INTRODUCTION
Parasites of the genus Trypanosoma are found in a wide variety of vertebrate and invertebrate hosts, with some of these species being the aetiological agents of important diseases, especially those that infect mammals (Hoare, Reference Hoare1972; Vickerman, Reference Vickerman, Lumsden and Evans1976). The close proximity of domestic dogs, humans and other domestic animals exposes them to the aetiological agents of these host diseases and may also render them reservoirs or carriers of pathogens. It has been well established that the domestic dog is the main reservoir of Leishmania (Leishmania) infantum (syn. L. (L.) chagasi), the aetiological agent of visceral leishmaniasis (Alvar et al. Reference Alvar, Cañavate, Molina, Moreno and Nieto2004). In addition, L. (Viannia) braziliensis, one of the causative agents of tegumentary leishmaniasis in Brazil, has also been identified in domestic dogs (Madeira et al. Reference Madeira, Serra, Schubach, Pereira, Figueredo, Soares, Quintella, Schubach and Marzochi2004, Reference Madeira, Schubach, Schubach, Serra, Pereira, Figueiredo, Confort, Quintella and Marzochi2005, 2006 b). However, the role of dogs as a reservoir is still a matter of discussion (Reithinger and Davies, Reference Reithinger and Davies1999; Dantas-Torres, Reference Dantas-Torres2007).
Domestic dogs are also important reservoirs of Trypanosoma cruzi, the aetiological agent of Chagas' disease in several American countries (Deane, Reference Deane1964; Montenegro et al. Reference Montenegro, Jiménez, Dias and Zeledón2002; Beard et al. Reference Beard, Pye, Steurer, Rodriguez, Campman, Peterson, Ramsey, Wirtz and Robinson2003; Gürtler et al. Reference Gürtler, Cécere, Lauricella, Cardinal, Kitron and Cohen2007). Dogs are also commonly infected with T. rangeli, another human parasite, in endemic areas of this species in Latin America (Pifano et al. Reference Pifano, Peñalver, Medina and Dominguez1948; D'Alessandro, Reference D'Alessandro, Lumsden and Evans1976). Other trypanosome species of veterinary and economic interest also infect domestic dogs, such as T. brucei brucei and T. congolense, the causative agents of Nagana or a similar disease in Africa and Asia (Hoare, Reference Hoare1972; Kaggwa et al. Reference Kaggwa, Munyua and Mugera1984; Harrus et al. Reference Harrus, Harmelin, Presenty and Bark1995), as well as T. evansi, the aetiological agent of Surra and the so-called ‘Mal de Cadeiras’ outside the African continent (Franke et al. Reference Franke, Greiner and Mehlitz1994; Savani et al. Reference Savani, Nunes, Galati, Castilho, Araújo, Ilha, Camargo, D'Auria and Floeter-Winter2005). Domestic dogs may also present mixed infections with the trypanosomatids cited above in geographical areas where these species overlap, which can complicate the diagnosis of these pathogens of medical, veterinary and economic interest based on routine serological tests (Bastrenta et al. Reference Bastrenta, Mita, Buitrago, Vargas, Flores, Machane, Yacsik, Torrez, Le Pont and Brenière2003; Madeira et al. Reference Madeira, Serra, Schubach, Pereira, Figueredo, Soares, Quintella, Schubach and Marzochi2004, Reference Madeira, Schubach, Schubach, Pacheco, Oliveira, Pereira, Figueredo, Baptista and Marzochi2006a; Savani et al. Reference Savani, Nunes, Galati, Castilho, Araújo, Ilha, Camargo, D'Auria and Floeter-Winter2005).
Cases of tegumentary and visceral leishmaniasis have been recorded in areas surrounding the Pedra Branca and Gericinó mountain chains (Municipality of Rio de Janeiro, Brazil), and infected domestic dogs are frequently found in these regions (Marzochi et al. Reference Marzochi, Coutinho, Souza, Toledo, Grimaldi, Momen, Pacheco, Sabroza, Souza, Rangel and Tramontano1985; Madeira et al. Reference Madeira, Schubach, Schubach, Pereira, Figueredo, Baptista, Leal, Melo, Confort and Marzochi2006b). During a routine study of leishmaniasis in these animals, we isolated a peculiar trypanosome species from axenic culture of an intact skin fragment from a dog (Canis familiaris) co-infected with Leishmania (Viannia) braziliensis (Madeira et al. Reference Madeira, Serra, Schubach, Pereira, Figueredo, Soares, Quintella, Schubach and Marzochi2004). This trypanosome was characterized using different approaches (biological, morphological, biometrical, biochemical and molecular) and was compared to T. cruzi and T. rangeli reference strains, as well as to trypanosome species isolated from dogs and other animals described in the literature. Our study indicates that this parasite is a new trypanosome species, which is described in the present paper.
