Introduction
Trypanosoma rangeli, a protozoan parasite that infects triatomines belonging to the Rhodnius genus and different species of mammals including humans, is broadly distributed in Latin America (Grisard et al., Reference Grisard, Steindel, Guarneri, Eger-Mangrich, Campbell and Romanha1999). The parasite has not been shown to be pathogenic for mammals. Besides, its development in these hosts is poorly understood but recent experimental evidence obtained in a murine model suggests it develops in secondary lymphoid organs (Ferreira et al., Reference Ferreira, Araújo, Martinelli, Teixeira-Carvalho, Alves-Silva and Guarneri2020). Trypanosoma rangeli is acquired by triatomines after ingesting the blood of infected mammals. On the other hand, mammals become infected via the saliva of infected triatomines. Even though mammal parasitemia is low or unapparent, transmission to bugs seems very efficient with rates close to 80% after a single meal on an infected mouse (Ferreira et al., Reference Ferreira, Pereira and Guarneri2015). In the anterior midgut, blood trypomastigotes differentiate into epimastigotes that colonize the whole intestinal tract of a bug after few days (Ferreira et al., Reference Ferreira, Teixeira, de Sousa and Guarneri2018). It is estimated that these parasites reach the haemolymph of 10–50% of insects presenting an intestinal infection. Once there, they multiply and later migrate to the salivary glands where metacyclogenesis takes place (D'Alessandro-Bacigalupo and Gore-Saravia, Reference D'Alessandro-Bacigalupo, Gore-Saravia and Gilles1999). Infected Rhodnius bugs present increased mortality rates, as well as several pathogenic effects, such as delayed and defective moulting, and decreased fecundity/fertility rates (reviewed in Guarneri and Lorenzo, Reference Guarneri and Lorenzo2017).
Interestingly, T. rangeli can also affect the behaviour of triatomines. Healthy bugs characteristically spend most of their life hidden inside shelters, seeking protection against the action of predators, as well as favourable microenvironmental conditions. A strong thigmotaxis associated with a negative phototaxis maintains the bugs in akinesis inside shelters during daytime. At the beginning of the night, and depending on their nutritional status (Ferreira et al., Reference Ferreira, Guarneri and Lorenzo2019), these insects can leave their refuges and engage in foraging activity. Under these conditions, bugs spend most of the night outside shelters, only returning a few hours before dawn, a behaviour endogenously controlled by their circadian clock (Lazzari, Reference Lazzari1992; Lorenzo and Lazzari, Reference Lorenzo and Lazzari1998). According to experiments using individual actometers, triatomines present a bimodal daily pattern of spontaneous activity, with a first peak occurring around lights on, and a second one during the first hours after lights off (Lazzari, Reference Lazzari1992; Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). Trypanosoma rangeli infection alters this spontaneous locomotory activity in R. prolixus (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). Even though the bimodal daily pattern of locomotion is not affected, T. rangeli promotes increased levels of locomotory activity during most of the daily cycle, i.e. including the photophase, and weakens the characteristic negative phototaxis of bugs. Infection also promotes a reduction in the expression of Rpfor, a gene encoding a protein kinase modulating the foraging profiles of insects of diverse orders (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). In the present work, we evaluated diverse parameters of shelter-related activity in T. rangeli-infected R. prolixus. These parameters were recorded for uninfected, as well as infected bugs, in the presence of a mammal host. Besides, we studied whether infection induced an increase in the predation rates endured by bugs. Finally, we evaluated whether the ingestion of T. rangeli-infected bugs can promote oral transmission to mice.
Materials and methods
Triatomines
The R. prolixus colony used in our study originated from insects collected in Honduras in the 90s. Triatomines were reared by the Vector Behaviour and Pathogen Interaction Group at the René Rachou Institute, and fed monthly on anaesthetized chickens (ketamine, 20 mg kg−1 – Cristália, Brazil; and detomidine, 0.3 mg kg−1 – Syntec, Brazil) and mice (ketamine, 150 mg kg−1 – Cristália, Brazil; and xylazine 10 mg kg−1 – Bayer, Brazil), according to the standards approved by the Committee for Ethics in Animal Use (CEUA), FIOCRUZ, under the license number LW-61/2012. The colony was maintained at 26 ± 1°C, 65 ± 10% RH and exposed to a natural illumination cycle.
