removes about 70% of debris from fecal samples and the remaining material is translucent. Another method for Schistosoma egg detection has been developed that is based on magnetic fractionation of parasite eggs from fecal matter (Fagundes Teixeira et al. Reference Fagundes Teixeira, Neuhauss, Ben, Romanzini and Graeff-Teixeira2007). With this approach, termed Helmintex, magnetic microspheres are mixed with fecal samples to make parasite egg–magnetic microsphere conjugates. The magnetic microspheres and eggs are then co-purified from other fecal material using a magnetic field and field gradient. The concentrated and purified eggs are more easily detectable by microscopy. This method is able to screen larger sample volumes, resulting in improved diagnostic sensitivity, although the mechanism of formation of the conjugates is unknown but may reflect the specific surface features of eggs and microspheres, or to their magnetic properties (Karl et al. Reference Karl, Gutierrez, Lucyk-Maurer, Kerr, Candido, Toh, Saunders, Shaw, Suvorova, Hofmann, House, Woodward, Graeff-Teixera, St Pierre and Jones2013).
Immunological methods
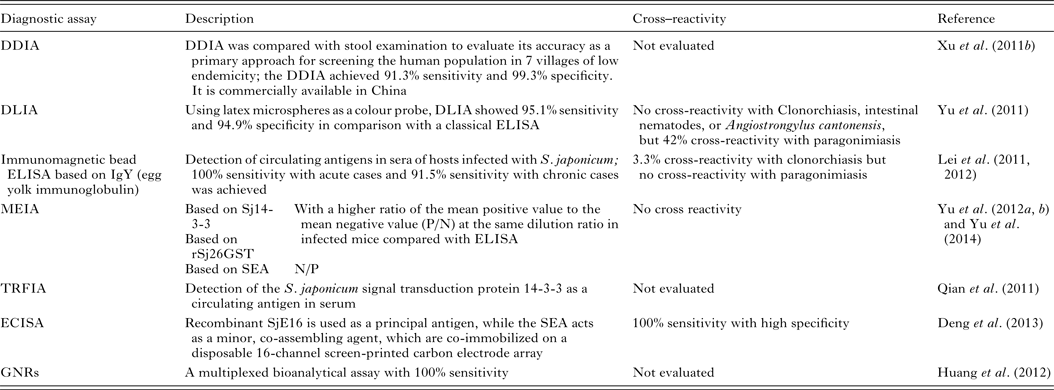
Patent schistosome infections are generally highly immunogenic, and anti-schistosome antibodies can be readily detected in subjects using a wide range of immunodiagnostic techniques. Many variations of indirect immunological approaches in schistosomiasis diagnosis include the circumoval precipitin test (COPT) and the IHA which have been widely used historically, and ELISA and dipstick dye immunoassays (DDIA), which have been used more frequently in the last 20 years. ELISA, using soluble egg antigen (SEA) as the source of target antigen, is the most widely used technique currently (Doenhoff et al. Reference Doenhoff, Chiodini and Hamilton2004). HSP70 and 78 kDa glucose-regulated protein, both present in SEA, may have diagnostic value for detecting early S. japonicum infections (Wang et al. Reference Wang, Song, He, Yin, Qian, Zhang, Xu, Cao, Ke and Yu2012). The modified fast ELISA (F-ELISA), which combines the 2-step routine ELISA into a single step making the assay process faster and simpler without sacrificing diagnostic accuracy (Hua et al. Reference Hua, Yu, Yin and Qian2011), may provide a rapid and practical method for schistosomiasis diagnosis in the field. Recently, more rapid, sensitive and portable diagnostic assays to detect human antibodies against S. japonicum, have been developed and tested in areas of low endemicity for schistosomiasis japonica in China (Table 