MATERIALS AND METHODS
Animals and collection of biological samples
A domestic dog (Canis familiaris) captured in the District of Campo Grande, Rio de Janeiro City (State of Rio de Janeiro, Brazil), which tested positive for Leishmania IgG antibodies (titre of 1:160) by indirect immunofluorescence, was euthanized (sodium thiopental overdose) according to recommendations of the Brazilian Program for Visceral Leishmaniasis Surveillance and Control (Ministério da Saúde, Reference Ministério2006). This animal presented an ulcerated lesion on the right external ear and no other dermatological alterations. Tissue fragments and venous blood (about 1·0 ml) were aseptically collected from this animal for parasite isolation from axenic culture. Tissue fragments were obtained from the following sites: (i) skin lesion, (ii) intact skin from the scapular and abdominal regions, (iii) cervical, popliteal and mesenteric lymph nodes, and (iv) liver, spleen and heart. The present study was approved by the Ethics Committee on Animal Experimentation of the Oswaldo Cruz Foundation (CEUA/FIOCRUZ/P0195-03).
Parasite isolation and cultures
For parasite isolation by culture, the collected tissue fragments were first immersed in saline containing 1000 U/ml penicillin, 200 μg/ml streptomycin, and 50 μg/ml 5′-fluorocytosine and stored at 4°C for 24 h. After this period, each fragment was seeded into screw-cap tubes containing blood-agar slants (NNN) overlaid with 1·5 ml of Schneider's Drosophila medium (Sigma) supplemented with 10% fetal calf serum (FCS). The venous blood sample was divided into ~0·3-ml aliquots and also seeded onto NNN/Schneider+10% FCS medium. All cultures were incubated at 27°C (±0·4°C) and examined weekly by light microscopy for 30 days. Positive cultures were cryopreserved in liquid nitrogen (stabilates) for deposition in 2 Culture Collections at the Oswaldo Cruz Foundation (FIOCRUZ). Parasite cultures were also kept in liver infusion-tryptose broth (LIT) supplemented with 20% FCS, and in 2 commercially available media, Dulbecco's Modified Eagle's Medium (DMEM) and Roswell Park Memorial Institute (RPMI) medium, both supplemented with 5% FCS and adjusted to pH 8·0. The following T. rangeli and T. cruzi stocks were used: H-14, Macias, SC 61 and 1562, and CL Brener, Y, Dm28c and CanIII, respectively. Trypanosoma rangeli stocks were maintained in LIT+20% FCS, whereas T. cruzi stocks were grown in LIT or RPMI+5% FCS. For biochemical and molecular analysis, parasite cells were harvested by centrifugation (1500 g, 15 min, 4°C), washed twice in saline plus 0·1 m EDTA, pH 8·0, and the pellets were stored in liquid nitrogen until the time of use.
Morphological and biometrical studies
For morphological and biometrical analysis, Giemsa-stained smears of the trypanosomatid isolated from the dog (stock A-27) and cultured in the media cited above were examined by light microscopy at 1000× magnification. Initially, we searched for evolutive stages and their peculiarities, including kinetoplast features. The frequency of the different stages was determined by counting 100–200 randomly chosen forms of 6 to 8-day-old cultures in each medium. The following biometrical parameters (Dias and Freitas-Filho, Reference Dias and Freitas-Filho1943) were chosen for analysis of stock A-27 and of the trypanosome reference strains: (i) total length, including the free flagellum (TL), and width (W) at the level of the nucleus of epimastigotes and trypomastigotes, (ii) length of the free flagellum (F) of epimastigotes and spheromastigotes, (iii) length of the main perpendicular axes (A-1 and A-2) of spheromastigotes, and (iv) longitudinal axis of the rod-like kinetoplast of epimastigotes (KL). All measurements are reported as centimetres (cm) and were made directly on camera lucida drawings of these stages and of the kinetoplasts of epimastigotes. These results were transformed into micrometers (μm) by a multiplication factor. This factor was deduced from the size of a scale bar (cm), corresponding to 10 μm, included in all drawing plates. For statistical analysis the results are reported as the mean, standard deviation, and range. The morphological and biometrical features of the isolate were compared to those of the T. cruzi and T. rangeli reference strains mentioned above, as well as to the features of other Trypanosoma species described in the literature.
