INTRODUCTION
Maternal provision for offspring is a crucial determinant for both female and offspring fitness (Smith and Fretwell, Reference Smith and Fretwell1974; Parker and Begon, Reference Parker and Begon1986; Roff, Reference Roff1992). Viviparity, i.e. prolonged maternal provision and offspring development inside the body of the mother, increases offspring survival by increasing offspring size and shortening the vulnerable juvenile phase. Production of extremely large offspring early in life has costs for females in terms of lower residual reproductive value as well as decreased survival (Stearns, Reference Stearns1992; Kindsvater et al. Reference Kindsvater, Alonzo, Mangel and Bonsall2010). Furthermore, as a result of high maternal provision per offspring, the period of offspring production is often long and asynchronous (Langley and Clutton-Brock, Reference Langley and Clutton-Brock1998; Härkönen et al. Reference Härkönen2012).
One consequence of a prolonged reproductive period is that the seasonal conditions that the young will experience vary according to offspring birth time. Warm-blooded hosts may offer a suitable thermal environment and resource supply for blood-feeding ectoparasites, even in winter (Tinsley, Reference Tinsley1999). The juvenile stages outside the host may depend totally on maternally derived resources until they find a host as adults. Thus, the relationship between maternal provision and the offspring environment determines offspring performance during the free-living stages (Langley and Clutton-Brock, Reference Langley and Clutton-Brock1998; Härkönen et al. Reference Härkönen2012). To our knowledge, there are no previous studies on how seasonal changes in this relationship affect offspring performance in viviparous ectoparasites with a long reproductive period.
Offspring size often varies with maternal age or as a result of seasonal phenotypic plasticity. First, in invertebrates, young females with high amount of resources available for reproduction often produce larger offspring with higher performance than old females (Mousseau and Dingle, Reference Mousseau and Dingle1991; Plaistow et al. Reference Plaistow, Clair, Grant and Benton2007; Kindsvater et al. Reference Kindsvater, Alonzo, Mangel and Bonsall2010). Second, females may adjust their offspring size in response to predictable environmental cues that signal future environmental conditions for the offspring (i.e. anticipatory or cued plasticity). For example, large offspring tend to perform better than small ones in cold environments (Colinet et al. Reference Colinet, Hance and Vernon2006; Härkönen et al. Reference Härkönen2012). Females may produce larger propagules if cold or otherwise poor conditions are expected for their offspring, or the size can vary according to the length of unfavourable conditions (Landa, Reference Landa1992; Fischer et al. Reference Fischer, Brakefield and Zwaan2003; Bownds et al. Reference Bownds, Wilson and Marshall2010). Alternatively, the seasonal variation in offspring size may be an immediate and unavoidable side effect of environmental heterogeneity for females (i.e. responsive or direct phenotypic plasticity): the resources available for reproduction may vary seasonally affecting the provisioning of the offspring (Langley and Clutton-Brock, Reference Langley and Clutton-Brock1998).
A viviparous ectoparasite, the deer ked (Lipoptena cervi), produces offspring highly asynchronously between autumn and spring. Blood-feeding females give birth to 1 pre-pupa at a time, which drops off the host after pupation (Haarløv, Reference Haarløv1964). The offspring rely totally on maternally derived resources through diapause in winter, pupal development in summer and also as they become host-searching adults in autumn. Due to high seasonal variation in offspring birth time, the young will encounter different environmental conditions: early-born pupae in autumn have a long winter ahead and their non-feeding period lasts almost a year, whereas late-born pupae have only a short winter, and a few non-feeding months ahead.
The aim of our study was to investigate whether offspring size varies seasonally in relation to the length of winter diapause, and how off-host performance depends on birth size. It was expected that an increase in offspring energy reserves (i.e. size) would promote survival during prolonged periods of low temperatures and starvation (Colinet et al. Reference Colinet, Hance and Vernon2006). We investigated whether young deer ked females in the autumn produce large propagules in order to ensure the survival of the offspring throughout their long diapause and off-host period. We also tested how individual variation in offspring size and diapause duration would contribute to offspring performance.