3). These methods include: (1) DDIA (Xu et al. Reference Xu, Feng, Lin, Wang, Tang, Wu, Guo, Peeling and Zhou2011a ) which is commercially available in China, and the dipstick with latex immunochromatographic assay (DLIA) (Yu et al. Reference Yu, Ding, Wen, Lou, Yan, Lin, Lu, Lin and Zhou2011); (2) magnetic affinity enzyme-linked immunoassays (MEIAs), based on the signal transduction protein 14-3-3 (Sj14-3-3), recombinant 26 kDa GST (rSj26GST) (Yu et al. Reference Yu, Yang, Feng, Yang and Zhu2012a ), or SEA-MEIA (Yu et al. Reference Yu, Yang, Feng, Zhu and Yang2012b ); (3) a time-resolved fluoroimmunoassay (TRFIA), established for detecting Sj14-3-3, as a circulating antigen in serum (Qian et al. Reference Qian, Huang, Yu, Zhang, Yin, Wang, Song, Zhang and Ke2011); (4) an electrochemical immunosensor array (ECISA) assay (Deng et al. Reference Deng, Xu, Hu, Li, Hu, Song, Feng and Fan2013) which uses a recombinant S. japonicum calcium-binding protein (SjE16), with SEA, co-immobilized on a disposable 16-channel screen-printed carbon electrode array. Antibodies in serum samples can be detected by a portable electrochemical detector; (5) a multiplexed bioanalytical assay which is developed by fusing 2 types of gold nanorods (GNRs) (Huang et al. Reference Huang, Liu, Huang, Yuan, Liao, Yi, Zeng and Chu2012). This technique allows the serum-based diagnosis of subjects infected with S. japonicum without the need for sample pretreatment and it can diagnose individuals co-infected with schistosomiasis and TB.
Table 3. Immunoassays for diagnosing S. japonicum infection
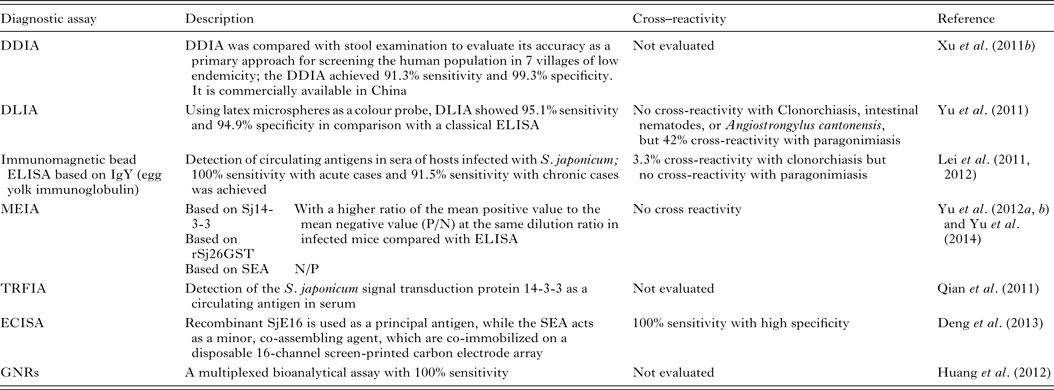
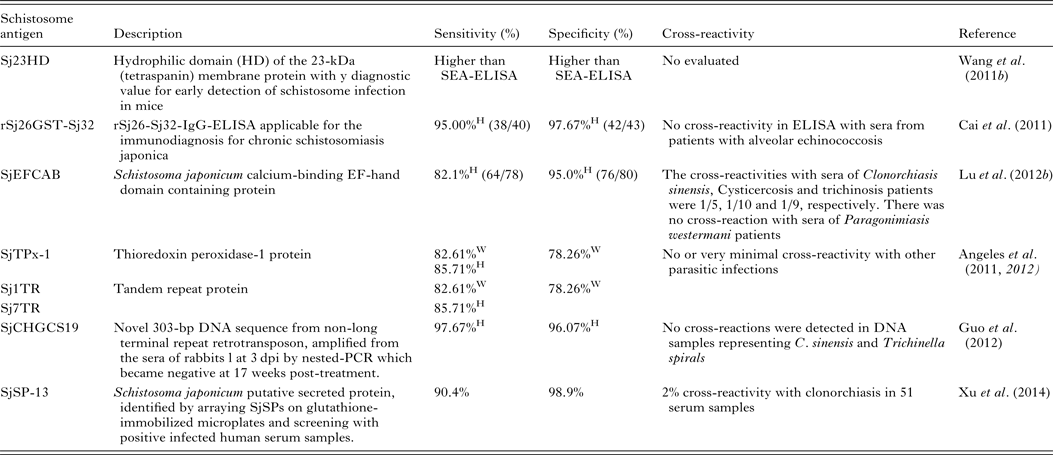
Serodiagnosis of schistosomiasis suffers from a number of drawbacks common to the antibody-based detection of other parasitic infections (Doenhoff et al. Reference Doenhoff, Chiodini and Hamilton2004; Rabello and Enk, Reference Rabello and Enk2006). One difficulty is in identifying an active from a previous infection, as parasite-specific antibodies remain detectable long after cure, and another is the inability to quantify infection intensity. A range of approaches to improve the accuracy of immunodiagnostic assays have been described. These include using specific parasite extracts such as cercarial antigens (Chand et al. Reference Chand, Chiodini and Doenhoff2010), using recombinant proteins as immunodiagnostic targets (Zhou et al. Reference Zhou, Wu, Huang, Kunnon, Zhu and Chen2010) or using a pool of synthetic peptides selected on the basis of the amino acid sequence of proteins from different