INTRODUCTION
Visceral leishmaniasis is a severe vector-borne disease of humans and other mammals caused by parasites of the Leishmania donovani complex. Clinically and epidemiologically there are two main forms: (1) zoonotic visceral leishmaniasis (ZVL), which affects mainly young children and has the domestic dog as its principal reservoir and (2) anthroponotic visceral leishmaniasis (AVL), which affects people of all ages, and is transmitted from human to human via infectious sandfly bites. There are an estimated 0·5 million cases of visceral leishmaniasis per year, concentrated in India, Nepal, Bangladesh, Sudan and Brazil, though this is likely to be an underestimate (Bern et al. Reference Bern, Maguire and Alvar2008; Reithinger, Reference Reithinger2008). While both forms are important public health problems, ZVL is also an important veterinary problem.
The viscerotropic Leishmania parasites have been classified as up to 4 species (L. donovani, L. infantum, L. chagasi and L. archibaldi), based largely on multilocus enzyme electrophoresis typing. The taxonomic situation has now been clarified after detailed molecular studies (Lukes et al. Reference Lukes, Mauricio, Schonian, Dujardin, Soteriadou, Dedet, Kuhls, Tintaya, Jirku, Chocholova, Haralambous, Pratlong, Obornik, Horak, Ayala and Miles2007). Phylogenetic analysis shows that the visceral parasites form two monophyletic groups, L. donovani and L. infantum, which diverged 1·2–0·7 Mya, with parasites from Sudan previously classified as L. infantum or L. archibaldi falling within L. donovani. The New World taxon chagasi lies within Old World L. infantum, and is not a distinct species (Mauricio et al. Reference Mauricio, Stothard and Miles2000), supporting a recent introduction to the New World. It has been argued that chagasi should continue to be recognized as a subspecies of L. infantum, on the basis on some phenotypic and genotypic differences (Lainson and Rangel, Reference Lainson and Rangel2005), but further sampling is required and the available molecular evidence does not suggest that L. chagasi and L. infantum form distinct clades (Mauricio et al. Reference Mauricio, Stothard and Miles2000). Differences between these parasites may be due to recent adaptation of chagasi to a new vector species (Shaw, Reference Shaw2006). The revised classification thus includes only two species, with L. infantum causing ZVL in Asia, the Middle East and Europe, and thence introduced to Latin America, and L. donovani causing AVL in Asia, the Middle East and Africa. This classification is followed here.
The principal transmission route for L. infantum, as for other Leishmania spp., is by the bite of blood-feeding female phlebotomine sandflies. The domestic dog is the main reservoir host and, unlike AVL, humans are considered as an accidental host that does not contribute to transmission. Control of ZVL transmission is thus focused on vector control and, in some areas, culling of infected dogs; there is currently no vaccine, and treatment of infected dogs is not usually curative (Baneth and Shaw, Reference Baneth and Shaw2002). However, the rising incidence of ZVL in Brazil and elsewhere suggests that existing control measures have not been effective (Maia-Elkhoury et al. Reference Maia-Elkhoury, Alves, Sousa-Gomes, Sena and Luna2008; Antoniou et al. Reference Antoniou, Messaritakis, Christodoulou, Ascoksilaki, Kanavakis, Sutton, Carson and Courtenay2009). A number of reasons have been suggested to explain this, including operational and logistical difficulties, but also the potential role of non-sandfly transmission routes and additional reservoir hosts. Here, we critically review aspects of the epidemiology and control of ZVL caused by L. infantum. We first consider (1) the epidemiology of transmission from the domestic dog, (2) the potential importance of non-sandfly transmission routes, and (3) the potential importance of other reservoir hosts, and the relevance of each to ZVL control. We then review studies examining the efficacy of current control methods and factors explaining their success or failure, considering dog culling, residual insecticide spraying, and topical insecticides aimed at preventing transmission from dogs. Throughout, we take a quantitative epidemiological approach, emphasizing those studies, or the lack of such studies, which allow firm, quantitative conclusions to be drawn about transmission and control of ZVL.
EPIDEMIOLOGY OF TRANSMISSION BY THE DOMESTIC DOG
Transmission of L. infantum from domestic dogs by the bite of infected sandflies was first demonstrated in the 1930s (Parrot et al. Reference Parrot, Donatien and Lestoquard1930; Adler and Theodor, Reference Adler and Theodor1932). Subsequently, many studies have confirmed the role of the domestic dog as the primary reservoir of ZVL: dogs often have a high prevalence of both infection and infectiousness, have long-lasting infections, and are common in the peridomestic environment in which most ZVL transmission occurs. At least a dozen sandfly species of the subgenus Larroussius have been incriminated as vectors of L. infantum in the Old World, including Phlebotomus perniciosus, P. ariasi, P. perfiliewi, P. neglectus and P. tobbi in Europe (Killick-Kendrick, Reference Killick-Kendrick1999). In Latin America, the most important vector is the Lutzomyia longipalpis species complex, with L. evansi suspected to be the important vector in some foci in Colombia and Venezuela (Travi et al. Reference Travi, Velez, Brutus, Segura, Jaramillo and Montoya1990; Montoya-Lerma et al. Reference Montoya-Lerma, Cadena, Oviedo, Ready, Barazarte, Travi and Lane2003), and L. cruzi incriminated in parts of Brazil (dos Santos et al. 1998).