Infectivity for macrophages
Macrophages were obtained from the peritoneum of adult Swiss-Webster mice, previously sacrificed in a CO2 chamber. Briefly, 6–8 ml of RPMI medium were inoculated into the peritoneal cavity of 4 mice and the resident cells with the medium were aspirated after gentle abdominal massage. Next, the cells were counted in a Neubauer haemocytometer and their concentration was adjusted to 2×106 cells/ml with RPMI. These suspensions were pooled and placed on sterile cell culture slides (Lab Test) and incubated for 2 h. After gentle washing, each slide with adherent cells was covered with RPMI+10% FCS and incubated for 24 h. Trypanosome stages in RPMI+5% FCS were added to the macrophage culture at parasite:macrophage ratios of 2–5:1. After 3 h of interaction, the cultures were washed and the medium was changed. All experiments were carried at 37°C in a 5% CO2 atmosphere. At different times (3, 24, 48, and 72 h), 1 slide was washed with phosphate-buffered saline (PBS, pH 7·2), fixed in methanol, stained with Giemsa, and examined under a light microscope at 1000× magnification.
Infectivity for triatomines
Thirty Rhodnius neglectus and Triatoma infestans specimens (4th and 5th instars), which had been deprived of blood for at least 1 month, were used throughout the study. Trypanosome cultures from NNN/Schneider+10% FCS, as well as rabbit erythrocytes, were washed twice with PBS, pH 7·2, mixed at a ratio of 1:1 (5-ml volumes each), and placed into an apparatus for artificial feeding of triatomines. At different times (15, 30, 45, and 50 days), the gut content and haemolymph of these insects were investigated for trypanosome stages by light microscopy at 400× magnification.
Isoenzyme analysis
The trypanosome isolate (stock A-27) and the T. cruzi and T. rangeli reference strains were investigated by multilocus enzyme electrophoresis on 1% agarose gels according to standard procedures as described by Cupolillo et al. (Reference Cupolillo, Grimaldi and Momen1994). The strains were tested for the activity of the following 8 enzymatic loci: malate dehydrogenase (E.C. 1.1.1.37; MDH), malic enzyme (E.C. 1.1.1.40, ME), glucose phosphate isomerase (E.C. 5.3.1.9; GPI), phosphoglucomutase (E.C. 2.7.5.1; PGM), isocitrate dehydrogenase (E.C. 1.1.1.42, IDH), mannose phosphate isomerase (E.C. 5.3.1.8, MPI), glucose 6-phosphate dehydrogenase (E.C. 1.1.1.49, G6PDH), and 6-phosphogluconate dehydrogenase (E.C. 1.1.1.44, 6PGDH).
Molecular analysis
For molecular analysis, several PCR assays were applied for parasite identification. The DNA target specificities, primers and PCR protocols were performed according the references given in Table 1. The PCR products and a DNA ladder molecular size marker were loaded onto the slots of agarose gels. Electrophoresis was performed at 70–73 V, for 1–2 h and the gels were stained with ethidium bromide, examined, and photographed under ultraviolet light.
Table 1. Applied PCR protocols for stock A27 identification

Sequencing of PCR product of stock A-27
DNA sequencing was performed on the PCR product obtained by amplification with D75/D76 primers from conserved sequences of Trypanosomatidae. For purification and sequencing procedures, the Quiackiq PCR purification kit (Quiagen®) was used according to the manufacturer's instructions. Nucleotide sequences were determined in a automated DNA sequencer (3730 DNA Analyzer, Applied Biosystems), at the Laboratory of Functionary Genomic and Bioinformatics, Oswaldo Cruz Foundation (Otto et al. Reference Otto, Vasconcellos, Gomes, Moreira, Degrave, Mendonça-Lima and Alves-Ferreira2008) and analysed with Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and MEGA 4.1 software (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). Sequence of stock A-27 was aligned with reference strains obtained from the GenBank.