MATERIALS AND METHODS
Life cycle of the study species
The deer ked is a louse-fly on several boreal cervids. The main host is moose (Alces alces; Välimäki et al. Reference Välimäki, Kaitala, Madslien, Härkönen, Várkonyi, Heikkilä, Jaakola, Ylönen, Kortet and Ytrehus2011). The lifespan of a deer ked may extend as long as 2 years. Reproducing adults remain active through the winter on the host. Females presumably produce a maximum of a few dozen offspring that overwinter on the ground at pupal diapause (Haarløv, Reference Haarløv1964). Diapause termination does not require a period of cold, but pupal development starts soon after exposure to high temperatures (Härkönen, Reference Härkönen, Kaitala, Kaunisto and Repo2012). Development takes approximately 3 months, adults emerge in late summer, and the main host search period takes place in September (Härkönen et al. Reference Härkönen, Härkönen, Kaitala, Kaunisto, Kortet, Laaksonen and Ylönen2010; Kortet et al. Reference Kortet, Härkönen, Hokkanen, Härkönen, Kaitala, Kaunisto, Laaksonen, Kekäläinen and Ylönen2010). After finding a host, adults drop their wings off. According to Ivanov (Reference Ivanov1981), reproduction begins a month after the adults have attached to the host. The majority of adults stay alive until early summer (May).
Material collection and preparation
All the collected deer ked pupae originated from the same moose population ranging in the surroundings of Siikalatva commune (64°N: 25°E), Central Finland. Deer ked pupae (N = 2030) were collected once a month between October 2009 and April 2010. The collection month was used as a proxy for maternal age as follows: females were considered young in late autumn and early winter (Oct–Dec), middle-aged in mid-winter (Jan–Feb), and old when approaching the end of their lifespan in late winter (Mar–Apr).
Reproductive adult deer keds cannot be reared in the laboratory due to their blood feeding habits and dependence on the host environment. After pupation, the pupae drop off the host fur often onto host bedding sites. The black pupae are easy to find in the wild during the snowy winter period, but difficult to locate in the soil without snow cover. Due to the lack of snow from October to December pupae were collected from the pelts of a few recently killed moose (killed by local hunters during the moose hunting season). The pupae (on average 300 pupae/moose) were picked from the pelt within 18 h after a moose had been shot. A deer ked female gives birth to a white third-instar larva, a pre-pupa. Pupation (including hardening and melanization of the puparium) takes several hours after birth (Bequaert, Reference Bequaert1953). The complete puparium is black in colour, but if pupation fails, the colour remains partly white/red/brown. Failed pupation leads to unviable pupa, and therefore only completely pupated individuals were selected for the following experiments. This selection guaranteed that, at the time of collection, newborn pupae were viable and, with high probability, voided before the moose were killed. From January to April the pupae were collected from the bedding sites of moose. We searched for recently used bedding sites (indicating that the pupae were born during the last few days) by following fresh moose tracks, and collected completely pupated individuals from the snow cover. The exact host individual was not known for all the pupae.
Offspring size
To test offspring size in relation to seasonal variation in their birth time and expected diapause duration, pupae were collected once a month throughout the main reproductive period on 12 October (n = 200), 17 November (n = 132), 9 December (n = 168), 15 January (n = 143), 18 February (n = 140), 18/23 March (n = 66) and 8 April (n = 67). Pupal mass was used as an indicator of the offspring size, and was measured with a precision balance (Mettler Toledo MT 5, accuracy of 0·001 mg).
We are aware that the pupae collected directly from the pelts may not be of similar quality to those collected from the bedding sites, but this was the only way to acquire material throughout the main reproductive period. In the field, pupae were collected from several different host individuals each month. In the beginning of the field season (January), we first investigated whether the pupal size varied between the bedding sites when collected at the same time. Visual examination of the bedding sites (see Kaunisto et al. Reference Kaunisto, Kortet, Härkönen, Härkönen, Ylönen and Laaksonen2009) showed that nearly all moose in the study area were heavily parasitized (see also Välimäki et al. Reference Välimäki, Kaitala, Madslien, Härkönen, Várkonyi, Heikkilä, Jaakola, Ylönen, Kortet and Ytrehus2011). However, although there was high variation in the number of pupae found on the bedding sites, this was most probably due to heavy predation by birds in the study area (Kaunisto et al. Reference Kaunisto, Välimäki, Kortet, Koskimäki, Härkönen, Kaitala, Laaksonen, Härkonen and Ylönen2012) rather than variation in parasite prevalence on the hosts. It is not yet known, for instance, whether the birds are more likely to prey on large pupae (since they may be easier to locate). Thus, to check the variation between bedding sites, we used only bedding sites with 15 or more pupae (n = 7, number of pupae ranging from 15 to 39) indicating that they were intact by avian predators (see Kaunisto et al. Reference Kaunisto, Välimäki, Kortet, Koskimäki, Härkönen, Kaitala, Laaksonen, Härkonen and Ylönen2012). These 7 bedding sites comprised 51% of all pupae (n = 361) collected in January.