antigenic schistosome preparations (de Oliveira et al. Reference de Oliveira, Kanamura, Takei, Hirata, Valli, Nguyen, de Carvalho Rodrigues, de Jesus and Hirata2008). Another approach that has been used with success in China is to combine information from serological surveys with parasitological data and to use a Bayesian statistical approach to develop accurate epidemiological maps of infection prevalence (Wang et al. Reference Wang, Wu and Zhou2006b ). So far, a number of candidate antigens have been tested for diagnosing S. japonicum infection; these include Sj23HD (Wang et al. Reference Lin, Liu, Hu, Li, Tao, Yuan, Xie, Huang, Jiang, Li, Gao and Wang2011b ), rSj26GST-Sj32 (Cai et al. Reference Cai, Li and Wang2011), SjEFCAB (Lu et al. Reference Lu, Xu, Ju, Mo, Chen, Feng, Wang and Hu2012b ), SjTPx-1 (Angeles et al. Reference Angeles, Goto, Kirinoki, Asada, Leonardo, Rivera, Villacorte, Inoue, Chigusa and Kawazu2012) and Sj1TR (Angeles et al. Reference Angeles, Goto, Kirinoki, Asada, Leonardo, Rivera, Villacorte, Inoue, Chigusa and Kawazu2012), Sj7TR (Angeles et al. Reference Angeles, Goto, Kirinoki, Leonardo, Tongol-Rivera, Villacorte, Inoue, Chigusa and Kawazu2011), SjCHGCS19 (Guo et al. Reference Guo, Zheng, Xu, Zhu, Wang and Xia2012), SjLAP (Zhang et al. Reference Zhang, Gao, Hou, Chen, Ji and Wu2011), whose characteristics are shown in Table 4. Recently, Xu et al. (Reference Xu, Zhang, Lin, Zhang, Xu, Liu, Hu, Qing, Xia and Pan2014) undertook a genome-wide survey to discover diagnostic protein markers for S. japonicum infection. In the study, 204 S. japonicum predicted secreted proteins (SjSPs) were arrayed on glutathione-immobilized microplates and screened with 302 patient serum samples that were diagnosed by the KK method as egg positive. One protein, SjSps-13, was identified as a potential diagnostic protein marker with 90·4% sensitivity and 98·9% specificity and its diagnostic validity was tested in ELISA using 1371 resident samples in a field study. The current scarcity of effective diagnostic tools is a dominant factor precluding the effective management of schistosomiasis (Colley et al. Reference Colley, Bustinduy, Secor and King2014) so the application of this newly developed sensitive, specific and affordable rSP13-ELISA method should help reduce schistosomiasis transmission through targeted treatment of individuals, particularly with low-intensity infections, and therefore support schistosomiasis control and elimination strategies (Xu et al. Reference Xu, Zhang, Lin, Zhang, Xu, Liu, Hu, Qing, Xia and Pan2014).
