INTRODUCTION
The ciliated protozoan Cryptocaryon irritans Brown, 1951 is an obligate parasite that causes cryptocaryonosis in a wide range of marine teleosts. Over the years, this disease has had a significant negative impact on the marine aquaculture industry worldwide, resulting in large economic losses (Colorni and Burgess, Reference Colorni and Burgess1997; Dickerson, Reference Dickerson, Woo and Leatherland2006). Many chemical and physical methods have been used to control this parasite, and several biological substances have been found to be lethal to C. irritans or useful as potential antigens for vaccine development. These exhibit various degrees of efficacy and frequently accompany with various drawbacks (Pironet and Jones, Reference Pironet and Jones2000; Hirazawa et al. Reference Hirazawa, Oshima, Hara, Mitsuboshi and Hata2001, Reference Hirazawa, Goto and Shirasu2003; Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007; Bai et al. Reference Bai, Xie, Zhu, Dan and Li2008; Wang et al. Reference Wang, Xie and Li2010; Huang et al. Reference Huang, Sun, Guo, Zheng, Xu, Yuan and Liu2012; Li et al. Reference Li, Dan and Li2013; Niu et al. Reference Niu, Jin, Xu, Qiao, Wu, Mao, Su and Wang2013; Rigos et al. Reference Rigos, Karagouni, Kyriazis, Athanasiou, Grigorakis, Kotou and Katharios2013).
The difficulty in eradicating C. irritans from the mariculture system partially arises from the complexity of its life cycle (Colorni and Burgess, Reference Colorni and Burgess1997; Rigos et al. Reference Rigos, Karagouni, Kyriazis, Athanasiou, Grigorakis, Kotou and Katharios2013). The life cycle of C. irritans is customarily divided into four stages, including the highly motile, infective theront; the parasitic and feeding trophont; the off-host and temporarily free-living protomont; and the reproductive cyst or tomont, which has a thick cyst wall (Yoshinaga and Dickerson Reference Yoshinaga and Dickerson1994; Colorni and Burgess, Reference Colorni and Burgess1997; Dickerson, Reference Dickerson, Woo and Leatherland2006; Lokanathan et al. Reference Lokanathan, Mohd-Adnan, Wan and Nathan2010). Treatment is particularly difficult owing to the existence of the thick cyst wall and asynchronous excystment of the infective theronts from tomonts (Colorni, Reference Colorni1985; Burgess, Reference Burgess1992; Hirazawa et al. Reference Hirazawa, Goto and Shirasu2003; Dickerson, Reference Dickerson, Woo and Leatherland2006). Studies aimed particularly at elucidating the tomont stage of C. irritans will therefore enhance our understanding of the basic features of the tomont, allowing for further exploration of targeted methods to prevent and control the parasitic disease.