Effect of diapause on survival
To study offspring survival from birth to adult emergence, we conducted 2 different diapause treatments. In the first treatment, survival was tested in relation to birth month, per se, by manipulating the pupae in such a way as to skip the diapause (i.e. to exclude the effect of varying diapause length). After measuring pupal mass (of the pupae described above), each pupa was enclosed individually in a transparent plastic cup (5 ml). These pupae were placed in a climate chamber at constant 17 °C with a relative humidity of 60%, with additional moisturising approximately every third day until the adults emerged.
In the second treatment, offspring survival was studied after natural, varying length of diapause by collecting another set of pupae. These were collected on the same dates as above [October (n = 206), November (n = 121), December (n = 117), January (n = 155), February (n = 125), March (n = 79) and April (n = 84)]. Overwintering took place in a dark cold room (+5 °C, as chilling at +5–7 °C has been used also for other Hippoboscid pupae to maintain their diapause; Kennedy et al. Reference Kennedy, Smith and Smyth1975) until the following spring (14 May, when temperatures in the wild often become high enough to start pupal development). The exposure to low temperature was thus the same for each pupa as they would have experienced in the wild (i.e. from 1 month for pupae produced in April to 7 months for October pupae). The mass of these pupae was measured after diapause.
Weight loss during diapause of varying length
To study the usage of energy reserves for body maintenance during diapause of varying length and its effects on survival, we used a set of pupae collected in January. Pupal mass was measured immediately after collection and the pupae were divided into 3 treatments with varying diapause duration. The control group consisted of 143 pupae that developed without a cold experience (see above), while for the second group we collected additional 64 pupae that overwintered for 1 month (30 days). The third group consisted of 64 pupae that overwintered for 4 months (i.e. natural diapause duration, see above). The pupal masses (immediately after collection) did not differ between the diapause treatments (One-way ANOVA: F 2,270 = 0·868, P = 0·421). We are aware that the above-zero temperature (+5 °C) used in this experiment may increase the rate of energy consumption. However, during diapause the metabolic rate should be suppressed at even higher temperatures (Tauber et al. Reference Tauber, Tauber and Masaki1986). After the experimental diapause period the pupae were weighed again and placed in the climate chamber to develop. Survival was assessed as adult emergence success.
Statistical analyses
Using SPSS for Windows (version 15.0), offspring performance was analysed in terms of survival from birth to adulthood, by (1) tracing first the factors predicting offspring survival, and then (2) examining in detail the seasonal variation in those factors contributing to offspring survival. To take into account both the collection method and maternal age in the analysis, we created an indicator factor as follows: Early season (pupae produced by young females and collected in Oct–Dec from pelts), Mid-season (pupae produced by middle-aged females and collected in Jan–Feb from bedding sites) and Late season (pupae produced by old females and collected in Mar–Apr from bedding sites). The category chosen for pair-wise comparisons was Mid-season, in order to estimate the differences, not only as a result of the change in collection method (between Early season and Mid-season) but also as a result of maternal aging (between Mid-season and Late season).
Binary logistic regression analysis was used to find the factors predicting offspring survival in general. Offspring survival was assessed as emergence success (thus including mortality during overwintering and/or pupal development). We first fitted diapause duration (1–7 months) and pupal size, and their interaction to the model. The moment of the reproductive season indicating both collection method and maternal age was also included to the model.
For detailed analysis of the factors contributing to offspring survival, we first analysed how offspring size varies along with the reproductive period. The mixed linear regression model was used to test offspring size variation by setting the birth month as a fixed factor and also including the indicator factor for maternal age. The host individual (determined as a pelt or a bedding site if known) was added as a random factor to the model. One-way ANOVA was used to further analyse whether the pupal masses varied between the bedding sites when collected at the same time (January). Second, a generalised linear model with binomial distribution and logit link function was used to examine how survival probability is affected by diapause compared to a control treatment in which the diapause period was omitted. In the model, all main effects and interactions were first included in a full model, which was then simplified by reducing the parameters according to the principle of parsimony in order to obtain the minimal sufficient model. We set birth month and diapause treatment (natural vs. no diapause) as fixed factors and pupal mass as a covariate. Using the same parsimonious protocol, a general linear model was applied to analyse whether the proportion of weight loss varied in relation to birth size or diapause treatment.
