Introduction
Toxoplasmosis is a global health issue prevalent both in developed and developing countries. Toxoplasma gondii, the causal agent of toxoplasmosis, is present everywhere and can theoretically infect all warm-blooded vertebrates (Galal et al., Reference Galal, Ajzenberg, Hamidović, Durieux, Dardé and Mercier2018). Since the first description in Ctenodactylus gundi, a species of rodent, in 1908 (Nicolle and Manceaux, Reference Nicolle and Manceaux1908), the parasite has progressively been reported in a wide range of animals, making it one of the most wide spread zoonotic diseases (Robert-Gangneux and Dardé, Reference Robert-Gangneux and Dardé2012). In the 1960s, the sexual reproductive phase of the parasite was described in cat (Hutchison et al., Reference Hutchison, Dunachie, Siim and Work1969; Dubey and Frenkel, Reference Dubey and Frenkel1972) and since then the cat has been known to play a central role in the transmission cycle of the parasite (Robert-Gangneux and Dardé, Reference Robert-Gangneux and Dardé2012). Toxoplasmosis has no restriction for boundary or race as significance of the disease has been reported in many countries of the world including the United States (11%), UK (9%), Singapore (17%), Chile (39%), China (11%), Brazil (50%), Nepal (55%) and Nigeria (78%) (Flegr et al., Reference Flegr, Prandota, Sovičková and Israili2014). Africa especially has become a focal point of transmission of T. gondii due to prevailing environmental factors and poor socioeconomic development.
While toxoplasmosis is a disease of all population strata, pregnant women and their newborns suffer most from the consequences of the disease (Bachmeyer et al., Reference Bachmeyer, Mouchnino, Thulliez and Blum2006; Andrade et al., Reference Andrade, Vasconcelos-Santos, Carellos, Romanelli, Vitor, Carneiro and Januario2009). The parasite T. gondii is haematogenously conveyed into the placenta to induce congenital infection in the growing foetus (Ander et al., Reference Ander, Rudzki, Arora, Sadovsky, Coyne and Boyle2018). This infection can result in abortion or cause permanent disabilities or defects in surviving children (Millar et al., Reference Millar, de Moura, Bastos, de Mattos, Fonseca, Sudré, Leles and Amendoeira2014). In several cases, the outcomes of in utero infections by T. gondii include ocular disease and developmental delays (Olariu et al., Reference Olariu, Remington, McLeod, Alam and Montoya2011).
This review reports the various epidemiological studies on maternal toxoplasmosis in Nigeria. Of importance is the disease contribution to maternal and fetal health and advocacy for inclusion of toxoplasmosis among prioritized diseases for routine screening during antenatal care in Nigeria and other sub-Saharan African countries.
The transmission cycle of Toxoplasma gondii: maternal-fetal route perspective
Hosts and vehicles
The life cycle of T. gondii, an obligate intracellular protozoan, is complex and involves essentially a feline definitive host e.g. domestic cats which harbour the sexual stage of the parasite, and numerous warm-blooded vertebrates as intermediate hosts for proliferation of asexual reproductive forms of the parasitic organism (Rouatbi et al., Reference Rouatbi, Amairia, Amdouni, Boussaadoun, Ayadi, Al-Hosary, Rekik, Ben Abdallah, Aoun, Darghouth, Wieland and Gharbi2019). Humans can become infected through ingestion of the parasite oocysts shed by felid definitive hosts, consumption of raw or undercooked meat containing tissue bradyzoites or vertically transmitted from infected pregnant woman across the placenta to the growing foetus (Tenter et al., Reference Tenter, Heckeroth and Weiss2000; Mahmoud et al., Reference Mahmoud, Saedi Dezaki, Soleimani, Baneshi, Kheirandish, Ezatpour and Zia-Ali2015).