Numerous studies have been conducted on the biology of C. irritans, including research into its life cycle, cellular morphology, proteome, transcriptome and immunogenicity (Brown, Reference Brown, Ludvik, Lom and Vavra1963; Nigrelli and Ruggieri, Reference Nigrelli and Ruggieri1966; Cheung et al. Reference Cheung, Nigrelli and Ruggieri1981; Colorni and Diamant, Reference Colorni and Diamant1993; Matthews et al. Reference Matthews, Matthews and Burgess1993; Colorni and Burgess, Reference Colorni and Burgess1997; Diggles, Reference Diggles1997; Huang et al. Reference Huang, Ma and Li2005; Dickerson, Reference Dickerson, Woo and Leatherland2006; Li et al. Reference Li, Huang, Ma and Xie2006; Ma et al. Reference Ma, Li, Xie and Huang2006; Bai et al. Reference Bai, Xie, Zhu, Dan and Li2008; Lokanathan et al. Reference Lokanathan, Mohd-Adnan, Wan and Nathan2010; Mai et al. Reference Mai, Li, Li, Li, Huang, Mo and Li2015; Ma et al. Reference Ma, Ni, Fan, Warren, Yin and Gu2016). However, information is still lacking with regards to the ultrastructural features of the tomont stage. While the fine structure of the cyst wall has been roughly described in several studies, very few details are available involving the formation of the cyst wall (Xu et al., Reference Xu, Jiang and Chen1992; Colorni and Diamant, Reference Colorni and Diamant1993; Matthews et al. Reference Matthews, Matthews and Burgess1993; Kesintepe, Reference Kesintepe1995; Huang et al. Reference Huang, Ma and Li2005). It is confused regarding whether somatic cilia are present during encystment (Nigrelli and Ruggieri, Reference Nigrelli and Ruggieri1966; Cheung et al. Reference Cheung, Nigrelli and Ruggieri1981; Colorni and Diamant, Reference Colorni and Diamant1993; Matthews et al. Reference Matthews, Matthews and Burgess1993; Huang et al. Reference Huang, Ma and Li2005). In addition, knowledge of the features of cell division in the tomont are restricted to the light microscopic level (Brown, Reference Brown, Ludvik, Lom and Vavra1963; Nigrelli and Ruggieri, Reference Nigrelli and Ruggieri1966; Xu et al., Reference Xu, Jiang and Chen1992), and there are few descriptions of cellular organelles in the cytoplasm of the tomont (Matthews et al. Reference Matthews, Matthews and Burgess1993; Huang et al. Reference Huang, Ma and Li2005).
In this study, the morphology of the tomont of C. irritans was investigated using light and electron microscopy. Detailed observations were made throughout encystment and subsequent cell-division progress.
MATERIALS AND METHODS
Culture and collection of C. irritans tomonts and theronts
Cryptocaryon irritans cells were isolated from a naturally infected host fish, Larimichthys crocea, in an aquaculture farm in Ningde, Fujian Province, China (Fufa Fisheries Co., Ltd.). Cells were propagated using a method modified from Yin et al. (Reference Yin, Gong, Ke and Li2015) with a water temperature of 25 ± 1 °C and L. crocea as the host fish. Cells at different stages of the life cycle of C. irritans were collected as follows. Trophonts were scraped from the skin or gills of hosts, and then collected using a glass pipette. Protomonts and tomonts were collected from petri dishes placed on the bottom of the aquarium containing infected L. crocea. Tomonts were collected once an hour in the 15 h after protomont attachment. They need to be fixed using 2·5% glutaraldehyde at 4 °C for 1 h before being brushed off by an eyelash pencil and collected with a glass pipette. Theronts were obtained by incubating collected tomonts at 25 °C in well-oxygenated, fresh sea water.
Observation of living tomonts
Living tomonts were isolated and directly observed in vivo using differential interference contrast microscopy.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
Sample preparation for SEM was conducted according to methods by Gu and Ni (Reference Gu and Ni1993). Collected cells were fixed in 2·5% glutaraldehyde at 4 °C for 24 h. Theronts were fixed in a 6:1 mixture of saturated HgCl2 and 1% OSO4 at 4 °C for 10 min. All fixing solutions were diluted in sodium cacodylate buffer (pH 7·2). Fixed cells were washed with buffer, dehydrated in a graded ethanol series, critical-point dried, placed on aluminium stubs, and sputter coated with platinum. To observe the cells inside tomonts, tomonts pasted on a sample stage were lacerated with a blade, and the cyst wall was stripped off with a needle. Prepared samples were observed with a Hitachi S-4800 SEM.
Sample preparation for TEM was performed according to Gu et al. (Reference Gu, Chen, Ni and Zhang2002). Collected cells were fixed in 3% glutaraldehyde at 4 °C for 24 h. After washing with buffer, samples were post-fixed in 1% OSO4 at 4 °C for 2 h. All fixing solutions were diluted in sodium cacodylate buffer (pH 7·2). Fixed cells were washed with buffer, dehydrated in a graded acetone series, embedded with Epon 812, and polymerized at 37 °C for 16 h, 45 °C for 24 h and 60 °C for 48 h. Ultrathin sections were created using a diamond knife and were then stained with uranyl acetate and lead citrate. Sections were examined with a Hitachi HT-7700 TEM.