RESULTS
Factors predicting offspring survival
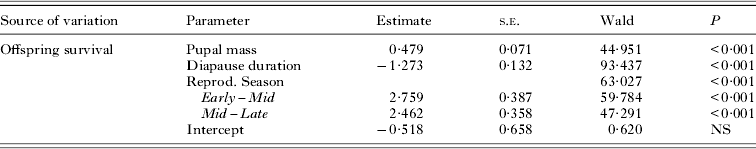
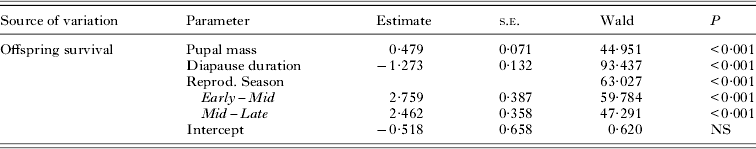
The offspring produced in October with the longest diapause had a very low survival rate (3%) compared to late-born ones in April with the shortest diapause (61%), whereas survival from November to March was relatively consistent (31–42%; Fig. 1). Both offspring size and diapause duration contributed to the probability of pupal survival from birth to adulthood (Table 1). Offspring collected from moose pelts in the Early season had on average the lowest survival, and survival was significantly higher in pupae collected later from bedding sites in Mid-season. The survival rate also increased significantly from Mid-season to Late season, suggesting that the time of reproductive season has a significant effect on offspring survival probability (Table 1).

Fig. 1. Survival of pupae from birth until adult emergence after direct development (i.e. no diapause) and natural diapause duration. In the latter, pupae produced in October by young females had the longest diapause (7 months, on the left), and pupae produced by old females in April had the shortest diapause (1 month, on the right).
Table 1. Binary logistic regression for pupal survival in relation to pupal mass and diapause duration (varying from 1 to 7 months depending on birth month)
Offspring size variation
Mean pupal mass increased gradually after the collection method was changed (Fig. 2). However, there was great variance in offspring size within the pelts and bedding sites. For example, the mass of pupae collected from a moose pelt in October ranged from 6·3 to 11·7 mg (n = 132; within one pelt) whereas pupae collected from a bedding site with highest number of them (n = 39) in January varied between 7·5 and 12·0 mg (n = 39). The mean pupal mass did not vary between the bedding sites (F 6,183 = 1·458, P = 0·195), the mean range being from 7·6 to 11·2 mg.

Fig. 2. Seasonal variation in pupal mass (mg: mean ± 95% CI) of the deer ked.
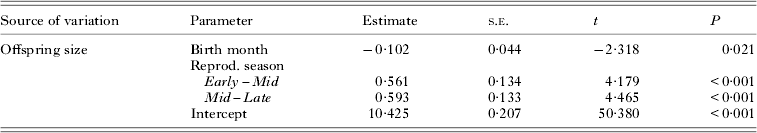
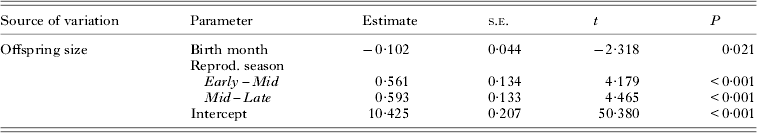
Despite the change in collection method and high variance in pupal mass within each birth month, the mean offspring size was significantly affected by birth month and maternal age (Table 2, Fig. 2). Pupae produced by young females (Oct–Dec) were the smallest (mg±s.e.: 9·67 ± 0·047, n = 475). Offspring size then increased along with maternal age: the pupae produced in Mid-season (Jan–Feb: 9·96 ± 0·052, n = 289) were larger than pupae produced by young females, but significantly smaller than pupae produced in Late season by old females (Mar–Apr: 10·36 ± 0·069, n = 133) (Table 2).