Parasites
Trypanosoma rangeli (CHOACHI strain) isolated from naturally infected R. prolixus salivary glands (Schottelius, Reference Schottelius1987) were used to infect insects. The parasites were cultured by twice a week passages in LIT (liver-infusion tryptose) medium supplemented with 15% fetal bovine serum, 100 mg mL−1 streptomycin and 100 units mL−1 penicillin.
Insect infection
Trypanosoma rangeli infection was performed as described by Ferreira et al. (Reference Ferreira, Lorenzo, Elliot and Guarneri2010). Briefly, third instar nymphs were fed on an artificial feeder with heat-inactivated (56°C, 30 min) citrated rabbit blood containing culture epimastigotes in a concentration of 1 × 106 parasites/mL. As systemic infections of T. rangeli do not occur in all individuals with intestinal infections (reviewed in Guarneri and Lorenzo, Reference Guarneri and Lorenzo2017; Guarneri and Schaub, Reference Guarneri, Schaub, Guarneri and Lorenzo2021), 7 days after moulting to the fourth instar, nymphs were inoculated 1 μL of sterile PBS (0.15 m sodium chloride in 0.01 m sodium phosphate, pH 7.4) containing approximately 100 parasites by inserting a needle of a Hamilton syringe (50 μL) through the thoracic pleura. One day after inoculation insects were fed on anaesthetized mice. To confirm haemocoelomic infection, a sample of haemolymph from the cut end of a hind tarsus was examined. In the experiment testing oral transmission, salivary gland infection was confirmed by examination of 10% of each bug cohort, which is sufficient according to Rodrigues et al (Reference Rodrigues, Lorenzo, Martins-Filho, Elliot and Guarneri2016). In addition, using insects with more than 30 days of infection guarantees the massive presence of metacyclic trypomastigotes in the gland (Paim et al., Reference Paim, Pereira, Araújo, Gontijo and Guarneri2013). Control group insects were fed on heat-inactivated citrated rabbit blood at their third instar, inoculated with sterile PBS after moulting to fourth instar and had a sample of haemolymph collected with the same procedure. Fifth instar nymphs starved for approximately 30 days were used for the experiment.
Experiments
Use of shelters
The dynamics of the use of shelters by R. prolixus, whether infected or not by T. rangeli, was evaluated according to the methodology described by Marliére et al. (Reference Marliére, Lorenzo and Guarneri2021). Two glass arenas (40 × 40 × 20 cm) presenting an artificial shelter in the central area were used to simultaneously record the behaviour of infected and uninfected nymphs. The shelters were made of a piece of corrugated cardboard (20 × 10 cm) folded in half to produce a 10 cm2 refuge with two ~0.5 cm high lateral accesses. Groups of 50 T. rangeli-infected and 50 uninfected nymphs were released in each arena 3 h before the end of the photophase. The insects were acclimatized in the arenas for 72 h. After acclimation, bugs found outside shelters were removed. One mouse (weighing ~40 g) held in a cylindrical plastic container (10 cm high × 8 cm in diameter) closed with a perforated plastic cap was placed in each of the arenas. The containers prevented physical contact between insects and mice but allowed chemical (odours) and physical (vibration and heat) stimuli to be emitted, signalling the presence of a host. The mice were placed in the arenas 3 h before the start of the scotophase and remained there until 3 h after the start of the following photophase, receiving water and food ad libitum during all the period (total assay duration = 18 h). Five replicate assays were done for each treatment (N = 250 per treatment). The assays were recorded by means of an infrared sensitive video camera (Panasonic digital video camera-recorder, model AG-DVC30P). The video records were analysed for the following parameters: insects inside and outside shelters during acclimatization, percentage of insects inside shelters before introducing the host, percentage of insects outside shelters in the presence of the host, and percentage of insects that remained outside the shelters 3 h after host removal. During the photophase, fluorescent tubes located overhead illuminated the chamber at a light intensity of ca. 160 LUX. Room temperature was kept at 24 ± 1°C and 12:12 L/D.