RESULTS
Protomonts that were naturally shed by host tissues moved freely for <2 h. They then gradually began to crawl more slowly and attach to the underwater substrate. Secretion of cyst wall materials and encystment began concomitantly. A typical palintomic cell division later occurred inside the tomont, and scores of theronts were then released.
Structure of the cyst wall
Tomonts of C. irritans were generally spherical with a relatively flat attachment area (Fig. 1A and B). The external surfaces of tomonts were usually peppered with bacteria and even small, sessile ciliates occasionally (Fig. 1B and C). The mature cyst wall was ~4 µm thick and could be easily stripped off in SEM sample preparation (Fig. 1D–F). The cyst wall was a multilayered structure that varied in layer number across different tomonts (Fig. 1E and F). In general, the middle portion of the cyst wall was compact, while the outer and inner portions were loose (Fig. 1E and F). The compact middle portion, which accounted for half of the total thickness, was composed of alternately arranged thin, electron-dense layers and thick, electron-lucent layers. There was no obvious stratification in the outer portion, but the inner portion was stratified finely (Fig. 1E and F). The cyst wall contained abundant fibrous materials in certain layers, which were irregularly interwoven (Fig. 1G and H). Cyst wall layers were not always tightly joined to each other but sometimes were widely spaced (Fig. 1A). In fact, multiple layers were rarely appressed when observed from the TEM cross-section. Many alveoli of various sizes were occasionally present between layers (Fig. 1I).

Fig. 1. Tomont of Cryptocaryon irritans viewed in vivo (A) and by SEM (B–E, G, H), TEM (F, I). (A, B) Holistic view of tomont. Arrowhead depicts the wide spaces between layers of cyst wall; arrow marks the relatively flat attachment area; (C) External surface of tomonts, showing bacterial attachments; (D) Cyst wall stripped off in SEM sample preparation; (E, F) Sectional view of cyst wall, showing the outer, middle and inner portion of the thick cyst wall; (G, H) Fibrous materials of the cyst wall. (I) Alveoli (arrowheads) present between layers of cyst wall. op, mp, ip, outer, middle and inner portion of cyst wall, respectively. Scale bars = 100 µm (A, B, D), 20 µm (G), 5 µm (C), 2 µm (E, F) and 1 µm (H, I).
Formation of the tomont
The secretion of sufficient cyst wall materials to form the thick cyst wall was a slow process, taking 14–15 h from the attachment of the protomont to the formation of a mature cyst wall. Secretion began even before movement had stopped and the protomont had attached. Over time, larger amounts of semi-transparent mucus material could be observed surrounding the cell in vivo, and cell locomotion was slowed and finally halted owing to the accumulation of cyst wall materials (Fig. 2A). These materials were a mixture of mucus and fibrous materials (Fig. 2B and C).

Fig. 2. Cells during encystment of C. irritans viewed in vivo (A) and by SEM (B–F, K, M), TEM (G–J, L, N). (A–C) Protomonts slowed down before attachment, with initial cyst wall materials surrounding the cell. (D–J) Cells during encystment showing the gradually thickened cyst wall and the cytoplasm underneath the pellicle at 1 h (D, G), 2 h (H), 4 h (E, I) and 10 h (F, J) after attachment of the encysting cells. Arrowheads indicate pellicular swells; (K, M) The flat cell surface after the cyst wall was stripped. Arrowheads mark the somatic cilia clinging to the pellicle, and arrow depicts the cytostome under the newly formed cyst wall; (L) Basal bodies of somatic cilia are present during encystment (arrowhead); (N) Circumoral cilia are also present (arrowheads). CW, cyst wall; op, mp, outer and middle portions of cyst wall, respectively. Scale bars = 100 µm (A), 5 µm (D, F, M), 2 µm (C, K), 1 µm (B, E, G–J, N) and 0·5 µm (L).