Table 2. Summary of mixed linear regression model for offspring size variation in relation to the time of birth
Effect of diapause on survival
Survival from birth to adulthood was affected by diapause (Treatment: Wald X 2 = 16·890, d.f. = 1, P < 0·001) and the time when a pupa was produced (Birth month: Wald X 2 = 25·221, d.f. = 6, P < 0·001, Fig. 1). Survival deteriorated after the pupae had experienced diapause of natural length (38·9%, N = 887) compared to treatment in which they developed directly after collection (45·8%, N = 924). Survival of late-born pupae did not differ as much between the two treatments as it did in early-born pupae (Birth month × Treatment: Wald X 2 = 94·896, d.f. = 6, P < 0·001, Fig. 1).
Large pupal mass at the beginning of developmental period increased survival (Pupal mass: Wald X 2 = 66·512, d.f. = 1, P < 0·001; B = 0·706 ± 0·207). Consequently, small early-born pupae had a lower survival rate than large late-born pupae (Pupal mass × Birth month: Wald X 2 = 27·264, d.f. = 6, P < 0·001; Fig. 1). After natural diapause, however, pupal mass was smaller than in pupae that started development without diapause (Pupal mass × Treatment: Wald X 2 = 14·648, d.f. = 1, P < 0·001; Fig. 3). The effect of large birth size on survival increases especially during the longest diapause: small October pupae survived relatively well after direct development but only the largest pupae (mean±s.e.: 11·47 mg ±0·99; n = 6) survived through their 7-month diapause (Figs 1 and 3).

Fig. 3. Pupal mass (mg: mean ± 95% CI) just before development, and in relation to birth month. Black circles represent the pupae that survived until adult emergence after direct development without diapause and, and white circles the survived pupae after natural diapause.
The effects of diapause duration and weight loss
An individual did not emerge if it had lost more than 21% of its mass during its diapause (Fig. 4). The proportion of pupal weight loss (%) decreased with the increasing length of diapause (Treatment: F 2,265 = 5·775, P = 0·004): when the pupae experienced no diapause, their post-diapause pupal mass was on average 10·06 mg ±0·774 (mean±s.e., n = 143). After 1 month in diapause the weight loss was on average 9·20% (−1·02 mg ±0·113, n = 64) and after 4 months it was 18·82% (−1·95 mg ±0·13, n = 64). The size at birth affected how much weight was lost during diapause (Birth size: F 1,265 = 4·193, P = 0·042): weight loss was relatively higher for small pupae than for large ones (Fig. 4). There was no interaction between size at birth and diapause duration (Birth size × Treatment: F 2,265 = 1·593, P = 0·205).

Fig. 4. Proportional decrease in pupal mass (%) after a diapause of either 1 month or 4 months in relation to mass at collection (i.e. before diapause). The weight loss and linear regression lines are presented for (a) only those pupae that survived until adult emergence and for (b) all pupae in the two treatments.
DISCUSSION
Seasonal variation in offspring size and diapause length contributed essentially to offspring survival. Diapause significantly reduced pupal energy reserves: if a pupa lost more than 21% of its weight during diapause it did not survive through the following developmental phase. Large offspring size increased survival into adulthood, especially during a long diapause. It was therefore surprising that the early-born offspring experiencing such conditions were provisioned with the lowest energy reserves. Our results thus did not support the prediction that females would adjust their offspring size according to the expected offspring environment outside the host.
Diapause is costly for the deer ked. The weight loss accumulated with prolonging diapause and it was relatively higher for small pupae. Small individuals often have a higher metabolic rate than do large individuals (Peters, Reference Peters1983; Matsuo, Reference Matsuo2006), so small deer ked pupae may suffer from relatively higher energetic costs, especially if they have a long diapause. It is often thought that the negative effects of resource loss on further lifespan following diapause (e.g. additional mortality and fewer reproductive opportunities) are only involved in a diapause that extends to several years (Leather et al. Reference Leather, Walters and Bale1993; Matsuo, Reference Matsuo2006). Our results show that diapause may have a crucial role in offspring performance even if it is short. This suggests that individual variation in diapause costs should be taken into account in future life-history studies. For instance, the deer ked does not feed until on the host and thus the variation in birth size and diapause duration may be a crucial factor in determining their host search success.