RESULTS
Trypanosomatid isolation and cultures
Promastigotes were isolated in NNN/Schneider+10% FCS medium from the ulcerated lesion found on the external ear of the dog and are characterized elsewhere. In addition, epimastigote forms (predominantly) were isolated from intact skin obtained from the scapular region of this animal. No Leishmania or Trypanosoma parasites were detected in the blood and other fragments analysed. Cryostabilates of the positive cultures of this trypanosomatid stock (named A-27) were deposited at the Leishmaniasis Surveillance Laboratory, Evandro Chagas Clinical Research Institute (IPEC) (code number R.847), and at the Trypanosomatid Collection of the Oswaldo Cruz Institute (code number CT-IOC 552), both at FIOCRUZ, Brazil.
Morphological and biometrical studies
Microscopic analysis (1000×) of Giemsa-stained smears of the trypanosomatid isolate (stock A-27) showed a predominance of epimastigotes (Fig. 1), followed by spheromastigotes (Figs 1B, F and 2B) and trypomastigotes (Fig. 2C, D), in addition to transitional stages between epimastigotes and trypomastigotes (Fig. 2A, arrow) or spheromastigotes. Epimastigotes (Fig. 1G) and spheromastigotes were the only dividing forms. The highest frequency of trypomastigotes was observed in DMEM+5% FCS and RPMI+5% FCS. Spheromastigotes were less frequent (0·9–2·8%) and usually presented a long free flagellum (Figs 1B, F and 2B). The frequency of the different stages of this isolate in the 4 culture media is shown in Table 2. Epimastigotes and trypomastigotes usually presented an elongated shape with pointed posterior ends, a round nucleus, and a rod-like kinetoplast (Figs 1A, C–E, H, I and 2A, C, D). These stages were frequently broader in the posterior portion of the body compared to the anterior part. In most epimastigotes, the kinetoplast was located next to the nucleus. In trypomastigotes, the kinetoplast was located closer to the nucleus than to the posterior end of the parasite (Fig. 2C, D). In spheromastigotes the kinetoplast was rod-like or round and was situated next to or behind the nucleus (Figs 1B, F and 2B). The total length, width, and free flagellum of the epimastigote forms of stock A-27 grown in the 3 culture media are shown in Table 3. Despite variations in the results obtained for each parameter analysed, the epimastigote forms of this isolate generally presented a long and broad shape, with a long free flagellum (Fig. 1). Marked differences were observed in the width of trypomastigote forms between this isolate (Fig. 2C, D) and T. rangeli (Fig. 2F, H) and T. cruzi (Fig. 2J), as well as in the total length of trypomastigotes between this isolate and T. cruzi. The size differences between spheromastigotes of the dog isolate (Figs 1F and 2B) and T. rangeli (Fig. 2G) were also striking. The peculiarities of the kinetoplasts of isolate A-27 (Fig. 2A–D), T. rangeli (Fig. 2E–H) and T. cruzi (Fig. 2J, I) can also be seen in these photomicrographs, but the measurements in Table 4 (see KL) show more clearly some important differences. The results of the biometrical analysis of trypomastigote and spheromastigote forms of stock A-27, T. rangeli and T. cruzi are also shown in Table 4.
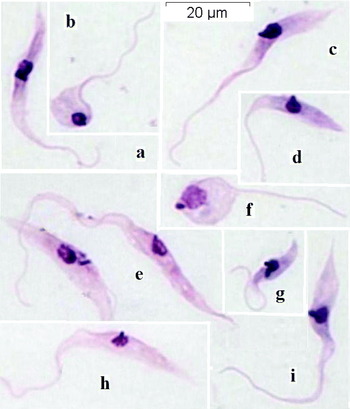
Fig. 1. Photomicrographs of culture stages of Trypanosoma caninum (stock A-27). Epimastigotes (a, c, d, e, h, i). Spheromastigotes (b, f). Dividing epimastigote (g). Note the rod-shaped kinetoplasts of epimastigotes, and the round one at a posterior position with respect to the nucleus in a spheromastigote (f). All forms are at the same magnification.

Fig. 2. Photomicrographs of culture stages of Trypanosoma caninum (stock A-27) (a–d) and reference strains of T. rangeli (H-14: e–g; SC-61: h), and T. cruzi (CL Brener: i, j). Note the differences in the width of the trypomastigotes from the dog trypanosome (c, d), T. rangeli (f, h) and T. cruzi (j). The spheromastigotes of the dog trypanosome (b) are also larger than those of T. rangeli (g). Note the remarkable differences between trypomastigotes of the dog trypanosome (c, d) and T. cruzi (i). All forms are at the same magnification.