Table 4. Novel diagnostic molecules to detect early S. japonicum infection
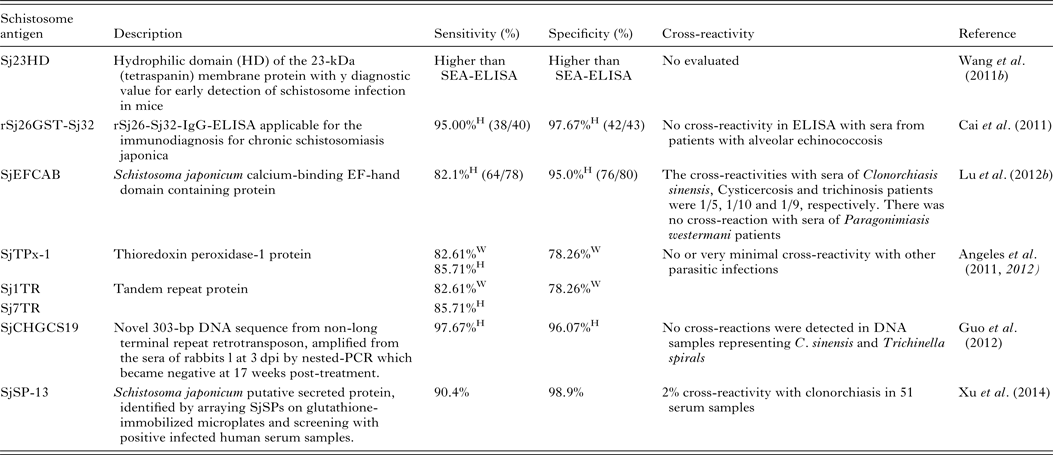
W, water buffaloes; H, human; SEA, soluble egg antigen.
An early study using immunoassays demonstrated the presence of schistosome-derived antigens in the circulation and/or excreta of hosts with schistosomiasis (Deelder et al. Reference Deelder, Klappe, van den Aardweg and van Meerbeke1976), and this report stimulated considerable research on antigen detection as a means of diagnosing the disease. Indeed, detection of schistosome circulating antigens (CAs) has now been shown to be an efficient method to differentiate between previous exposure and current infection. In one approach, AWA egg yolk immunoglobulin (IgY) was generated by immunization of hyline chicken hens with AWA. The purified IgY was then immobilized onto resin to immune-precipitate CAs in sera from infected subjects. Four proteins including protein BUD31 homologue, ribonuclease, SJCHGC06971 protein and SJCHGC04754 protein were isolated from the precipitated proteins that could be employed as diagnostic biomarkers (Lu et al. Reference Lu, Xu, Ju, Mo, Chen, Feng, Wang and Hu2012a ). A novel immunomagnetic bead ELISA, based on IgY, has also been used for detection of circulating CAs in sera or urine of hosts infected with S. japonicum (Lei et al. Reference Lei, Su, Xu, Shen, Guan, Feng, Li, Xu and Liu2011, Reference Lei, Guan, Xu, Chen, Su, Zhou, Wang, Li and Liu 2012 ) (Table 3).