Predation rates
Bug predation rates by mice were evaluated using the same experimental design of the previous experiment, with modifications. In this case, mice were kept inside steel cages (10 × 6 × 10 cm) that allowed interactions between them and bugs. Video records were analysed for the following parameters: insects inside and outside shelters during acclimation, percentage of insects inside shelters before introducing the host, percentage of insects predated, and percentage of fed insects. Fed insects were transferred to a BOD chamber (26 ± 1°C) to evaluate moulting rates.
Oral transmission of T. rangeli
To evaluate whether T. rangeli can be orally transmitted to the vertebrate host, infected fifth nymphs were maintained in a glass arena (40 × 40 × 20 cm) with Balb/c strain mice (starved for 3 h). One day before the experiment, nymphs had their proboscis removed to prevent bugs from biting mice, and therefore to avoid parasite transmission through bug feeding. For assays (n = 10), one mouse and three nymphs were kept in the arena for 1 h to allow the mouse to eat the insects. On days 1–6 and 15 after this, mice were then anaesthetized and offered as a food source to three uninfected fifth instar nymphs (starved for 30 days). Fed nymphs were maintained at 26 ± 1°C for 21 days, when their intestinal tracts, haemolymph and salivary glands were examined for parasites (Ferreira et al., Reference Ferreira, Pereira and Guarneri2015). Thirty days after each assay, mice were anaesthetized, bled and their blood transferred to culture tubes containing 2 mL of LIT medium. After 20 days, these blood cultures were examined for T. rangeli presence.
Statistical analysis
A GLM model (repeated-measures ANOVA) was used to determine whether infection status affected the percentage of insects found outside shelters during host presentation (GraphPad Prism 5). Data were arcsine square root-transformed before the analysis. Comparisons between the number of insects found inside/outside shelters, and between predation rates were made by means of a χ 2 test. Odd ratio calculations estimated the probabilities of bugs being exposed/predated. The level of significance was set to P ⩽ 0.05 (95%).
Results
Use of shelters
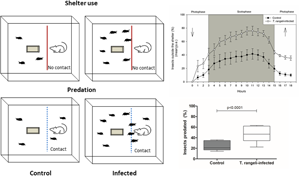
Trypanosoma rangeli infection induced changes in all shelter use-related parameters evaluated. After acclimatization, significantly less T. rangeli-infected nymphs had entered shelters compared to uninfected ones (Fig. 1, χ 2, P < 0.0001). Trypanosoma rangeli infection increased 2.7 times the probability of R. prolixus nymphs to become exposed by being outside the shelter. Infection by T. rangeli also altered the dynamic of shelter entries and exits in the presence of the host. A significantly larger number of T. rangeli-infected insects were found outside shelters during the whole interval in which the host was presented (Fig. 2; GLM F = 15.38, d.f. = 1, P < 0.01). An intense movement of bugs around the container was also observed (Supplementary video). For most of the scotophase, more than 70% of infected nymphs remained exposed in the arena, while only 40% of uninfected nymphs did so. Finally, 3 h after host removal, a significantly higher percentage of T. rangeli-infected nymphs remained outside shelters (Fig. 3, χ 2; P < 0.0001). The infection increased by 4.7 times the probability of a nymph to remain outside the shelter after it has perceived the presence of a host.

Fig. 1. Trypanosoma rangeli infection induced a decrease in the number of Rhodnius prolixus nymphs that enter the shelter in the absence of a host. Percentage of T. rangeli-infected or uninfected R. prolixus nymphs found outside shelters after acclimatization (χ 2, P < 0.0001). Data depicted represent the median (horizontal line) of the percentage of insects inside the shelter from five independent assays (25–75%, Max–Min).

Fig. 2. Trypanosoma rangeli infection increased the number of Rhodnius prolixus nymphs that left shelters in the presence of a host. Arrows represent host introduction and removal from the arena. Data depicted represent the hourly means of five independent assays.

Fig. 3. Trypanosoma rangeli infection induced increased exposure in Rhodnius prolixus nymphs. Percentage of T. rangeli-infected or uninfected R. prolixus nymphs found outside shelters 3 h after host removal. Data depicted represent the median (horizontal line) of the percentage of insects found outside shelters in five independent assays (25–75%, Max–Min).