Understanding the epidemiology of infection in the domestic dog is essential to plan the control of human and canine infection. In particular, we need to know (1) the intensity of transmission between dogs, usefully summarized by the basic reproduction number, R0; (2) the proportion of the dog population that is infectious to sandflies, and (3) whether infectious and non-infectious dogs can be differentiated, to allow targeted control of infectious dogs. R0 is defined as the average number of secondary cases arising from a primary case in a susceptible population. Thus the magnitude of R0 provides a measure of the difficulty of disease control, since the effort (E) required to eliminate infection (i.e. to drive R0<1) is E>1−1/R0. R0 can be estimated using the prevalence of infection in dogs, or the incidence of infection and dog life expectancy, but accurate estimates require the use of sensitive methods to detect infection (e.g. multiple diagnostic tests), detailed longitudinal studies, or both (Dye et al. Reference Dye, Killick-Kendrick, Vitutia, Walton, Killick-Kendrick, Harith, Guy, Canavate and Hasibeder1992; Hasibeder et al. Reference Hasibeder, Dye and Carpenter1992). Using such methods, it has been estimated that R0=11 in Malta and R0=9 in Amazon Brazil (Dye et al. Reference Dye, Killick-Kendrick, Vitutia, Walton, Killick-Kendrick, Harith, Guy, Canavate and Hasibeder1992; Quinnell et al. Reference Quinnell, Courtenay, Garcez and Dye1997; Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b). This suggests that control of ZVL will be difficult, requiring a >89% reduction of transmission to eliminate infection. Longitudinal studies in France and Italy have shown high incidences of 40–92% per transmission season, suggesting that R0 in these areas may be similarly high (Dye et al. Reference Dye, Vidor and Dereure1993; Oliva et al. Reference Oliva, Scalone, Foglia Manzillo, Gramiccia, Pagano, Di Muccio and Gradoni2006). In contrast, other Mediterranean studies have produced much lower estimates of R0 (Amela et al. Reference Amela, Mendez, Torcal, Medina, Pachon, Canavate and Alvar1995; Zaffaroni et al. Reference Zaffaroni, Rubaudo, Lanfranchi and Mignone1999; Keck and Dereure, Reference Keck and Dereure2003). However, the latter studies were cross-sectional and used only a single diagnostic method, the indirect fluorescent antibody test (IFAT), to detect infection, so are likely to have underestimated the prevalence of infection and thus R0 (Baneth et al. Reference Baneth, Koutinas, Solano-Gallego, Bourdeau and Ferrer2008). Prevalence estimates based on PCR diagnosis can be much higher than seroprevalences in cross-sectional studies, e.g. 60–80% by PCR, compared to <30% seropositive (Solano-Gallego et al. Reference Solano-Gallego, Morell, Arboix, Alberola and Ferrer2001; Lachaud et al. Reference Lachaud, Chabbert, Dubessay, Dereure, Lamothe, Dedet and Bastien2002). Further estimates of R0 from diverse endemic areas would be useful.
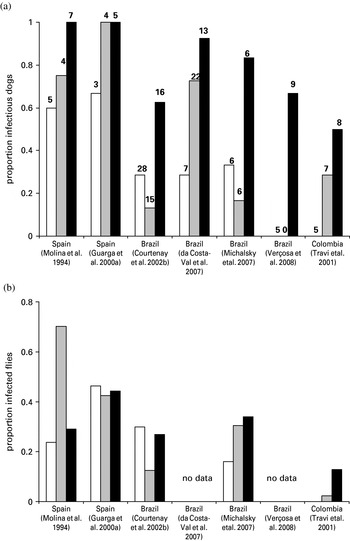
The assessment of infectiousness currently requires xenodiagnostic studies using uninfected, laboratory-bred sandflies. Such studies have been performed on dogs presenting varying severity of clinical disease in both Europe and South America. The results show that a high proportion of infected dogs are infectious (Fig. 1a), and these infectious dogs infect a high proportion of sandflies feeding on them (Fig. 1b). To test the relationship between infectiousness and clinical condition, we carried out a meta-analysis of 7 published studies by logistic regression. The outcome measure was infectiousness to sandflies, with the continuous predictor being number of symptoms defined as 0 (asymptomatic), 1 (oligosymptomatic) and 2 (polysymptomatic), according to the authors' definitions of these clinical classes; study was included as a random effect in the model. The proportion of infectious dogs increased significantly with increasing clinical severity, from 0·29 of asymptomatic dogs through to 0·80 of polysymptomatic dogs (Odds Ratio (OR)=3·09, 95% CL 1·96–4·88, P<0·0001, for each increase in clinical category). There was no significant difference in this relationship between European (OR=6·98) and South American (OR=2·94) studies (continent*symptoms interaction, P=0·399). In contrast, the proportion of sandflies that are infected by infectious dogs does not seem to vary with clinical status: infectious asymptomatic dogs infect similar proportions of flies to infectious sick dogs (Fig. 1b). Though the relationship between infectiousness and clinical severity was similar in both continents, the overall proportion of infectious dogs was higher in Europe (0·86) than in South America (0·45) (OR=8·02, 95% CL 1·92–33·5, P=0·004), reflected also in a higher proportion of flies infected in Europe. This may indicate a greater susceptibility of P. perniciosus to infection compared to L. longipalpis, and perhaps differences between dog populations (e.g. breeds). The proportion of sandflies infected also varies between South American studies, with a particularly low proportion infected in the one Colombian study, perhaps reflecting variation across the L. longipalpis species complex. There is also a high degree of variation in the proportion of sandflies infected when exposed to dogs repeatedly over short intervals e.g. between consecutive days (O. Courtenay, unpublished data). Studies that directly compare the infectiousness of dogs to different vector species would be useful.

Fig. 1. Infectiousness to sandflies of domestic dogs infected with Leishmania infantum. The relationship between clinical severity and (a) proportion of infectious dogs and (b) proportion of sandflies infected by infectious dogs, from published xenodiagnostic studies using the sandflies Phlebotomus perniciosus (Spain) or Lutzomyia longipalpis (Brazil and Colombia). Dogs were classified as asymptomatic (open bars), oligosymptomatic (grey bars) or polysymptomatic (black bars) according to the authors' definitions. Sample sizes (number of dogs) are given above each bar. For Courtenay et al. (Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b), a single feed per dog/clinical category was selected at random. Da Costa-Val et al. (Reference Da Costa-Val, Cavalcanti, Gontijo, Michalick, Alexander, Williams and Melo2007) included dogs which infected very few sandflies in the non-infectious group. The proportions of infected sandflies for Michalsky et al. (Reference Michalsky, Rocha, Lima, Franca-Silva, Pires, Oliveira, Pacheco, Dos Santos, Barata, Romanha, Fortes-Dias and Dias2007) were estimated assuming equal numbers of flies dissected per dog.
The results of the meta-analysis confirm that infectiousness increases with clinical severity, but that the infectiousness of asymptomatic dogs is sufficiently high that control needs to be directed at both asymptomatic and symptomatic dogs. Accurate assessment of the population prevalence of infectiousness from the existing studies is difficult, as dogs were not randomly sampled from endemic areas, and such bias may explain some of the results of the analyses performed above. However, we can use these results to crudely estimate the proportion of transmission that is due to asymptomatic dogs. Cross-sectional studies suggest that about 50% of infected dogs are symptomatic (e.g. Gradoni et al. Reference Gradoni, Gramiccia, Mancianti and Pieri1988); if so, then about 30% of transmission will be due to asymptomatic dogs. There is evidence from Brazil that most asymptomatic infectious dogs go on to develop progressive disease, so are more accurately described as pre-symptomatic (Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b). This longitudinal study demonstrated that ‘true’ asymptomatic dogs contribute very little to transmission compared to symptomatic and pre-symptomatic dogs (Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b).