One hour after the attachment of encysting cells, the cyst wall was quite thin (Fig. 2D and G), and 2 h later, it had thickened slightly (Fig. 2H). Four hours after attachment, the cyst wall was ~1 µm thick, and the formation of the outer portion was completed (Fig. 2E and I). Ten hours after attachment, the cyst wall was ~3 µm thick, and the distinctly layered middle portion had formed completely (Fig. 2F and J). The formation of the inner portion took additional 4–5 h. Throughout the encysting process, there were no prepackaged secretory cytoplasmic vesicles or specific extrusomes observed under the pellicle accompanying the accumulation of the cyst wall materials (Fig. 2G–J). In addition, no foci of secretion or exocytosis were visible during SEM or TEM observations (Fig. 2G–J, K and M).
The close-set pellicular swells, which cover the cell surface of the trophont and protomont, dwindled in size and were immersed in the pellicle after the start of encystment (Fig. 2G–J). When the cyst wall was stripped off, swells were compressed and the pellicle was flat (Fig. 2K and M). The cytostome was present during encystment (Fig. 2C and M). Both circumoral and somatic cilia, with their basal bodies, existed during encystment. These were overwhelmed by abundant cyst wall materials and clung to the pellicle, all of which were covered by the newly formed cyst wall (Fig. 2B, C and K–N).
Palintomic division inside the tomont
In most cases, the typical palintomic cell division in the C. irritans tomont began 1–2 days after cyst wall formation and was completed in 2–4 days. The metrocyte experienced continuous asymmetrical cleavage inside the tomont, and the daughter cells did not separate from each other immediately, but formed a long, coiled cell chain of variously sized daughter cells. At the end of this division process, 200–400 small, dissociative tomites had been produced. The overall process involved the following stages. (1) One metrocyte first divided into a large daughter cell and a small daughter cell (Fig. 3A and J); the large cell then successively divided out several smaller daughter cells (Fig. 3B and K). (2) More small daughter cells were generated, about 20–30 of which were stacked into 2–3 spirals. The large daughter cell diminished further as it divided (Fig. 3C and L). (3) A large cell was no longer visible, and small daughter cells similar in size accumulated as a cell chain (Fig. 3D and M). (4) These daughter cells continued to divide, and 200–400 dissociative spherical tomites were produced. They moved slowly in the tomont (Fig. 3E and N). These tomites were obviously more and smaller than the former daughter cells, but we failed to recognize the cleavage during this process was asymmetrical or symmetrical (Fig. 3D, E, M and N). (5) Small, spherical tomites developed further and subsequently transformed into ellipsoidal theront precursors (Fig. 3F and O). The motion of the theront precursors was so rapid that it was difficult to clearly recognize cell profiles (Fig. 3F and G). The fast-moving theront precursors broke through the cyst wall and exited the tomont (Fig. 3G), leaving behind an empty cyst wall (Fig. 3H). At times, a few small cells, which divided or matured abnormally, remained in the empty cyst wall (Fig. 3I).

Fig. 3. Cell division inside the C. irritans tomont viewed in vivo (A–I) and by SEM (J–O). Arrowheads indicate artificial broken joints between dividing daughter cells; (P) Cilia on the dividing daughter cells are of various lengths; (Q) Theront precursors; (R) Cleavage ring (asterisk) between dividing cells; (S) Dissociative spherical tomites with oral primordia (arrowheads). CW, cyst wall; T, Theront; TP, theront precursor; To, dissociative spherical tomite. Scale bars = 200 µm (G, L), 50 µm (A–F, H–K, M–O), 10 µm (Q, S) and 5 µm (P, R).