Roles of placenta
In eutherian vertebrates, the primary function of the placenta is for gaseous, nutrient and waste exchange between maternal and fetal compartments. In addition, it restricts exposure of the foetus to infectious agents (Ander et al., Reference Ander, Rudzki, Arora, Sadovsky, Coyne and Boyle2018). The specialized trophoblasts, syncytiotrophoblast and cytotrophoblast, which form the components of the placenta are known to form a primary barrier to the passage of pathogens that may infect the foetus by the haematogenous route (Ander et al., Reference Ander, Rudzki, Arora, Sadovsky, Coyne and Boyle2018). Although these trophoblast layers play an important role in placental pathogen transmission restriction, the pathways of this restriction are still not well understood. The strategic location of trophoblasts which favours its close interaction with maternal and fetal blood has, however, inextricably favoured the parasite transmission to the foetus (Robbins et al., Reference Robbins, Zeldovich, Poukchanski, Boothroyd and Bakardjiev2012). The risk of transmission to the foetus may increase in pregnant women with the first experience of T. gondii infection (Tenter et al., Reference Tenter, Heckeroth and Weiss2000). The transplacental invasion of tachyzoites and eventual penetration of fetal tissues or bloodstream may result in congenital infection (Pardini et al., Reference Pardini, Bernstein, Carral, Kaufer, Dellarupe and Gos2019). Common outcomes of congenital toxoplasmosis include fetal or neonatal death, defects mainly in the ocular region and neuromuscular system (Hayde and Pollak, Reference Hayde and Pollak2000). In immunocompromised individuals, the common clinical signs are pneumonia, encephalitis and ophthalmologic disorders (Tenter et al., Reference Tenter, Heckeroth and Weiss2000).
Epidemiology of toxoplasmosis during pregnancy
Sub-Saharan African countries perspective
Generally, more than one-third of the human population with T. gondii infection present an asymptomatic situation because of the roles played by the immune system (Flegr et al., Reference Flegr, Prandota, Sovičková and Israili2014). Toxoplasmosis could be latent when there is no overt clinical presentation of the disease but chronic infection oftentimes results in clinical symptoms (Flegr et al., Reference Flegr, Prandota, Sovičková and Israili2014). Several factors which include environmental and socioeconomic status are known to influence the seroprevalence of T. gondii which could vary from 4 to 80% in women (Lelong et al., Reference Lelong, Rahelimino, Candolfi, Ravelojaona, Villard and Kien1995; Dubey et al., Reference Dubey, Tiao, Gebreyes and Jones2012; Lim et al., Reference Lim, Lee, Jung, Kim, Lee, Nam, Shin, Yun, Cho, Shin and Chai2012).
According to Uttah et al. (Reference Uttah, Ogban and Okonofua2013), ‘toxoplasmosis is an epidemiological paradox; it is one of the most prevalent and most widespread parasitic infections, yet one of the most ignored of all human infections’. Lindstrom et al. (Reference Lindstrom, Kaddu-Mulindwa, Kironde and Lindh2006) had earlier noted that toxoplasmosis often remains undetected and untreated due to insufficient diagnostic procedures in sub-Saharan African countries. It has also been observed that toxoplasmosis is not routinely screened in pregnant women in most countries in sub-Saharan Africa (Linguissi et al., Reference Linguissi, Nagalo, Bisseye, Kagoné, Sanoud, Tao, Benao, Simporé and Koné2012). Epidemiological studies available in different sub-Saharan African countries, however, suggest that seroprevalence of T. gondii infection in pregnant women varies greatly (Table 1).
Table 1. Seroprevalence of toxoplasmosis in pregnant women in sub-Saharan Africa

Note: Seroprevalence is determined as a measure of chronic infection i.e. total serum anti-Toxoplasma IgG.
The seroprevalence of T. gondii among pregnant women in sub-Saharan Africa ranges from 5.9 to 85.4%. A considerable variation in the incidence of T. gondii among pregnant women in sub-Saharan Africa may be ascribed to food (consumption of raw or undercooked meats or contaminated water) and environmental sources (exposure to soil or cat litter) (Elmore et al., Reference Elmore, Jones, Conrad, Patton, Lindsay and Dubey2010; Walle et al., Reference Walle, Kebede, Tsegaye and Kassa2013).