Table 2. Evolutive stages found in Giemsa-stained smears from axenic cultures of Trypanosoma caninum (stock A-27) isolated from a domestic dog
(The parasite was maintained in different media at 27°C (±0·4°C), and the cultures examined at 6–8 days after seeding. Epi=epimastigotes, Trypo=trypomastigotes, Sphero=spheromastigotes.)

Table 3. Measurements (μm) of epimastigotes from Giemsa-stained smears of cultures in different media of Trypanosoma caninum (stock A-27) isolated from a domestic dog
(Biometrical parameters analysed: total length (TL, free flagellum included), width at the level of the nucleus (W), and length of the free flagellum (F). Results are in μm (means, standard deviations, and ranges in parentheses); n=30.)

Table 4. Measurements of evolutive stages and kinetoplasts found in Giemsa-stained smears from axenic cultures of Trypanosoma caninum (stock A-27) isolated from a domestic dog, together with reference strains of T. rangeli and T. cruzi
(Biometrical parameters analysed: total length (TL, free flagellum included) and width (W, at the level of the nucleus) of trypomastigotes, length of the main perpendicular axes of spheromastigotes (A-1 and A-2), free flagellum (F), and length of the longitudinal axis of rod-like kinetoplasts of epimastigotes (KL). Results are in μm (means, standard deviations, and ranges in parentheses). Samples: n=20 (trypomastigotes), n=10 (spheromastigotes), and n=30 (kinetoplasts), unless indicated.)

All cultures were maintained at 27°C (±0·4°C) and were from: (a) RPMI+5% FCS, (b) NNN+Schneider's+10% FCS, (c) NNN/LIT+20% FCS, (d) LIT+20% FCS, and (e) LIT. (f) n=14. (g) n=15. ND, not determined: no or low number of this stage.
Infectivity for macrophages
After 3 h of interaction, culture stages of stock A-27 were found either adhered to or inside mouse peritoneal macrophages (Fig. 3A, B). After 48 h, parasite forms completely disappeared and no free swimming or internalized forms were seen. These observations are shown in Fig. 3.

Fig. 3. Photomicrographs of Giemsa-stained smears showing in vitro cellular interactions between culture stages of T. caninum and mouse peritoneal macrophages. Note the parasites adhered and internalized at 3 h of interaction (a, b), and the final steps of their destruction within macrophages at 24 and 72 h (c–e). All forms are at the same magnification.
Infectivity for triatomines
No trypanosome stages were found in the gut or haemolymph of the triatomine bugs examined.
Isoenzyme analysis
Figure 4 shows a schematic representation of the isoenzyme patterns for MDH, ME, GPI, PGM, IDH, MPI, G6PDH, and 6PGDH obtained for isolate A-27 and for the T. rangeli and T. cruzi reference strains. The banding pattern of the trypanosome isolate differed markedly from those of the reference stocks.

Fig. 4. Diagrammatic representation of isoenzyme patterns displayed by Trypanosoma caninum, Trypanosoma rangeli and Trypanosoma cruzi reference strains. T. rangeli strains: A (H-14), B (Macias) and C (SC-61). T. caninum: D (stock A-27). T. cruzi: E (CL Brener clone), F (Y strain) and G (Dm28c clone). Isoenzymes: (MDH) malate dehydrogenase, (ME) malic enzyme, (GPI) glucose phosphate isomerase, (PGM) phosphoglucomutase, (IDH) isocitrate dehydrogenase, (MPI) mannose phosphate isomerase, (G6PDH) glucose 6-phosphate dehydrogenase, and (6PGDH) phosphogluconate dehydrogenase.
Molecular analysis
The results obtained for the different PCR assays indicated that the stock A-27 belongs to the family Trypanosomatidae the assay of which amplified conserved sequences within the Trypanosomatidae genomes (Souto et al. Reference Souto, Vargas and Zingales1999). Using primers D75/D76 one product of approximately 250 bp was observed for stock A-27 (Fig. 5A), therefore, no amplification products were obtained when primers D71/D72 and primers to the mini-exon gene (T. cruzi specific) (Fig. 5B, C) or R1/R2 (T. rangeli specific) (Fig. 5D) were used. Curiously, 2 distinct products of about 330 and 350 bp, the smallest one being similar to those of the T. cruzi strains were obtained with primers 121/122 (Fig. 5E).