Cilia were present in the tomont throughout the cell division process. During cell division, cilia on the daughter cells were of various lengths, with the longest ones 7–8 µm long (Fig. 3P). Cilia beat actively when observed in vivo. Cilia on the dissociative spherical tomites and theront precursors were all 7–8 µm in length, which is equal to the length of theront cilia (Fig. 3Q). The cleavage rings, which did not contain cilia, ran perpendicular to the ciliary rows of the dividing cells (Fig. 3R). No oral primordia were observed on the dividing cell chains, appearing on the dissociative tomites only after cytokinesis (Fig. 3S).
Specific activities of some tomont cellular organelles
In protomonts and encysting cells, we observed numerous nascent mitochondrial autophagosomes, in which the mitochondria began to be fused and digested by the primary lysosomes (Fig. 4A). The arrangement of the mitochondrial cristae of the dividing cells inside the tomont was less compact than those of trophonts and theronts (Fig. 4B–D).

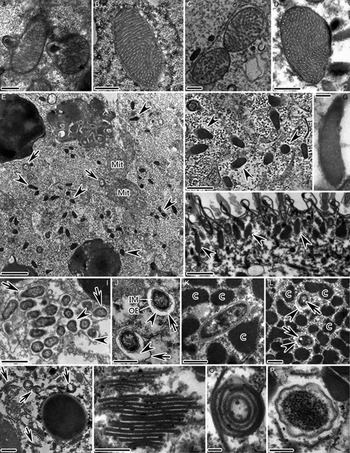
Fig. 4. TEM images of some cellular organelles in the cytoplasm of C. irritans tomonts. (A) Nascent mitochondrial autophagosomes in the encysting cells; (B) Trophont mitochondria; (C) Mitochondria in tomont dividing cells, (D) Theront mitochondria showing different arrangements of cristae; (E, F) Appearance of cytoplasm in the dividing cells inside the tomont. Arrowheads indicate mucocyst precursors; (G, H) Mucocysts beneath the pellicle of theronts (arrowheads); (I, J) Endosymbionts in the cytoplasm of tomont. Arrowheads indicate electron-lucent halos. Arrows mark membranous vacuoles; (K, L) Endosymbionts in the macronucleus of tomont. Arrowheads depict electron-lucent halos. Arrows indicate circular, electron-lucent patches; (M, N) Golgi-like cisternae scattered throughout the cytoplasm of dividing cells inside tomont (arrows); (O) Semi-circular to circular Golgi-like cisternae; (P) Endosymbiont surrounded by several Golgi-like sacs arranged in a monolayer. Mit, mitochondria; IM, inner cytoplasmic membrane of endosymbionts; OE, outer envelope of endosymbionts; C, chromatin patches. Scale bars = 1 µm (E, H, I), 0·5 µm (A–D, F, J–M), 0·2 µm (P, N) and 0·1 µm (G, O).
Mucocysts were not observed in the trophonts or protomonts. However, numerous mucocyst precursors appeared as electron-dense pyriform structures bounded by a single membrane in the cytoplasm of the dividing cells inside the tomont (Fig. 4E and F). These matured in the theronts, appearing as elliptical or elongated saccular structures regularly located beneath the pellicle (Fig. 4G and H).
Endosymbionts were present in the cytoplasm at all stages of the C. irritans life cycle; however, numbers clearly increased in tomonts, with endosymbionts even clustering in groups (Fig. 4I). Endosymbionts were oblong or rod-shaped and contained a granular cytoplasm, inner cytoplasmic membrane and wavy outer envelope (Fig. 4I–L). They were always surrounded by a prominent electron-lucent halo and were sometimes individually contained in membranous vacuoles (Fig. 4I and J). Endosymbionts were also commonly observed in the macronuclei of tomonts; these were never surrounded by membrane vacuoles, however, and only some were surrounded by an electron-lucent halo (Fig. 4K and L). Moreover, nuclear endosymbionts exhibited circular electron-lucent patches according to observations of transverse sections (Fig. 4L).