Since not all infected dogs become infectious, control targeted at infectious dogs could be effective, if a reliable test for infectiousness was available that, unlike xenodiagnosis, could be used at the population level. Infectiousness is positively associated with clinical severity, high anti-parasite antibody responses, and low CD4+ T cell count (Guarga et al. Reference Guarga, Moreno, Lucientes, Gracia, Peribanez, Alvar and Castillo2000b; Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b; da Costa-Val et al. Reference Da Costa-Val, Cavalcanti, Gontijo, Michalick, Alexander, Williams and Melo2007). These correlations probably reflect an underlying association with parasite burden, and infectiousness is reduced as parasite loads decline after treatment (Gradoni et al. Reference Gradoni, Maroli, Gramiccia and Mancianti1987; Ribeiro et al. Reference Ribeiro, Moura, Pimentel, Sampaio, Silva, Schettini, Alves, Melo, Tafuri, Demicheli, Melo, Frezard and Michalick2008). However, these clinical and immunological correlates do not provide a sensitive and specific test for infectiousness (Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b). A more specific marker might be provided by parasite load in the skin, but studies comparing infectiousness and parasite detection in skin biopsies are few and have so far not shown a correlation in dogs (Travi et al. Reference Travi, Tabares, Cadena, Ferro and Osorio2001). More accurate assessment of skin parasite load using quantitative PCR may be more informative.
POTENTIAL IMPORTANCE OF NON-SANDFLY TRANSMISSION ROUTES
Sandfly transmission has a central role in maintaining L. infantum infection. The spatial and temporal overlap of ZVL cases and incriminated vector species show that sustained transmission does not generally occur in the absence of sandfly vectors. Thus, for example, the recent occurrence of autochthonous human and canine cases in northern Italy is associated with the northerly expansion of two vectors, P. perniciosus and P. neglectus (Maroli et al. Reference Maroli, Rossi, Baldelli, Capelli, Ferroglio, Genchi, Gramiccia, Mortarino, Pietrobelli and Gradoni2008). Further evidence for the importance of sandflies comes from the observed reductions in ZVL incidence during controlled intervention studies using e.g. insecticide-impregnated dog collars (reviewed below), and evidence that widespread use of DDT against malaria vectors was associated with a marked decline in ZVL and AVL human cases, with case resurgence following cessation of DDT use (Alexander and Maroli, Reference Alexander and Maroli2003). However, cases of autochthonous transmission of L. infantum have been described from northerly latitudes in Europe where sandfly vectors are absent (Harris, Reference Harris1994). More recently, the sustained transmission of ZVL in foxhounds from 58 hunt clubs in 18/35 USA states and in 2/4 Canadian provinces from 1999 onwards (Duprey et al. Reference Duprey, Steurer, Rooney, Kirchhoff, Jackson, Rowton and Schantz2006), in areas where sandflies occur at low densities, or where their vectorial competence is thought to be poor, has renewed interest in other potential transmission routes. The lack of spread from foxhounds to the wider dog population or local wild canid population suggests that sandfly transmission is not involved, and thus that under some circumstances infection can be maintained by non-sandfly transmission routes. Fourteen species of Lutzomyia are found in North America north of Mexico including L. anthorphora, L. diabolica, L. cruciata, and L. shannoni, each occurring at higher densities in the southern states; some species including L. vexator extend north into Canada (Young and Perkins, Reference Young and Perkins1984; Haddow et al. Reference Haddow, Curler and Moulton2008). However, to date no sandfly species has been implicated in transmission of ZVL in these foxhounds.
Severe canine leishmaniasis is characterized by widespread dissemination of parasites not only where accessible to biting vectors in peripheral blood and in the skin, but also in internal organs, saliva, semen, conjunctiva and the genital tract. Thus transmission by transfer of infected body fluids (e.g. by biting, blood transfusion, needles or sexual contact) or congenitally, have been suggested to explain non-sandfly transmission. Congenital transmission to puppies has been confirmed experimentally (Rosypal et al. Reference Rosypal, Troy, Zajac, Frank and Lindsay2005), and there have been a number of case reports of congenital transmission of AVL and ZVL in humans, including from asymptomatic mothers with ZVL (Meinecke et al. Reference Meinecke, Schottelius, Oskam and Fleischer1999; Pagliano et al. Reference Pagliano, Carannante, Rossi, Gramiccia, Gradoni, Faella and Gaeta2005; Boehme et al. Reference Boehme, Hain, Novosel, Eichenlaub, Fleischmann and Loscher2006). Additional transmission routes include infection through blood transfusion from infected to uninfected hosts, both in foxhounds (Owens et al. Reference Owens, Oakley, Marryott, Hatchett, Walton, Nolan, Newton, Steurer, Schantz and Giger2001) and humans (Otero et al. Reference Otero, Da Silva, Luz, Palatnik, Pirmez, Fernandes and Palatnik de Sousa2000), human organ transplantation (Antinori et al. Reference Antinori, Cascio, Parravicini, Bianchi and Corbellino2008) and sexual transmission in dogs (Silva et al. Reference Silva, Oliveira, Silva, Xavier, Nascimento and Santos2009) and humans (Symmers, Reference Symmers1960). Transmission via shared syringes is another possibility, and has been indicated amongst IV-drug users in southwest Europe (Cruz et al. Reference Cruz, Morales, Noguer, Rodriguez and Alvar2002; Alvar et al. Reference Alvar, Aparicio, Aseffa, Den Boer, Canavate, Dedet, Gradoni, Ter Horst, Lopez-Velez and Moreno2008). The possibility of transmission by non-sandfly vectors also has been considered (Dantas-Torres, Reference Dantas-Torres2006; Coutinho and Linardi, Reference Coutinho and Linardi2007). Blanc and Caminopetros (Reference Blanc and Caminopetros1930) first demonstrated trans-stadial infection with Leishmania in experimentally infected brown dog ticks Rhipicephalus sanguineus, confirmed recently by Coutinho et al. (Reference Coutinho, Bueno, Sterzik, Fujiwara, Botelho, De Maria, Genaro and Linardi2005). These studies also demonstrate mechanical transmission from infected dogs to ticks, however neither dog-to-dog transmission by ticks, nor metacylogenesis within ticks (i.e. biological development from amastigote to infectious promastigote), have yet been proven. A similar incomplete scenario is true for Leishmania infections in Ctenocephalides fleas (Coutinho and Linardi, Reference Coutinho and Linardi2007).