Predation
Similar to the previous experiment, a significantly lower proportion of infected insects entered shelters after acclimation (69.2 ± 5.8 vs 86.4 ± 4.3% for infected and uninfected nymphs, respectively, χ 2, P < 0.0001). In addition, T. rangeli-infected insects were significantly more predated than uninfected ones (Fig. 4; χ 2, P < 0.0001). Trypanosoma rangeli infection increased 2.7 times the probability of a bug being predated. Feeding success was also reduced in T. rangeli-infected bugs (Fig. 5, χ 2, P < 0.05). No differences in moulting rates were found when fed insects of both groups were compared, as 40% of infected insects and 58.5% of uninfected ones moulted to the adult stage (Fig. 5, χ 2, n.s).

Fig. 4. Infection by T. rangeli increased predation of R. prolixus nymphs. Data depicted represent the median (horizontal line) of the percentage of predated insects from five independent assays (25–75%, Max–Min).

Fig. 5. Infection by T. rangeli decreased bug feeding success. Data represent the mean percentage of surviving insects that succeeded to feed and moult (as a percentage of fed bugs) in five independent assays.
Trypanosoma rangeli oral transmission
Eight mice ate all nymphs offered, while two of them ate two out of the three insects presented. A total of 217 triatomines were examined after the xenodiagnosis and were all found negative (23 out of 240 died or did not feed and were therefore excluded). All haemocultures made with the blood of experimental mice were also found negative for T. rangeli.
Discussion
Our results show that T. rangeli infection modifies R. prolixus behavioural traits affecting its interaction with vertebrate hosts. The infection weakened the hiding tendency of the bugs, decreasing their protection from predation. This decreased avoidance of open places increased interactions with the host and, consequently, the predation rates while bugs attempted to feed.
Infected and uninfected R. prolixus nymphs showed a similar and expected behaviour when exposed to an illuminated area; after a short period of intense movement, insects searched for a hiding place. Nevertheless, while 85% of the uninfected insects (mean for both experiments) entered shelters after acclimation, only 69% of the infected ones expressed this behaviour. Triatomines are nocturnal insects that remain hidden during the photophase to avoid predation, as their vertebrate hosts usually feed on them. These insects are very sensitive to light and exhibit an intense negative phototaxis, always avoiding illuminated environments. This behaviour, which is under circadian control (Reisenman et al., Reference Reisenman, Lazzari and Giurfa1998), can be modulated by several factors, including the intensity and the spectral composition of the light (Reisenman et al., Reference Reisenman, Lazzari and Giurfa1998; Reisenman and Lazzari, Reference Reisenman and Lazzari2006). The decrease of negative phototaxis observed in T. rangeli-infected R. prolixus (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015) could explain a decreased avoidance of illuminated areas. Further studies should address whether infection reduces bug sensitivity to light intensity (Reisenman et al., Reference Reisenman, Lazzari and Giurfa1998), promoting higher exposure. Additionally, potential alterations of the intense thigmotaxis of this species by T. rangeli infection deserve consideration in future experiments (Mosquera and Lorenzo, Reference Mosquera and Lorenzo2020).
Infection by T. rangeli also impacted bug responses to the presence of a host. Rhodnius prolixus is known to express low levels of spontaneous foraging, mostly leaving the protection of shelters in the presence of robust cues of host presence (Ferreira et al., Reference Ferreira, Guarneri and Lorenzo2019; Marliére et al., Reference Marliére, Lorenzo and Guarneri2021). Indeed, ~40% of uninfected bugs left the shelter during the interval in which mice were present, while this almost doubled for infected bugs. Furthermore, half of these infected individuals did not come back to shelter safety after lights on and host removal, reinforcing that infection decreases negative phototaxis (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). Indeed, R. prolixus infected with T. rangeli presents an infection-induced increase in locomotory activity in the absence of host cues (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). Interestingly, T. rangeli infection also induces a reduction in the expression of Rpfor, a gene coding a protein kinase modulating foraging intensity (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). In fact, a reduced expression of this gene correlates to increased locomotory activity in this species (Marliére et al., Reference Marliére, Lorenzo, Villegas and Guarneri2020). The increase in the proportion of bugs that leave the shelter in the presence of host cues could be the result of a decrease in the nutritional reserves of infected insects, as T. rangeli-infected bugs usually bear massive amounts of parasites. Nevertheless, nutritional status seems not to trigger the decision of starting forage in R. prolixus, as the proportion of uninfected bugs that leave shelters does not change between 30 and 60 days of starvation (Ferreira et al., Reference Ferreira, Guarneri and Lorenzo2019). Alterations at the sensory level, increasing infected-bug perception of the host presence could be happening, as observed in dengue-infected Aedes aegypti (Tallon et al., Reference Tallon, Lorenzo, Moreira, Martinez Villegas, Hill and Ignell2020).