Fig. 5. Products amplified by PCR assays. (A) Family Trypanosomatidae-specific with primers D75/D76. (M) 100 bp ladder; (1) stock A-27; (2) Trypanosoma cruzi II – Y strain; (3) Trypanosoma cruzi I – Dm28c clone; (4) Trypanosoma rangeli – H14 strain. (B) Trypanosoma cruzi-specific with primers D71/D72. (M) 100 bp ladder; (1) stock A-27; (2) Trypanosoma cruzi II – Y strain; (3) Trypanosoma cruzi I – Dm28c clone; (4) Trypanosoma rangeli – H14 strain. (C) Trypanosoma cruzi-specific with primers to mini-exon gene. (M) 100 bp ladder; (1) Trypanosoma cruzi I – Dm28c clone; (2) Trypanosoma cruzi II – Y strain; (3) Trypanosoma cruzi Z3 – CanIII strain; (4) Trypanosoma rangeli – 1562 strain; (5) stock A-27. (D) Trypanosoma rangeli-specific with primers R1/R2. (M) 100 bp ladder; (1) stock A-27; (2) Trypanosoma cruzi II – Y strain; (3) Trypanosoma cruzi I – Dm28c clone; (4) Trypanosoma rangeli – H14 strain. (E) KDNA minicircle of kinetoplastid with primers 121/122. (M) ϕX174 DNA-HaeIII Digest; (1) Trypanosoma rangeli – H14 strain; (2) stock A-27; (3) Trypanosoma cruzi I – Dm28c clone; (4) Trypanosoma cruzi II – Y strain.
Sequencing of PCR products of stock A-27 and alignment with other species
A fragment of 208 bp was obtained. Nucleotide Blast search against GenBank retrieved 8 high-scoring database matches (100% similarity, with e-value=6×10−56) that aligned with 55% of the query sequence (query coverage of 55%), corresponding to 24Sα rDNA sequences of Trypanosoma kuseli (Accession number AB175626), Trypanosoma otospermophili (Accession number AB175625), Trypanosoma grosi isolate: AKHA (Accession number AB175624), Trypanosoma grosi isolate: HANTO (Accession number AB175623), Trypanosoma grosi isolate: SESUJI (Accession number AB175622), Trypanosoma otospermophili (Accession number AB190228), Trypanosoma cruzi (Accession number L22334 ) and Trypanosoma rangeli (Accession number U73612). These sequences were aligned with Mega software (Fig. 6A, B). An UPGMA dendrogram was constructed from this alignment, showing that stock A-27 is separated from the other parasites studied (Fig. 6C).

Fig. 6. Alignment of the 24sα rDNA sequence from D75 to D76 amplified DNA of stock A-27 and reference sequences obtained from the GenBank: Trypanosoma kuseli (AB175626), T. otospermophili (AB175625), T. grosi isolate: AKHA (AB175624), T. grosi isolate: HANTO (AB175623), T. grosi isolate: SESUJI (AB175622), T. otospermophili (AB190228), T. cruzi (L22334) and T. rangeli (U73612). Region with (A) high and (B) low similarity. (C) UPGMA dendrogram constructed with the alignment obtained. Bar represents evolutionary distance.
DISCUSSION
The genus Trypanosoma comprises numerous parasite species, some of them causing important disease in humans and animals. The present paper reports the isolation and characterization of new species of this genus, obtained from domestic dog, called Trypanosoma caninum.
The isolation and maintenance of cultures are crucial conditions for the study and description of new trypanosomatid species. The strain described here was easily isolated and cultured on different axenic media, with the observation of all evolutive stages characteristic of the genus Trypanosoma under these conditions (Hoare, Reference Hoare1972). One interesting aspect of this organism was its isolation by culture of intact skin fragments of the animal, whereas the blood culture was negative. This characteristic is uncommon and is described for the first time for the isolation of parasites of this genus, suggesting that this protozoan may preferentially inhabit tissues and vessels of the peripheral circulation. Although T. caninum seems not to cause any clinical or dermatological alterations, the understanding of how this parasite is maintained and transmitted under natural conditions is of fundamental importance in terms of different aspects. The impossibility of this isolate to infect triatomine bugs such as R. neglectus and T. infestans may suggest that other arthropods act as vectors, as do fleas in the cycle of T. lewisi (Hoare, Reference Hoare1972) or ticks in the cycle of T. theileri (Shastri and Deshpande, Reference Shastri and Deshpande1981). Ectoparasites such as fleas and ticks are widely distributed in many areas and the common characteristic of changing hosts during the life cycle facilitates the transmission of potential disease-causing organisms (Wilson, Reference Wilson2002). In visceral leishmaniasis areas, ticks have been suspected to participate in the transmission of Leishmania chagasi from dog to dog (Coutinho et al. Reference Coutinho, Bueno, Sterzik, Fujiwara, Botelho, De Maria, Genaro and Linardi2005). However, the vector competence of mosquitoes and sandflies should also be investigated in this region.