Transmission by non-sandfly routes is likely to be rare, except under specific dog husbandry conditions. Iatrogenic transmission (by blood transfusion or vaccination), fighting and sexual transmission may explain the US foxhound epidemic, though a role for sandflies has not been definitively excluded, but the epidemiological importance of these non-sandfly transmission routes in most endemic areas will be low. Nonetheless, some recent studies have shown a relatively high frequency of sexual and congenital transmission in dogs. Sexual transmission was demonstrated in 58% (7/12) of uninfected bitches mated to multiple infected dogs (Silva et al. Reference Silva, Oliveira, Silva, Xavier, Nascimento and Santos2009), and congenital transmission to 26% (8/31) of puppies born to 7 bitches in Italy (Masucci et al. Reference Masucci, De Majo, Contarino, Borruto, Vitale and Pennisi2003). In contrast, none of 56 puppies born to 18 infected bitches in a Brazilian study were infected by congenital transmission (Andrade et al. Reference Andrade, De Toledo, Marques, Silva, Tafuri, Mayrink and Genaro2002). There is no evidence that sexual and congenital routes can sustain transmission in the absence of sandflies.
POTENTIAL IMPORTANCE OF OTHER RESERVOIR HOSTS
Theoretical framework for reservoir host incrimination
For a parasite species to persist in a reservoir host population, it must have a basic reproduction number (R0) ⩾1 (Anderson and May, Reference Anderson and May1991). Where multiple host species can be infected, as in ZVL, it is possible to divide host species on epidemiological grounds into primary, secondary and accidental reservoir hosts. A primary reservoir host can maintain R0 above 1 in the absence of other hosts, so the parasite can persist indefinitely in this host alone. Secondary reservoir hosts can transmit infection, so that R0 is increased, but cannot maintain parasite transmission in the absence of the primary host(s). Accidental hosts can be infected, but do not usually transmit the parasite, and thus have no effect on R0. Control of parasite transmission by the primary reservoir can eliminate the disease by reducing R0 below 1, control directed at secondary reservoirs can reduce R0 but cannot lead to elimination, whereas control directed at accidental reservoirs will not affect R0. More complex reservoir scenarios are discussed elsewhere (Haydon et al. Reference Haydon, Cleaveland, Taylor and Laurenson2002).
Differentiating primary and secondary reservoirs is notoriously difficult. Direct evidence that a species is a primary reservoir requires the demonstration that the parasite can persist in areas where only that species is infected, or that control methods preventing transmission from that species are effective in interrupting transmission. In the absence of such data, incrimination of reservoirs will depend on demonstrating (1) the prevalence of infection in sympatric hosts, (2) where transmission is seasonal, chronic infection lasting through the non-transmission period and (3) the relative infectiousness of sympatric hosts to the vector. The duration of infection (2) in potential ZVL reservoirs has rarely been assessed. Here we review the evidence for a high prevalence of L. infantum infection and infectiousness in potential reservoir hosts. Where available, such data can be mathematically modeled to quantify the estimated contributions by different reservoirs to parasite maintenance. Although supportive, association studies e.g. of human and reservoir host infection or of human infection and reservoir host density, or molecular studies demonstrating shared parasite strains in the reservoir and humans, do not indicate the source of infection, the direction of transmission, or differentiate reservoir importance. There is also the possibility that peridomestic and sylvatic transmission cycles operate concurrently, involving a different primary reservoir species (e.g. a domestic and a wildlife host respectively), and with a link between the two cycles via a common sandfly vector.
Prevalence of L. infantum infection in potential reservoir hosts
A large number of studies have investigated L. infantum infection in both domestic and wild mammals. Traditionally such studies have used parasitological methods, such as direct examination of tissues or isolation of the parasite using in vitro or in vivo culture. The most complete studies were those of workers in Brazil, such as Deane and Deane, and Alencar, in the state of Ceará, and Lainson and Shaw in the state of Pará (Table 1). More recently, PCR-based methods to detect parasite DNA have provided increased sensitivity and are logistically easier. Serological tests can also be used, though are not generally species-specific, and do not distinguish active from past infection.
Table 1. Leishmania infantum infection in New World wild mammals. The table lists only positive reports, since negative findings are much less likely to be published, and thus only illustrates local rather than global prevalence

1 102 Didelphis albiventris and 10 Didelphis aurita captured. Species-specific (FML) ELISA results presented here.
2 6/12 confirmed as L. infantum.
3 For nomenclature of the foxes examined in this study see Courtenay et al. (1996).
4 Captive animals.
Domestic mammals
While infection in humans and domestic dogs has long been known, infection in other domestic animals has received less attention. Several domestic species have now been shown to be have a high prevalence of infection in some areas, reviewed by Gramiccia and Gradoni (Reference Gramiccia and Gradoni2005). In particular, a number of European studies have shown a high prevalence in domestic cats, for example 26% of 183 cats tested in a Spanish study were PCR positive (Martin-Sanchez et al. Reference Martin-Sanchez, Acedo, Munoz-Perez, Pesson, Marchal and Morillas-Marquez2007). In contrast, earlier Brazilian studies found very few infected cats (Chagas et al. Reference Chagas, Da Cunha, Ferreira, Deane, Deane, Guimaraes, Von Paumgartten and Sa1938; Sherlock, Reference Sherlock1996), though a recent study in Brazil found 2 of 8 asymptomatic cats positive by serology and PCR (da Silva et al. Reference Da Silva, Candido, Pereira, Brazil and Carreira2008). The apparent discrepancy in these results may reflect generally low parasite burdens in cats, which may not have been detectable by parasitological techniques. Infections have also been reported in horses in Europe, with seroprevalences of up to 14% (16/112) in Spain (Fernandez-Bellon et al. Reference Fernandez-Bellon, Solano-Gallego, Bardagi, Alberola, Ramis and Ferrer2006), and in pigs in Brazil (Moraes-Silva et al. Reference Moraes-Silva, Antunes, Rodrigues, Juliao, Dias-Lima, Lemos-de-Sousa, De Alcantara, Reis, Nakatani, Badaro, Reis, Pontes-de-Carvalho and Franke2006). Symptomatic infection has been reported in both cats and horses, though appears to be rare; in contrast, experimental infection of pigs was asymptomatic, with positive serology post-infection but no parasites detectable by culture or PCR (Moraes-Silva et al. Reference Moraes-Silva, Antunes, Rodrigues, Juliao, Dias-Lima, Lemos-de-Sousa, De Alcantara, Reis, Nakatani, Badaro, Reis, Pontes-de-Carvalho and Franke2006).
New World wild mammals
Published reports of L. infantum infection in wild mammals from the New World are detailed in Table 1. The crab-eating fox (Cerdocyon thous) has long been known to have a high prevalence of infection in endemic areas of Brazil, and more recently PCR studies have shown infection in a range of other carnivores, rodents and a bat. Opossums (Didelphis spp.) have a high prevalence of infection in Colombia and parts of Brazil, though L. infantum was not found in hundreds of opossums examined in Amazonian Brazil (Lainson et al. Reference Lainson, Shaw, Silveira and Braga1987). Unlike the situation in dogs, the vast majority of natural infections in wild mammals appear to be asymptomatic (Table 1). Experimental infections in Didelphis marsupialis and Proechimys semispinosus were also largely asymptomatic (Travi et al. Reference Travi, Osorio, Guarin and Cadena1998b, Reference Travi, Arteaga, Leon and Adler2002), though the clinical outcome in experimental infection may depend on the route and dose of infection.