There were many more Golgi-like cisternae scattered throughout the cytoplasms of the dividing cells inside the tomont than in other life cycle stages (Fig. 4E and M). These appeared as flattened, membrane-bound sacs and several to dozens were stacked in parallel arrays (Fig. 4M–O). In cross-section, most were banded in shape, while some were semi-circular to circular (Fig. 4N and O). Sometimes an endosymbiont was surrounded by several Golgi-like sacs arranged in a monolayer (Fig. 4P).
DISCUSSION
No secretion of encystation-specific secretory vesicles or extrusomes during formation of C. irritans cyst wall
Protist cyst walls exhibit a great deal of morphological diversity. Many are composed of two or more distinct layers with different compositions. For example, the resting cyst wall of Histriculus muscorum consists of four layers: the ectocyst, mesocyst, endocyst and granular layer (Matsusaka and Hongo, Reference Matsusaka and Hongo1984; Lynn, Reference Lynn2008; Aguilar-Díaz et al. Reference Aguilar-Díaz, Carrero, Argüello-García, Laclette and Morales-Montor2011; Verni and Rosati, Reference Verni and Rosati2011). However, the three portions of the C. irritans cyst wall could not be distinguished to several nature distinct layers and might be of similar composition. Since the three portions exhibit similar morphology with the exception of electron-dense, thin layers in the middle portion.
During encystment, many protists secrete cyst wall materials via exocytosis of encystation-specific secretory vesicles from the cytoplasm (Ruthmann and Kuck, Reference Ruthmann and Kuck1985; Greco et al. Reference Greco, Bussers, Van Daele and Goffinet1990; Chávez-Munguía et al. Reference Chávez-Munguía, Cedillo-Rivera and Martínez-Palomo2004, Reference Chávez-Munguía, Omaña-Molina, González-Lázaro, González-Robles, Cedillo-Rivera, Bonilla and Martínez-Palomo2007; Lynn, Reference Lynn2008; Aguilar-Díaz et al. Reference Aguilar-Díaz, Carrero, Argüello-García, Laclette and Morales-Montor2011). In other protists, the cyst wall is formed by secretion of specific extrusomes, such as mucocysts or clathrocysts (Holt and Chapman, Reference Holt and Chapman1971; Hausmann, Reference Hausmann1978; McArdle et al. Reference McArdle, Berquist and Ehret1980; Ewing and Ewing, Reference Ewing and Ewing1983; Lynn, Reference Lynn2008). During encystment of C. irritans, however, there were no prepackaged secretory cytoplasmic vesicles or visible foci of secretion observed, even though cells were sampled every hour throughout the entire encystment process. Moreover, there were no mucocysts or other extrusomes observed during development of the mature trophont, protomont, or earlier tomont, suggesting that cyst wall formation in C. irritans does not involve secretory vesicles or extrusome secretion.
Instead, the mechanism of cyst wall formation in C. irritans may be similar to that of some apostome, peritrich and hypotrich ciliates, and some flagellates. The cyst wall materials of these protozoa originate in the cytosol and polymerize at the plasma membrane without formation or secretion of membrane-limited cyst wall precursors (Herth et al. Reference Herth, Mulisch, Zugenmaier, Muzzarelli, Jeuniaux and Gooday1986; Greco et al. Reference Greco, Bussers, Van Daele and Goffinet1990; Landers, Reference Landers1991; Calvo et al. Reference Calvo, Fernandez-Aliseda, Garrido and Torres2003). The synthesis and construction of the cyst wall materials of C. irritans may also involve molecular events in the pellicle, which are difficult to observe during routine electron microscopy.