The other biological parameters analysed also revealed interesting findings. Attempts to evaluate the growth of this parasite in murine peritoneal macrophage culture were equally unsuccessful. Flagellate forms had disappeared completely after 48 h of interaction and no phagocytosed or free forms were observed in the supernatant. In this respect, it should be remembered that only parasites of the subgenus Schizotrypanum are able to invade and infect mammalian cells. On the other hand, the biometric results also demonstrated peculiar and marked characteristics (e.g., a long body and flagellum of epimastigote and trypomastigote forms), which permitted the easy differentiation of the isolate from T. cruzi and T. rangeli strains. However, the wide morphobiometric heterogeneity among parasites of the genus Trypanosoma restricts, to some extent, the use of this parameter, although it has been employed as a complementary tool for the differentiation and identification of various species of this genus (Ziccardi and Lourenço-de-Oliveira, Reference Ziccardi and Lourenço-de-Oliveira1998), especially the differentiation between T. cruzi and T. rangeli (Sousa et al. Reference Sousa, Fonseca, Santos, Santos-Pereira, Carvalhal and Hasslocher-Moreno2008). In a recent study, elegantly exploring these parameters, Lainson et al. (Reference Lainson, Da Silva and Franco2008) demonstrated the circulation of a new Trypanosoma species of the subgenus Megatrypanum.
No PCR products were raised with primers D71/D72 and mini-exon gene specifics for T. cruzi (Souto et al. Reference Souto, Vargas and Zingales1999; Fernandes et al. Reference Fernandes, Santos, Cupolillo, Mendonça, Derre, Junqueira, Santos, Sturm, Naiff, Barret, Campbell and Coura2001) and R1/R2 for T. rangeli (Vargas et al. Reference Vargas, Souto, Carranza, Vallejo and Zingales2000), whose results had pointed to another species. In addition, using primers designed to amplify a conserved sequence within all Trypanosomatids showed a product of about 250 bp in the stock A-27, that differed fom the amplification products obtained with T. cruzi and T. rangeli. Comparison of the nucleotide sequences of 24α rDNA genes indicated that T. caninum is separated from the other parasites studied when an UPGMA dendrogram was constructed from that alignment.
Another peculiarity of this organism is the large size of its spheromastigotes, which are usually greater than those found in T. rangeli. These results were confirmed by isoenzyme analysis which showed differences compared to the banding patterns of the T. cruzi and T. rangeli stocks for all enzymatic loci studied.
The distinction of this parasite from species of salivarian trypanosomes found in Brazil is very easy since, in contrast to the dog trypanosome, those parasites do not grow in axenic cultures (Hoare, Reference Hoare1972). Other salivarian trypanosomes found in dogs from the African and Asian continents, such as T. brucei brucei and T. congolense, besides requiring special culture media for growing, their developmental stages are generally restricted to trypomastigotes, which are typically found in the midgut of their vectors (Vickerman, Reference Vickerman1965; Taylor and Baker, Reference Taylor and Baker1968; Hoare, Reference Hoare1972).
Githure et al. (Reference Githure, Anjili, Ngumbi, Mwanyumba, Lugalia, Koech and Kinoti1995) reported the isolation of a trypanosome from the spleen of a domestic dog from Kenya, but this parasite was insufficiently characterized, although considered distinct from the Herpetosoma species used as references in isoenzyme analyses. It is interesting to mention that species of the subgenus Herpetosoma can be easily isolated in axenic cultures from the blood and viscera of their hosts (Hoare, Reference Hoare1972), in contrast to the dog trypanosome under study.
It is known that different species of the family Trypanosomatidae cross-react in serological tests due to the shared expression of numerous proteins. This characteristic is a complicating factor for correct diagnosis, especially in areas where endemic species overlap as observed for T. cruzi and T. rangeli (Saldana and Souza, Reference Saldaña and Souza1996; Caballero et al. Reference Caballero, Sousa, Marques, Saez-Alquezar and Umezawa2007) and L. braziliensis and L. chagasi (Madeira et al. Reference Madeira, Schubach, Schubach, Pereira, Figueredo, Baptista, Leal, Melo, Confort and Marzochi2006b).