Old World wild mammals
L. infantum infection in the Old World has been reported from a range of carnivores and rodents, with a single case report from a seal (Table 2). Numerous surveys have been reported for foxes and jackals, with infected animals found in many endemic areas. The prevalence of infection by PCR in the red fox (Vulpes vulpes) can be as high as 40–75% in Mediterranean countries (Criado-Fornelio et al. Reference Criado-Fornelio, Gutierrez-Garcia, Rodriguez-Caabeiro, Reus-Garcia, Roldan-Soriano and Diaz-Sanchez2000; Dipineto et al. Reference Dipineto, Manna, Baiano, Gala, Fioretti, Gravino and Menna2007). Reported parasite prevalences in the golden jackal in the Middle East are typically lower, up to 13% in Iran (Hamidi et al. Reference Hamidi, Nadim, Edrissian, Tahvildar-Bidruni and Javadian1982; Mohebali et al. Reference Mohebali, Hajjaran, Hamzavi, Mobedi, Arshi, Zarei, Akhoundi, Naeini, Avizeh and Fakhar2005), though there have been no published PCR studies. Most rodent studies have concentrated on the black rat (Rattus rattus), for which reported parasite prevalence is only 1–2% (Bettini et al. Reference Bettini, Gradoni and Pozio1978, Reference Bettini, Pozio and Gradoni1980; Pozio et al. Reference Pozio, Gradoni, Bettini and Gramiccia1981), but only limited PCR studies have been performed. As in the New World, infections of wild mammals are usually asymptomatic. The low prevalence of symptomatic infection generally amongst wild mammals might be attributed to sampling bias towards healthy individuals. Only one study to our knowledge has ruled out this possibility by performing behavioural studies alongside sampling by live-mark-recapture (Courtenay et al. Reference Courtenay, Macdonald, Lainson, Shaw and Dye1994).
Table 2. Leishmania infantum infection in Old World wild mammals, updated from Ashford (Reference Ashford1996)

Infectiousness of potential reservoirs of L. infantum
There have been few xenodiagnostic studies of hosts other than the domestic dog, and most of these have examined very small numbers of infected animals (Table 3). Theoretical models of ZVL transmission are not sufficiently detailed to provide a cut-off value of infectiousness which would determine whether a host species is a primary reservoir. However, comparative studies allow us to partition transmission, and thus R0, between species. The ability to transmit infection has been confirmed in humans, crab-eating foxes, opossums, black rats and domestic cats, which thus have the potential to act as primary or secondary reservoirs. Notably, no xenodiagnosis results have been published for any Old World wild carnivore.
Table 3. Xenodiagnosis studies of Leishmania infantum in mammalian hosts, other than dogs. The proportion of infectious hosts (number of infectious hosts/total number xenodiagnosed) and the proportion of sandflies infected in feeds on infectious hosts

1 Proportion of flies infected in feeds on infectious hosts only; 2 estimated assuming equal numbers of sandflies dissected per infectious and non-infectious host; 3 HIV-coinfected; 4 experimentally infected; 5 cortisone-treated.
Infectiousness has only been assessed in >10 individuals of two species other than dogs: humans and crab-eating foxes. A proportion of people with symptomatic ZVL are infectious to sandflies (Table 3). However, symptomatic human ZVL cases (excluding HIV-coinfections) are less infectious than symptomatic patients with AVL, or post-kala-azar dermal leishmaniasis (PKDL), considered the important anthroponotic reservoir in the Indian subcontinent (Table 4). Both the proportion of infectious ZVL patients (0·27), and the average proportion of sandflies infected by infectious patients (0·14), are much lower than the respective figures for AVL/PKDL patients (0·88 and 0·26) (Tables 3 and 4). According to the original data sources, the majority of L. donovani xenodiagnosis experiments were conducted on patients selected for high parasitaemia and therefore these values probably represent upper estimates. Nevertheless, they are similar to the average values for dogs (Fig. 1b), the known primary reservoir of ZVL. Asymptomatic people infected with L. infantum have not been shown to be infectious, although parasites can be demonstrated in the skin (Costa et al. Reference Costa, Gomes, Silva, Garcez, Ramos, Santos, Shaw, David and Maguire2000). Given the low incidence of symptomatic human infection, the relative contribution of symptomatic humans to transmission will be much less than that of infected dogs. The higher infectiousness of HIV-coinfected individuals suggests that this situation may change with increasing HIV-coinfection (Molina et al. Reference Molina, Gradoni and Alvar2003).
Table 4. Xenodiagnostic studies of Leishmania donovani in humans with symptomatic kala-azar and PKDL. The proportion of infectious hosts (number of infectious hosts/total number xenodiagnosed) and the proportion of sandflies infected in feeds on infectious hosts

1 Patients reported to be selected on basis of high parasitaemia, in most cases; flies usually exposed twice to subject, once before, once after oviposition.
2 Proportion of flies infected in feeds on infectious hosts only;
3 PKDL patients; all others presenting kala-azar.
4 Assumes that all of the unspecified number of patients exposed to flies were infectious.
Crab-eating foxes have a high prevalence of infection, but none of 21 wild-caught infected foxes from northern Brazil were found to be infectious in 37 serial sandfly feeds (Courtenay et al. Reference Courtenay, Quinnell, Garcez and Dye2002a). Mathematical modeling using the upper 95% confidence limit on infectiousness showed that these foxes contributed <9% of transmission in this setting, and could not maintain infection in the absence of domestic dogs: R0 in foxes was ≪1 (Courtenay et al. Reference Courtenay, Quinnell, Garcez and Dye2002a). This study highlights the fact that a high prevalence of infection cannot be taken as evidence for a role as a reservoir, and demonstrates the importance of assessing infectiousness at the population rather than individual level to confirm a role as a reservoir. Single foxes have been found to be infectious in other studies, suggesting that further population studies would be useful (Table 3).