Cilia are present during encystment and cell division of the tomont
It is well known that C. irritans trophonts, protomonts and theronts have somatic cilia (Cheung et al. Reference Cheung, Nigrelli and Ruggieri1981; Colorni and Diamant, Reference Colorni and Diamant1993; Matthews et al. Reference Matthews, Matthews and Burgess1993; Diggles, Reference Diggles1997; Huang et al. Reference Huang, Ma and Li2005; Li et al. Reference Li, Huang, Ma and Xie2006; Ma et al. Reference Ma, Li, Xie and Huang2006, Reference Ma, Ni, Fan, Warren, Yin and Gu2016). Previous studies reported that the cilia were also present on the dividing cells inside the tomont (Colorni and Diamant, Reference Colorni and Diamant1993; Huang et al. Reference Huang, Ma and Li2005; Ma et al. Reference Ma, Ni, Fan, Warren, Yin and Gu2016), and our study confirms this. However, there has been some confusion in the literature regarding whether somatic cilia are present during encystment. Theories have suggested that they are absorbed (Nigrelli and Ruggieri, Reference Nigrelli and Ruggieri1966), gradually internalized (Cheung et al. Reference Cheung, Nigrelli and Ruggieri1981), mostly shed (Matthews et al. Reference Matthews, Matthews and Burgess1993), embedded in the thickened cyst wall with bacteria and cellular debris (Colorni and Diamant, Reference Colorni and Diamant1993) or lost (Huang et al. Reference Huang, Ma and Li2005). In the present study, we found that the somatic cilia are never shed and do not disappear during encystment; instead, they are present under the cyst wall materials.
During subsequent cell divisions in the tomont, numerous new, somatic cilia of various lengths are constantly being created between old cilia; this is similar to what happens in morphogenesis in many free-living ciliates (Lynn, Reference Lynn2008). During encystment, the retained, old somatic cilia may be resorbed or distributed to daughter cells intact; in the latter case, they may play an important role in determining the locations of new somatic cilia, as in hypotrich ciliates (Gu and Zhou, Reference Gu and Zhou1990).
Huang et al. (Reference Huang, Ma and Li2005) briefly mentioned that the buccal apparatus was lost in the early stage of tomont development. In this study, however, we found that both the cytostome and its circumoral cilia were present under the newly formed cyst wall during encystment. These may subsequently be disintegrated and resorbed, as the dividing cell chains exhibited no oral primordia. Tuffrau (Reference Tuffrau1952) reported that in dividing colpodids, all parental oral structures dedifferentiated, and the ciliates were astomatous during palintomy. A previous study claimed that in C. irritans, oral primordia were apparent until after cytokinesis of the cell chains during telophase of cell division (Ma et al. Reference Ma, Ni, Fan, Warren, Yin and Gu2016). This was not accompanied by stomatogenesis during cell division in the tomont. New oral primordia in the offspring likely formed before the dividing cell chains split into dissociative spherical tomites.
Cilia are also present during encystment and cell division inside the reproductive cysts of Hyalophysa chattoni and Ichthyophthirius multifiliis (Landers, Reference Landers1991; Matthews, Reference Matthews2005; Dickerson, Reference Dickerson, Woo and Leatherland2006). We speculate therefore that this may represent a common feature in these typical histophagous ciliates.
Dividing daughter cells in the tomont form temporary cell chains before separation into dissociative spherical tomite precursors
The phenomenon of reproduction and cell division inside a cyst has been reported in numerous ciliates, including some free-living ciliates [tetrahymenids, hypotrichs and colpodids (Williams, Reference Williams1960; Foissner, Reference Foissner and Matthes1993; Benčaťová et al. Reference Benčaťová, Tirjaková and Vďačný2016)] and some histophagous ciliates [apostomes, ophryoglenids and prostomes (Savoie, Reference Savoie1962a, Reference Savoie1962b; Bradbury, Reference Bradbury1966; Hiller and Bardele, Reference Hiller and Bardele1988; Leipe, Reference Leipe1989; Bradbury et al. Reference Bradbury, Song and Zhang1997; Molloy et al. Reference Molloy, Lynn and Giamberini2005)]. It is worth noting that in these cases, two to eight tomites or filial products are produced. In contrast, the two parasitic ciliates C. irritans and I. multifiliis produce up to 200 and 1000 tomites, respectively, from a single cyst (Ewing and Ewing, Reference Ewing and Ewing1983; Diggles and Lester, Reference Diggles and Lester1996; Colorni and Burgess, Reference Colorni and Burgess1997; Lynn, Reference Lynn2008). It may be that the number of filial products created by a reproductive cyst is related to the feeding habits of the ciliate, with parasitic ciliates able to produce many more tomites.