The mean offspring size increased during the last 4 months of the study period. The smaller size before mid-winter may partly be due to the collection method: host stress during the hunting season or death of the host individual may have increased physiological stress for the ectoparasite female, and consequently affected the offspring size (Clutton-Brock, Reference Clutton–Brock1984). Further work is thus needed to control the seasonal effects of different host individuals on reproductive performance. Offspring size varied greatly also within the host individuals. Care should thus be taken when considering the effects of maternal age on offspring size in deer ked, as also other factors, such as female body size, are likely to affect offspring size (Marshall et al. Reference Marshall, Heppell, Munch and Warner2010).
In the deer ked, offspring size increased with maternal age. This was an unexpected pattern since it is rarely found in invertebrates (but see Kindsvater et al. Reference Kindsvater, Alonzo, Mangel and Bonsall2010; Marshall et al. Reference Marshall, Heppell, Munch and Warner2010). However, old females have been reported to produce large offspring if they have sufficient resources to do so (Fox, Reference Fox1993). Females with low residual reproductive value may also be able to transfer an increasing proportion of remaining resources to offspring provisioning (referred to as terminal investment in vertebrates; Clutton-Brock, Reference Clutton–Brock1991). Each propagule that a viviparous deer ked female produces is extremely large among invertebrates: a pupa weighs approximately the same as the blood-consuming female herself (see Paakkonen et al. Reference Paakkonen, Mustonen, Roininen, Niemelä, Ruusila and Nieminen2010). A high reproductive effort early in life may shorten adult lifespan and reduce their lifetime fecundity (Stearns, Reference Stearns1992). If also the survival probability of offspring is low, females may be expected to postpone their reproduction to an older age (Stearns, Reference Stearns1992; Marshall and Uller, Reference Marshall and Uller2007). In viviparous species a relatively small increase in the size of already large offspring rarely exceeds the benefits of increasing the offspring number (Schrader and Travis, Reference Schrader and Travis2008). Due to large propagule size and low fecundity, we think that the deer keds may be selected rather to guarantee a long reproductive lifespan and to increase the offspring number than to increase offspring size or postpone reproduction.
In ectoparasites, seasonal variation in offspring size may result from direct effects of the on-host condition and seasonal changes in the quality of host blood (Langley and Clutton-Brock, Reference Langley and Clutton-Brock1998). The body condition of moose declines as the winter progresses due to decreased food availability (Sæther and Gravem, Reference Sæther and Gravem1988). When the host condition declines, it is less able to develop and maintain costly immunological or physiological defence mechanisms, and its lowered resistance increases the quality of resources for blood-feeding ectoparasites (Roulin et al. Reference Roulin, Brinkhof, Bize, Richner, Jungi, Bavoux, Boileau and Burneleau2003; Tschirren et al. Reference Tschirren, Bischoff, Saladin and Richner2007). Accordingly, it was found that offspring size in the deer ked increased from midwinter towards the spring, which may indicate seasonal changes in the host effect on an ectoparasite's resource quality and thus on reproductive performance.
To conclude, seasonal variability among offspring in their size and diapause duration is an important component of the life-history evolution of the deer ked. The unexpected variation in offspring size is likely to result from direct effects of seasonal changes in female condition and on-host environment: old females in spring may be able to allocate more resources to offspring size as they approach the end of their life span, or, on the other hand, weakening moose condition may increase resource quality for females, resulting in higher reproductive performance. Further work on long-living ectoparasites is still needed to distinguish the effects of maternal reproductive costs and host effects on offspring performance in seasonal environments.
ACKNOWLEDGEMENTS
We are indebted to Juho-Antti Junno and Kivimäki Hunting Club, Panu Välimäki and Heidi Kosunen for their help in the material collection. We owe special thanks to Tero Klemola, Tiit Teder and the reviewers for their careful work with the manuscript, and also to Jouni Aspi, Jukka Forsman, Sirpa Kaunisto, Petri Niemelä, Katri Ronkainen and Maria Tuomaala for their helpful comments on earlier drafts of the manuscript. We thank Rosemary Mackenzie and Marko Pyhähuhta for checking the English. This study complies with the current laws of Finland.
FINANCIAL SUPPORT
This study was funded by the Academy of Finland (A.K., E.H., L.H.) and by personal grants to L.H. (University of Oulu, Ella and Georg Ehrnrooth Foundation, Oskar Öflund Foundation and Multidisciplinary Environmental Graduate Net school).