Other evidence
Proof for a sylvatic reservoir could come from evidence of transmission in the absence of dogs – this has not been demonstrated, and there are few populated areas of the world without dogs, though human cases have been found where dogs are rare (Burney et al. Reference Burney, Wazir and Lari1979). A sylvatic cycle would also be indicated if distinct parasite genotypes were found in dogs and wild mammals; this was suggested by a recent study of dogs and foxes in Spain, though the number of parasites typed was low (Sobrino et al. Reference Sobrino, Ferroglio, Oleaga, Romano, Millan, Revilla, Arnal, Trisciuoglio and Gortazar2008). The increasing availability of markers for strain typing by PCR should allow more extensive studies. The failure of dog culling to reduce the number of human cases in Brazil has been suggested to indicate the existence of other reservoirs, but this failure can be readily explained by logistical and dog demographic factors (see below). Risk factor studies can suggest sylvatic reservoirs, such as the association between human infection and opossums in a Brazilian study (Cabrera et al. Reference Cabrera, Paula, Camacho, Marzochi, Xavier, Da Silva and Jansen2003), but ruling out other confounding factors is essential. Spatial scale is also important for risk factor studies since the vector is mobile: there is often no association between human infection and dog ownership at the household level, whereas such an association is more likely observed at the village level, as demonstrated in Iran (Mazloumi Gavgani et al. Reference Mazloumi Gavgani, Mohite, Edrissian, Mohebali and Davies2002b).
Thus, there remains no clear evidence for any important reservoir of ZVL other than the domestic dog. This does not mean there are no other reservoirs, and the infectiousness of all potential reservoirs requires further study. The evolutionary history of L. infantum implies there must have been a sylvatic cycle in the Old World, since L. infantum diverged from L. donovani around 1 Mya, well before the domestication of dogs around 15 000 years ago. This ancestral reservoir is often assumed to be a wild canid, though rodents have also been suggested (Ashford, Reference Ashford2000). In the New World there may be no primary sylvatic reservoir, as genetic evidence supports a recent introduction of L. infantum to Latin America (Mauricio et al. Reference Mauricio, Stothard and Miles2000). One notable feature of studies of domestic and wild hosts other than the domestic dog is the low proportion of symptomatic infection. Thus only 6 of 75 infected crab-eating foxes, and 2 of 30 infected red foxes, showed any symptoms of ZVL (Table 1; Courtenay et al. Reference Courtenay, Quinnell and Dye2001). The absence of disease is likely to be reflected in a low prevalence of infectiousness following the dog and human models. It has been suggested that one criterion for a good reservoir host is that infection is long-lived but asymptomatic, reflecting evolution towards reduced parasite virulence in a long-established host-parasite association. However, there is no clear link between virulence and persistence (Haydon et al. Reference Haydon, Cleaveland, Taylor and Laurenson2002). Lack of virulence may simply reflect an evolutionarily novel association, where the parasite has not yet evolved mechanisms to ensure transmission, thus causing asymptomatic infection with low parasite burdens. Moreover, both empirical and theoretical studies show that host-pathogen co-evolution does not necessarily lead to low virulence: if transmission depends on virulence, there will be a trade-off between decreasing host survival and increasing the rate of transmission, leading to evolution of intermediate virulence (Anderson and May, Reference Anderson and May1982; May and Anderson, Reference May and Anderson1983). Such a trade-off could exist with visceralizing Leishmania parasites, where increased transmission may depend on widespread dissemination of large numbers of parasites, leading also to increased disease.
EFFICACY OF CURRENT CONTROL METHODS
Control of ZVL has relied on human case detection and treatment, residual insecticide spraying and removal of the source of infection by culling of infected dogs. Treatment of canine infection is not considered to be a useful control method due to the high rate of treatment failure, though mass treatment was associated with a decline in incidence in one observational study (Gradoni et al. Reference Gradoni, Gramiccia, Mancianti and Pieri1988). Where coverage has been high, a combination of dog culling and insecticide spraying has been reported to have been successful, for example in China and some regions of Brazil (Magalhaes et al. Reference Magalhaes, Mayrink, Da Costa, Melo, Dias, Batista, Michalick and Williams1980; Zhi-Biao, Reference Zhi-Biao1989). However, the increasing number of human cases in Brazil, despite culling of large numbers of infected dogs and insecticide spraying, has led to a re-evaluation of control methods (Costa and Vieira, Reference Costa and Vieira2001; Oliveira et al. Reference Oliveira, Morais and Machado-Coelho2008). Detailed analysis of variation in control effort and incidence within the existing Brazilian national control programme may be informative, but the available data have been variably interpreted as showing both some efficacy and minimal efficacy (Vieira and Coelho, Reference Vieira and Coelho1998; Palatnik-de-Sousa et al. Reference Palatnik-de-Sousa, Dos Santos, Franca-Silva, Da Costa, Reis, Palatnik, Mayrink and Genaro2001). Robust conclusions about the relative efficacy of control methods require well-designed intervention trials. The strength of evidence depends critically on the study design, with the gold standard being a cluster-randomized controlled trial, including replication at the community level, random assignment of communities to control or treatment groups, sufficient statistical power to detect a predetermined degree of efficacy, and appropriate analysis taking into account the non-independence of individuals within communities (Kirkwood et al. Reference Kirkwood, Cousens, Victora and De Zoysa1997; Donner and Klar, Reference Donner and Klar2000). Other study designs can also be useful, such as pre- and post-intervention comparisons, particularly where it is not ethical to include communities with no control methods, but replication remains essential (Kirkwood et al. Reference Kirkwood, Cousens, Victora and De Zoysa1997). Unfortunately, many of the existing intervention studies have not been replicated at the community level, which means that secular changes in incidence in control or treatment communities cannot be excluded as explanations for either efficacy or lack of efficacy. Such changes in ZVL incidence through time are commonly observed. Other considerations for an intervention trial are the choice of outcome variable, and the length of the study. From a public health perspective, the most important outcome is the incidence of human disease, though as this is much lower than the incidence of human or canine infection, these intermediate outcomes are more often used. Effects of control measures are expected to increase over a number of years, as there will be a cumulative decrease in transmission (Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b; Reithinger et al. Reference Reithinger, Coleman, Alexander, Vieira, Assis and Davies2004). For logistical reasons, most studies have been relatively short (<2 years).
Dog culling
Despite the large investment in dog culling, very few randomized, controlled studies assessing its efficacy have been performed (Table 5a). Two published studies have directly investigated the effect of dog culling, but neither was replicated at the community level. In the first study, culling 42–73% of seropositive dogs annually for 5 years was associated with a reduction in the incidence of human cases, and a variable reduction in canine incidence (Ashford et al. Reference Ashford, David, Freire, David, Sherlock, Eulalio, Sampaio and Badaro1998). In the second study, culling of all seropositive dogs at 6 month intervals was not associated with reductions in either human or canine incidence (Dietze et al. Reference Dietze, Barros, Teixeira, Harris, Michelson, Falqueto and Corey1997). Temporal changes in incidence may explain the variable outcomes in these studies. More recently, a cluster-randomized controlled trial comparing the effect of dog culling and residual insecticide spraying to that of insecticide alone has been carried out in Piaui, NE Brazil (Costa et al. Reference Costa, Tapety and Werneck2007). The results were variable, suggesting an effect of dog culling when insecticide was applied inside houses, but not when applied inside and outside houses (Table 5a). Combining the data, human seroconversion declined by around 38% in clusters where dogs were killed compared to those where only insecticide was used. Although replicated and randomized, the study was underpowered as the size of clusters used was very small, and the clusters were close together.