To our knowledge, no trypanosomatid species other than leishmanial parasites have been detected in dogs from the municipality of Rio de Janeiro and the occurrence of T. caninum may have epidemiological consequences, especially in terms of aspects related to its diagnosis. In this municipality, tegumentary and visceral leishmaniasis overlap and the diagnosis of canine leishmaniasis is made by serological tests. Seroreactive dogs are referred for euthanasia as one of the control measures of visceral leishmaniasis (Ministério da Saúde, Reference Ministério2006) and the circulation of this new species may confound the results of serological surveys conducted in this region.
The present results indicate that T. caninum is a new species of the genus Trypanosoma that circulates among dogs in the municipality of Rio de Janeiro. This assumption is based on the fact that other stocks resembling T. caninum were isolated from the skin of 20 other leishmaniasis-seroreactive dogs, demonstrating cross-reactivity between the two parasites (unpublished data). This finding should alert the epidemiological surveillance staff within the Leishmaniasis Control Program, particularly in the municipality of Rio de Janeiro.
DIAGNOSIS
Name:Trypanosoma caninum n. sp.
Mammalian host:Canis familiaris (Canidae, Carnivora, Mammalia).
Locality: District of Campo Grande, Rio de Janeiro City, State of Rio de Janeiro, Brazil.
Vector: unknown.
Biology and morphology: This species grows well in different commercially available media supplemented with fetal calf serum (FCS), overlaying, or not blood-agar slants (NNN). The evolutive stages found in axenic cultures were epimastigotes (predominantly), trypomastigotes, spheromastigotes, transitional stages from epimastigotes to spheromastigotes, and from epimastigotes or trypomastigotes. Dividing forms seen were epimastigotes or spheromastigotes. Epimastigotes from different culture media averaged 31·7–39·1 μm in total length, 2·3–2·5 μm in width, and 12·3–15·6 μm in free flagellum. The highest rates of differentiation to trypomastigotes occurred in RPMI and DMEM cultures supplemented with 5% FCS. Trypomastigotes from the former medium presented 43·0 (±6·9) μm in total length. Spheromastigotes averaged 5·9 μm× 4·9 μm, usually having a long free flagellum (up to 20·9 μm). The kinetoplasts of these stages were mainly rod-like shaped, but also could be rounded, and sometimes placed behind the nucleus. Kinetoplasts of epimastigotes averaged 1·3 μm in length (0·8–2·3 μm). The culture stages of this trypanosome infected neither mouse peritoneal macrophages nor the gut and haemolymph of triatomine bugs (Triatoma infestans and Rhodnius neglectus).
Other features: The electrophoretic mobilities of isoenzymes MDH, ME, GPI, PGM, IDH, MPI, G6PDH, and 6PGDH were very distinct from those of T. cruzi (strains CL Brener, Y and Dm28c) and T. rangeli (strains H-14 and Macias). This trypanosome presented amplified products of about 250 bp using primers D75/D76, but no T. cruzi-specific and T. rangeli-specific bands were detected using primers D71/D72, mini-exon gene and R1/R2.
Type materials: Cryostabilates of the axenic cultures of this trypanosome (named stock A-27) are deposited at the Laboratory of Leishmaniasis Surveillance (code number LVL-847), Evandro Chagas Clinical Research Institute (IPEC), and at the Trypanosomatid Collection of the Oswaldo Cruz Institute (code number CT-IOC 552), both at the Oswaldo Cruz Foundation, Brazil.
Comments: This trypanosome was isolated from the intact skin of the scapular region of a dog, but not from the intact abdominal skin, cutaneous lesions, venous blood, lymphnodes, liver, spleen and heart. This species does not appear to be pathogenic for the dog. It can be easily distinguished from T. cruzi by the size of the trypomastigotes and kinetoplasts, besides its inability to infect mouse peritoneal macrophages. It can be distinguished from T. rangeli with regard to the body width and size of spheromastigotes. In contrast to T. cruzi and T. rangeli, this trypanosome was unable to infect triatomines.
This study was supported by grants from Fundação de Amparo a Pesquisa do Rio de Janeiro (FAPERJ). The authors thank the Laboratory of Triatomines, Instituto Oswaldo Cruz, Rio de Janeiro, for the donation of the triatomine bugs used in this study.