Molecular methods
Some of the deficiencies of currently available diagnostic methods for intestinal schistosomiasis are that: (1) early diagnosis of the disease using egg detection methods is problematical. Generally, egg production within the host takes several weeks as does the passage of eggs through the gut wall, their discharge into the intestinal lumen and their release in feces; (2) variability in egg shedding and problems in distribution of eggs in the analysed sample often leads to unreliable results when microscopic examination for eggs is performed; (3) as emphasized earlier, serological tests, based on antibody detection, do not discriminate between active infection and previous exposure. Accordingly, the application of the polymerase chain reaction (PCR) as a tool for the diagnosis of schistosomiasis has been explored for detection of schistosome DNA in human and bovines feces (Fung et al. Reference Fung, Xiao, Wang and Carlton2012), in serum/plasma (Wichmann et al. Reference Wichmann, Panning, Quack, Kramme, Burchard, Grevelding and Drosten2009) and in urine (Obeng et al. Reference Obeng, Aryeetey, de Dood, Amoah, Larbi, Deelder, Yazdanbakhsh, Hartgers, Boakye, Verweij, van Dam and van Lieshout2008) and the approach has proven to be of value (Xu et al. Reference Xu, Liu, Guo, Wang, Qiu, Sun, Guan, Zhu, Xia and Wu2013). Some of these highly sensitive and specific PCR-based methods have been assessed in areas with medium or low intensity of schistosome infection. A combination of real-time PCR (qPCR) and the earlier described FEA-SD technique (Xu et al. Reference Xu, Gordon, Hu, McManus, Chen, Gray, Ju, Zeng, Gobert, Ge, Lan, Xie, Jiang, Ross, Acosta, Olveda and Feng2012) was used in the Philippines to determine the prevalence and intensity of S. japonicum, thereby providing a more accurate diagnosis to evaluate the potential role of bovines in the transmission of S. japonicum (Gordon et al. Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012). It should be stressed, however, that PCR-based methods give positive results only if the analysed fecal sample contains schistosome DNA and inhibitors present in feces may affect the PCR assay working optimally.
In another diagnostic approach, Wichmann et al. (Reference Wichmann, Panning, Quack, Kramme, Burchard, Grevelding and Drosten2009) used real-time PCR to detect cell free parasite DNA (CFPD) in human plasma according to the principle that Schistosoma worms contain DNA copies, which may be released due to parasite turnover and reach the blood, in stoichiometrical excess over parasite count and that schistosome DNA. This method may provide a new laboratory tool to detect schistosome infection in all phases of clinical disease (Wichmann et al. Reference Wichmann, Panning, Quack, Kramme, Burchard, Grevelding and Drosten2009).
Another molecular approach is loop-mediated isothermal amplification (LAMP), a highly sensitive DNA detection method that is proving of value for the diagnosis of a number of pathogenic organisms including schistosomes. A LAMP assay has been established that detects S. japonicum DNA in fecal and serum samples of rabbits and in sera of infected human subjects. Based on the sequence of a highly repetitive retrotransposon, SjR2, the LAMP method was considerably more sensitive than traditional PCR being able to measure 0·08 fg S. japonicum and appropriate for clinical diagnosis and therapeutic evaluation of human schistosomiasis (Xu et al. Reference Xu, Rong, Zhang, Shi, Zhu and Xia2010). LAMP appears suitable for the detection of early S. japonicum infection in certain patients including travellers, migrants, military personnel and younger aged subjects but it is likely to be of less value for determining the efficacy of drug treatment in the early stages because of its high sensitivity (Wang et al. Reference Wang, Chen, Yin, Hua, Hou, Ji, Yu and Wu2011a ).
Recently, circulating microRNAs (miRNAs) have attracted attention as novel biomarkers for the diagnosis of schistosomiasis and also for further understanding the host–schistosome interaction. Deep sequencing identified 5 schistosome-specific miRNAs (Bantam, miR-3479, miR-10, miR-3096 and miR-0001) in the plasma of S. japonicum-infected rabbits, 4 of which were detectable by real-time reverse transcription-PCR in the plasma of mice infected with S. japonicum (Cheng et al. Reference Cheng, Luo, Hu, Cao and Jin2013). Another miRNA molecule, miR-223, was identified by He et al. (Reference He, Sai, Chen, Zhang, Xu, Zhang and Pan2013) as a potential new biomarker of S. japonicum infection and the assessment of the response to PZQ treatment. Parasite-derived miRNAs have also been identified (Anna et al. Reference Hoy, Lundie, Ivens, Quintana, Nausch, Forster, Jones, Kabatereine, Dunne, Mutapi, MacDonald and Buck2014) as novel markers of S. mansoni infection in mice and humans, some of which could distinguish ‘egg-negative’ from ‘egg-positive’ individuals with high specificity and sensitivity with potential diagnostic value. However, this study showed that, whereas several host miRNAs were shown to be dysregulated in the livers of mice during S. mansoni infection, they were unable to serve as reliable serum biomarkers of infection in humans (Anna et al. Reference Hoy, Lundie, Ivens, Quintana, Nausch, Forster, Jones, Kabatereine, Dunne, Mutapi, MacDonald and Buck2014). These data contrast with those of Han et al. (Reference Han, Peng, Hong, Zhang, Han, Liu, Fu, Shi, Xu, Tao and Lin2013) who applied a miRNA microarray to investigate differences in miRNA expression in different tissues, including the liver, of mice before and 10 dpi with S. japonicum and detected a total of 220 miRNAs in the different tissues whose functions correlated with nutrient metabolism, the immune response, apoptosis, signalling pathways and cell differentiation (Han et al. Reference Han, Peng, Hong, Zhang, Han, Liu, Fu, Shi, Xu, Tao and Lin2013). As pointed out by Hoy et al. (Reference Hoy, Lundie, Ivens, Quintana, Nausch, Forster, Jones, Kabatereine, Dunne, Mutapi, MacDonald and Buck2014), these conflicting results may have been due to the very early time point (10 dpi) used in the S. japonicum study.