Table 5. Randomized controlled trials of intervention measures against ZVL. Efficacy is expressed as the percentage reduction in incidence in the treatment group compared to the control group after intervention

1 Number sampled post-intervention;
2 spraying inside houses;
3 spraying inside houses and outbuildings;
4 combined (incidence estimated assuming equal sample sizes);
5 prevalence not incidence;
6 not randomized;
7 Fisher's exact test;
8 average sample size per cluster.
The available evidence thus does not allow an estimate of the efficacy of dog culling. The only firm conclusions that can be drawn are that dog culling during intervention trials did not reduce transmission to zero, and that dog culling as part of the national control programme has not prevented a rise in the number of human or canine cases in Brazil (Costa and Vieira, Reference Costa and Vieira2001; Maia-Elkhoury et al. Reference Maia-Elkhoury, Alves, Sousa-Gomes, Sena and Luna2008). Theory suggests that dog culling is likely to be less effective than other control methods (Dye, Reference Dye1996). The main reason is that culled dogs are likely to be replaced by uninfected susceptible dogs, which rapidly acquire infection and become infectious. In contrast, a canine vaccine is likely to be more efficacious, as vaccinated dogs are not replaced by susceptibles (Dye, Reference Dye1996). A high replacement rate after culling has been confirmed in field studies, with culled dogs being replaced a mean of 4 months after culling (Nunes et al. Reference Nunes, De Lima, De Paula, Perri, De Andrade, Dias and Burattini2008). Where culling is localized, replacement dogs from surrounding areas may already be infected, further limiting the effects of culling: 15% of replacement dogs in one study were seropositive (Moreira et al. Reference Moreira, De Souza, Sreenivasan, Nascimento and De Carvalho2004). The efficacy of dog culling would be increased if only infectious dogs could be identified and killed, since only a proportion of replacement dogs would become infectious (Fig. 1a). As discussed earlier, currently there are no diagnostic methods that reliably distinguish infectious and non-infectious dogs.
Despite the rapid dog replacement rate, culling has the potential to reduce infection if a high proportion of dogs are culled. Low coverage, i.e. the removal of a low proportion of infectious dogs, is a major reason for the low efficacy of control. Low coverage results from a number of factors: culling dogs only in households near to human cases rather than throughout endemic areas, use of a relatively insensitive method to detect infected dogs (IFAT on filter paper blood eluates), and long delays between dog sampling and culling. Mathematical modeling of current control strategies shows that low coverage due to either low sensitivity or delays between detection and culling is sufficient to greatly reduce the efficacy of dog culling: successful control is predicted to require both a highly sensitive test and no delays (Courtenay et al. Reference Courtenay, Quinnell, Garcez, Shaw and Dye2002b). The efficacy of such an optimized dog control programme was tested in a cluster-randomized control trial in Ceará, Brazil (Braga et al. Reference Braga, Coelho, Pompeu, Evans, Macaullife, Teixeira and Lima1998). There was a 43% reduction in canine prevalence in areas with optimized culling (ELISA, culling within 7 days) compared to areas where culling was performed according to existing Ministry of Health methods (IFAT, culling 80 days after diagnosis) (Table 5a). This study provides the strongest evidence to date for the potential effectiveness of dog culling, though the analysis did not take into account the clustered nature of the data. In contrast, two uncontrolled observational studies of optimized culling produced variable results, with either no decline in canine incidence or a variable decline in human and canine incidence (Moreira et al. Reference Moreira, De Souza, Sreenivasan, Nascimento and De Carvalho2004; Palatnik-de-Sousa et al. Reference Palatnik-de-Sousa, Batista-de-Melo, Borja-Cabrera, Palatnik and Lavor2004). Implementation of such an optimized programme would depend on the availability of a rapid, cheap diagnostic test that could be used in the field. The use of immunochromatographic dipstick tests has been suggested, since these are a highly sensitive test for symptomatic human and canine infection (Da Costa et al. Reference Da Costa, Franca, Mayrink, Nascimento, Genaro and Campos-Neto2003; Chappuis et al. Reference Chappuis, Rijal, Soto, Menten and Boelaert2006). Such tests are currently expensive, and their sensitivity in asymptomatic dogs is lower (Lemos et al. Reference Lemos, Laurenti, Moreira, Reis, Giunchetti, Raychaudhuri and Dietze2008).
Residual insecticide spraying
Sandfly control by residual insecticide spraying has been used as part of national ZVL control campaigns in Brazil since the 1950s, initially using DDT, and more recently using synthetic pyrethroids (Lacerda, Reference Lacerda1994). Theory shows that sandfly control by insecticides can be a highly effective control method, since increasing sandfly mortality has a non-linear effect on disease transmission (Dye, Reference Dye1996). Since insecticide spraying is typically limited to the intra- or peri-domiciliary environment, spraying aims to reduce biting rates within and around houses rather than reduce overall sandfly population numbers, so will be most successful against indoor-biting vectors. The decline in ZVL cases in Italy and AVL cases in India after widespread DDT spraying directed primarily against mosquito vectors of malaria suggests that residual insecticide use can be effective (reviewed by Alexander and Maroli, Reference Alexander and Maroli2003). However, the overall failure of the Brazilian national control programme suggests that sandfly control as much as dog culling has been ineffective in Brazil. Residual spraying reduces the numbers of L. longipalpis within houses (reviewed by Alexander and Maroli, Reference Alexander and Maroli2003), but there have been no published community-based controlled studies of the effect on the incidence of ZVL. Possible reasons for a low efficacy of residual insecticide spraying in Brazil include low coverage, for example only spraying houses within 200 m of a human case, the relatively short-lived residual activity of pyrethroids compared to DDT (Oliveira and Melo, Reference Oliveira and Melo1994), the higher densities of vectors outside houses (e.g. in animal sheds) and vector behaviour, as L. longipalpis is active in the early evening while people are outside houses (Quinnell and Dye, Reference Quinnell and Dye1994; Courtenay et al. Reference Courtenay, Gillingwater, Gomes, Garcez and Davies2007).