The higher predation rates seen may simply be a consequence of the increased bug exposure reported above, as an elevated number of T. rangeli-infected insects left the shelters as a response to the presence of a host, probably approaching it more frequently. An increase in predation rates was recently shown in R. prolixus infected with Trypanosoma cruzi, the etiological agent of Chagas disease, but the observed increase in that case was related with a bolder behaviour expressed by infected individuals, as the number of insects that left the shelter was similar between infected and uninfected bugs (Marliére et al., Reference Marliére, Lorenzo and Guarneri2021). Trypanosoma cruzi oral transmission is highly efficient (Shikanai-Yasuda and Carvalho, Reference Shikanai-Yasuda and Carvalho2012; Coura, Reference Coura2015) and epidemiologically relevant in sylvatic and domestic Chagas disease cycles (Reithinger et al., Reference Reithinger, Ceballos, Stariolo, Davies and Gürtler2005; Jansen et al., Reference Jansen, das Chagas Xavier and Roque2018). In the case of T. rangeli, oral transmission has not been proven to date and our results suggest that it may not happen, as we failed to demonstrate this form of transmission in our experimental model. Nevertheless, it is important to consider that sylvatic animal models (Dario et al., Reference Dario, Pavan, Rodrigues, Lisboa, Kluyber, Desbiez, Herrera, Roque, Lima, Teixeira and Jansen2021) need to be tested for oral transmission before it can be discarded for this parasite. The bite of infected bugs is extremely efficient for T. rangeli transmission, as it was estimated that 50 000 metacyclic trypomastigotes are released during a single feeding event (Ferreira et al., Reference Ferreira, Pereira and Guarneri2015). Indeed, 90% of mice become infected in a single feeding event and can subsequently transmit T. rangeli to healthy insects (Ferreira et al., Reference Ferreira, Pereira and Guarneri2015). If increased activity translates into higher numbers of insects leaving shelters, this could increase the probability of biting, ensuring parasite transmission. Therefore, it is reasonable to suggest that T. rangeli can increase its transmission rates through simply increasing the host-seeking motivation of bugs. In addition, our results reinforce that T. rangeli infection compromises the feeding performance of bugs, as fewer nymphs succeeded feeding (Añez and East, Reference Añez and East1984; Garcia et al., Reference Garcia, Mello, Azambuja and Ribeiro1994).
Although many of the behavioural/physiological changes promoted by parasites are a mere byproduct of infection, some have been suggested to represent cases of host manipulation (Thomas et al., Reference Thomas, Poulin and Brodeur2010). These effects can be considered a form of host manipulation if the molecules responsible for inducing altered host behaviour are produced by the parasites and increase parasite transmission rates (Hurd, Reference Hurd2003). Our results suggest bug manipulation by T. rangeli, but more evidence is necessary to propose this hypothesis. This idea is also supported by the fact that T. rangeli alters the expression of a gene controlling insect foraging behaviour (Marliére et al., Reference Marliére, Latorre-Estivalis, Lorenzo, Carrasco, Alves-Silva, Rodrigues, Ferreira, Lara, Lowenberger and Guarneri2015). Next steps should identify the processes governing the transmission of this parasite, characterizing the mechanisms that underlie the behavioural alterations presented here.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001682.
Author contributions
A.A.G. and M.G.L. conceived and designed the study. N.P.M. conducted data gathering. N.P.M. and A.A.G. performed statistical analyses. N.P.M., M.G.L. and A.A.G. wrote the article.
Financial support
A.A.G. and M.G.L were supported by CNPq productivity grants (A.A.G. grant number 303546/2018-2; M.G.L. grant number 311826/2019-9). This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG (A.A.G., grant numbers APQ-00569-15 and PPM-00162-17), Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular, INCTEM/CNPq (A.A.G. and M.G.L., grant number 465678/2014-9). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.