FUTURE DIRECTIONS
The key to eliminating schistosomiasis is to reduce transmission combined with morbidity control, an approach that is more cost effective than treating the clinical outcome of continued re-infection, and which is currently being used successfully in China (Sun et al. Reference Sun, Wang, Liang, Tian, Hong, Yang, Yang, Dai and Gao2011). Central to this goal is to integrate traditional control measures – PZQ treatment, the use of molluscicides, environmental modification, health education/promotion, enhanced water supply (Kosinski et al. Reference Kosinski, Adjei, Bosompem, Crocker, Durant, Osabutey, Plummer, Stadecker, Wagner, Woodin and Gute2012) and improved sanitation – with an effective vaccine, a tool that is needed to accelerate intervention efforts to eliminate a disease that has existed for many centuries. In this respect, the Chinese experience serves as a good model, whereby a comprehensive control approach based on interventions to block transmission of S. japonicum infection from bovines and humans to snails has proven highly effective (Seto et al. Reference Seto, Remais, Carlton, Wang, Liang, Brindley, Qiu, Spear, Wang, Wang, Chen, Dong, Wang, Hao, Bergquist and Zhou2011). As emphasized earlier, it has been established that bovines are the major animal reservoir host for S. japonicum in China and the Philippines (McManus and Loukas, Reference McManus and Loukas2008) and this fact underpins efforts to develop a bovine transmission-blocking vaccine against S. japonicum as an effective and feasible objective. Recent developments in the preclinical and clinical testing of existing anti-schistosome vaccine candidates have been encouraging (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). Furthermore, the most recent comprehensive understanding of schistosome genomes and proteomes has equipped us with all the information for antigen choice as novel vaccine targets. Based on the fact that the membrane proteins located on the tegument of the schistosomulum and the adult worm are rational vaccine targets (Loukas et al. Reference Loukas, Tran and Pearson2007), we need to select the best antigen or combined antigens as a schistosomiasis vaccine using other innovative tools. Defining a clear target product profile (TPP) for an optimum schistosomiasis vaccine and using new technologies and tools which are available for new antigen discovery, clinical research and vaccine efficacy assessment will all contribute to accelerated progress (Mo et al. Reference Mo, Agosti, Walson, Hall and Gordon2014). Effective schistosomiasis vaccines and new drugs that act on both adult and larval schistosomes would help to achieve and sustain disease control and eventual elimination. Importantly as well, further research, similar to that described by Xu et al. (Reference Xu, Zhang, Lin, Zhang, Xu, Liu, Hu, Qing, Xia and Pan2014), is required to develop improved diagnostic tests that are able to identify light S. japonicum infections so as to determine the extent of the interruption of transmission in an endemic area and to establish whether control efforts have led to schistosomiasis elimination.
ACKNOWLEDGEMENTS
DPM is a National Health and Medical Research Council (NHMRC) of Australia Senior Principal Research Fellow and HY is a NHMRC Early Career Fellow. DPM acknowledges project and programme grant support from NHMRC for his studies on schistosomiasis.
FINANCIAL SUPPORT
This review was supported by the National Health and Medical Research Council of Australia (grant number APP1037304).