Insecticide-treated nets
The potential of insecticide-treated nets (ITNs) to control leishmaniasis has recently been reviewed (Ostyn et al. Reference Ostyn, Vanlerberghe, Picado, Dinesh, Sundar, Chappuis, Rijal, Dujardin, Coosemans, Boelaert and Davies2008). There are no published studies of ITNs as an intervention against ZVL, though some efficacy against AVL in Sudan has been demonstrated (Ritmeijer et al. Reference Ritmeijer, Davies, Van Zorge, Wang, Schorscher, Dongu'du and Davidson2007). For ZVL, ITNs may be useful to provide individual protection for humans, though will not affect transmission between dogs. Only one field study has investigated the entomological efficacy of ITNs against ZVL vectors. ITNs provided a high degree of protection against L. longipalpis in Brazil while people were under the nets, and also reduced biting rates on unprotected hosts in the same room. Despite this, the potential effectiveness of bednets against transmission in the study region was low since sandflies were active in the early evening before children would be protected by bednets (Courtenay et al. Reference Courtenay, Gillingwater, Gomes, Garcez and Davies2007). Further studies in other endemic areas are needed.
Topical insecticides – collars and pour-ons
Recently, much attention has been paid to the potential use of topical insecticide treatment of dogs as a control strategy for ZVL. Deltamethrin-impregnated collars have been shown experimentally to have long-lasting anti-feeding and lethality effects on both L. longipalpis and P. perniciosus, with effects lasting for at least 6 months. Direct topical application of insecticide (pour-ons) has similar but shorter-lived effects, lasting for only around a month (reviewed by Alexander and Maroli, Reference Alexander and Maroli2003). Controlled field trials of the use of collars or monthly application of pour-ons for individual protection of dogs have demonstrated significant protection against infection in Europe (Table 5b). In 3 of 4 studies the level of protection was high, >83% reduction in incidence, as might be expected from experimental studies, though a lower level of protection (51%) was seen in one study of deltamethrin collars (Table 5b). Community-wide use of topical insecticides would be expected to have a greater effect on incidence in protected dogs, and to reduce the incidence in unprotected dogs and humans, since as well as providing individual protection to treated dogs the overall transmission rate should be reduced. The only cluster-randomized controlled trial showed a significant reduction in the incidence of infection in humans and collared dogs 1 year after intervention in 9 pairs of Iranian villages (OR=0·46–0·57) (Mazloumi Gavgani et al. Reference Mazloumi Gavgani, Hodjati, Mohite and Davies2002a) (Table 5a). Other controlled community-level intervention studies have shown a variable efficacy of 32–86%, but did not include replication at the community level (Maroli et al. Reference Maroli, Mizzoni, Siragusa, D'Orazi and Gradoni2001; Giffoni et al. Reference Giffoni, De Almeida, Dos Santos, Ortega and De Barros2002; Reithinger et al. Reference Reithinger, Coleman, Alexander, Vieira, Assis and Davies2004). Loss of collars has proved to be a problem in some studies, with up to 41% of collars lost in a single season in a Brazilian study (Reithinger et al. Reference Reithinger, Coleman, Alexander, Vieira, Assis and Davies2004).
Mathematical modeling predicts that annual application of deltamethrin collars could result in effective control of transmission if coverage is high, even allowing for high collar loss rates (Reithinger et al. Reference Reithinger, Coleman, Alexander, Vieira, Assis and Davies2004). The high cost of collars is an issue for less developed countries. Topical pour-on insecticides generally require monthly application, which is logistically very difficult for a national control programme. Insecticidal baths may have longer-lasting efficacy; in China, deltamethrin baths protected dogs for at least 3·5 months against Phlebotomus chinensis (Xiong et al. Reference Xiong, Jin, Cheng, Su and Hong1994), and have been reported to reduce the number of human cases in an uncontrolled field intervention trial (Xiong et al. Reference Xiong, Jin, Hong, Su, Xue, Xie, Zhang, Li and Gao1995). We have recently tested two deltamethrin formulations for their efficacy against L. longipalpis when used as an insecticidal bath in Brazil. Both formulations protected dogs, with the anti-feeding and sandfly lethality effects of one formulation remaining high (>50%) for 6 months (Courtenay et al. Reference Courtenay, Kovacic, Gomes, Garcez and Quinnell2009). The epidemiological impact of such formulations has yet to be tested, but the relatively long residual efficacy suggests that they may be useful for community control.
CONCLUSIONS
A large number of studies have been carried out on the transmission routes, reservoir hosts and control of ZVL. Whilst the central importance of sandfly transmission from the domestic dog remains clear, a number of other potential transmission routes and reservoir hosts have been identified. This review highlights the lack of studies which produce firm conclusions on the relative importance of these transmission routes and hosts, compared to sandflies and dogs. From an epidemiological and control perspective, there are two important questions: what is the proportion of transmission in an endemic area attributable to each transmission route and reservoir host, and are these proportions high enough to suggest that infection can be maintained in the absence of either sandflies or dogs? There is currently no evidence that non-sandfly transmission can maintain infection, except perhaps for US foxhounds, where dog husbandry is very different from endemic populations. Similarly, there is no strong evidence that reservoir hosts other than the domestic dog are an important source of human infection, though sylvatic reservoirs are likely to exist in the Old World. Further quantitative studies of the prevalence of infectiousness in potential reservoir hosts are needed, and the identification of a reliable marker for infectiousness would be a very useful epidemiological tool.
Control programmes for ZVL have been in place for over 50 years, with varying but limited efficacy. Both operational and theoretical weaknesses have been identified in the current control methods of dog culling and residual insecticide spraying, and their effectiveness has been widely questioned. The current review highlights the need for large, replicated community intervention studies, though we do not underestimate the difficulties, both logistical and financial, in carrying out such trials. In the absence of such evidence, it is not possible to provide clear recommendations for control. The cost of control is another important consideration, and cost-benefit or cost-effectiveness analyses for leishmaniasis are few. Mathematical models have been useful in comparing control methods, for instance the relative efficacies of different culling strategies, and deltamethrin-impregnated collars. However, only limited field data are available to confirm these predictions. Integrated control programmes may use several interventions concurrently, and models have not yet addressed this. Reports of successful ZVL control using current methods suggest that further studies to address the issues of implementation of insecticidal control, using spraying, collars or ITNs, would be very useful whilst awaiting new tools for ZVL control, such as human and canine vaccines (reviewed by Palatnik-de-Sousa, Reference Palatnik-de-Sousa2008).
ACKNOWLEDGEMENTS
We thank the Wellcome Trust for funding of field studies over many years, and our collaborators at the Instituto Evandro Chagas, Brazil and elsewhere. Connor Carson and Richard Reithinger commented on earlier drafts of the manuscript.