Previous studies have indicated that C. irritans tomonts undergo asymmetric palintomic division, culminating in numerous daughter tomites (Colorni and Burgess, Reference Colorni and Burgess1997; Huang et al. Reference Huang, Ma and Li2005; Dickerson, Reference Dickerson, Woo and Leatherland2006). Beyond this, our study found that the dividing daughter cells form temporary cell chains in the tomont, rather than being pinched off or separated from each other with each binary fission. In certain species of astomes, apostomes, hymenostomes and scuticociliates, chains of daughter cells are also formed during the reproduction process (Molloy et al. Reference Molloy, Lynn and Giamberini2005; Lynn, Reference Lynn2008; Long and Zufall, Reference Long and Zufall2010). Our findings indicate a similar characteristic in prostomes. This suggests that the formation of cell chains may represent either an ancestral feature in these groups or a convergent trait that has arisen multiple times independently. Other ciliates that have not been intensively studied, especially those with a polymorphic life cycle, may also share such a trait.
Activities of some cellular organelles in the C. irritans tomont cytoplasm are not identical to those in resting cysts
Autophagic activity, in which numerous autophagic vacuoles containing membranous materials and some cytoplasmic organelles are generated, is a common phenomenon during encystment for many resting cysts (McArdle et al. Reference McArdle, Berquist and Ehret1980; Gutierrez and Perez-Silva, Reference Gutierrez and Perez-Silva1983). Mitochondrial autophagy was also observed in encysting cells of C. irritans; this process may reflect lower-energy requirements when protomonts stop moving and begin to encyst.
The activities of some cellular organelles in the cytoplasm of C. irritans tomont were clearly different from those in resting cysts of other protists. Similar to the development of toxicysts (Ma et al. Reference Ma, Ni, Fan, Warren, Yin and Gu2016), mucocysts matured underneath the pellicle in theronts, with mucocyst precursors first developing in the dividing cells inside the tomont. These organelles may contribute to the processes of theronts escaping from the tomont or invading host tissues. In addition, there were numerous endosymbionts and Golgi structures in the cytoplasm of dividing cells inside the tomont, with some Golgi structures associated with endosymbionts. Cryptocaryon irritans may provide a suitable environment as a host for endosymbiont growth and proliferation, and our findings implied an active interaction between endosymbionts and their host cells in this stage. The Golgi apparatus is the central sorting organelle in eukaryotic cells and is involved in many important cell activities (Farquhar and Palade, Reference Farquhar and Palade1981; Rothman, Reference Rothman1981; Kurz and Tiedtke, Reference Kurz and Tiedtke1993; Morgado-Diaz et al. Reference Morgado-Diaz, Nakamura, Agrellos, Dias, Previato, Mendonca-Previato and De Souza2001). The appearance of additional Golgi-like cisternae indicates increased cell activity and an increase in material synthesis in the tomont. Yin et al. (Reference Yin, Sun, Wang and Gao2016) reported that numerous genes were activated before the tomont began cell division. In conclusion, unlike a resting cyst, cellular metabolic activity in the C. irritans tomont was quite high, with large amounts of materials or cellular organelles synthesized and prepared for the subsequent infective theront stage.
FINANCIAL SUPPORT
This work was funded by the National Natural Science Foundation of China (grant numbers. 31101932 and 31572223) and the Special Scientific Research Funds for Central Non-profit Institutes of China (grant no. 2014M01, East China Sea Fisheries Research Institute; grant no. 2015B05XK01, Chinese Academy of Fishery Sciences).