Unlike malaria and other infectious diseases, studies on maternal toxoplasmosis are relatively scarce in sub-Saharan African countries. The research neglect on this parasitic disease is detrimental considering its burden on pregnant women and their growing foetuses. All the regions of sub-Saharan Africa are at risk with the Central African countries showing the highest seroprevalence of maternal toxoplasmosis (Fig. 1). Data on Southern African countries are limited; therefore the seroprevalence presented in this review may significantly deviate from the true infection status in the region. The higher occurrence of toxoplasmosis in the Central and East Africa countries may be due to their geographical locations. Many of the countries in these regions are located along the equator and therefore receive maximum rainfall and sunshine. An increase in temperature and rainfall has been linked to an increase in transmission of T. gondii among pregnant women. A positive correlation has been reported between the prevalence of toxoplasmosis in pregnant women and the average annual temperature in the corresponding area (Ljungström et al., Reference Ljungström, Gille, Nokes, Linder and Forsgren1995). Terrestrial animals such as rodents thrive in warmer temperature and an increase in their population makes them prone to predation by cats, dogs and pigs (Jiang et al., Reference Jiang, Sullivan, Su and Zhao2012). Thus, higher temperatures may afford these hosts the opportunity to increase the transmission dynamics of T. gondii and extend its range of distribution (Gubler et al., Reference Gubler, Reiter, Ebi, Yap, Nasci and Patz2001). Rain, on the other hand, helps to create a moist environment for oocysts' survival and increase food supply to the rodent hosts (Gubler et al., Reference Gubler, Reiter, Ebi, Yap, Nasci and Patz2001; Afonso et al., Reference Afonso, Germain, Poulle, Ruette, Devillard, Say, Aubert and Gilot-Fromont2013). Increased incidence of T. gondii infection in felids has also been linked to increasing rainfall (Afonso et al., Reference Afonso, Thulliez and Gilot-Fromont2010).

Fig. 1. Seroprevalence of maternal toxoplasmosis by sub-Saharan African regions. Note: Data computed from all available literature from 1980 to date.
Ethiopia recorded the highest seroprevalence (85.5%) of maternal toxoplasmosis in the sub-Saharan Africa region (Gelaye et al., Reference Gelaye, Kebede and Hailu2015) and no study elsewhere in the world has recorded such high prevalence in the recent times among pregnant women. It is difficult to monitor transmission patterns or dynamics in pregnant women in sub-Saharan Africa because some countries experienced relatively stable transmission patterns while others showed a gradual increase or decrease from the 1990s to 2000s. In other instances, the transmission was rather sporadic. In Republic of Benin for example, a gradual decrease from 53.6 to 48.7% was recorded (Rodier et al., Reference Rodier, Berthonneau, Bourgoin, Giraudeau, Agius, Burucoa, Hekpazo and Jacquemin1995; Ogouyèmi-Hounto et al., Reference Ogouyèmi-Hounto, Agbayahoun-Chokki, Sissinto Savi de Tove, Biokou, Adinsi de Souza, Assogba, Kinde-Gazard and Massougbodji2014) while the reverse situation was recorded in Burkina Faso i.e. an increase from 25.3% in 2006 to 31.1% in 2017 (Simpore et al., Reference Simpore, Savadogo, Ilboudo, Nadambega, Esposito, Yara, Pignatelli, Pietra and Musumeci2006; Bamba et al., Reference Bamba, Cissé, Sangaré, Zida, Ouattara and Guiguemdé2017). In Cameroon, transmission dynamics among pregnant women showed no particular pattern while in Central Republic of Africa and Nigeria (after the 78% seroprevalence report by Onadeko et al., Reference Onadeko, Joynson and Payne1992), transmission appeared to be stable (Morvan et al., Reference Morvan, Mambely, Selekon and Coumanzi-Malo1999; Akinbami et al., Reference Akinbami, Adewunmi, Rabiu, Wright, Dosunmu, Dada and Adeyemo2010; Gamba et al., Reference Gamba, Nambei and Kamandji2013; Nasir et al., Reference Nasir, Aderinsayo, Mele and Aliyu2015; Oboro et al., Reference Oboro, Obunge and Wariso2016). The transmission trend observed in these countries is attributed to the peculiar features of each study area, behavioural attitudes and exposure to risk factors of transmission of T. gondii. Some countries such as Somalia and Togo where there are no records of maternal toxoplasmosis were found, however, to have recorded seroprevalence of toxoplasmosis in other population groups (Ahmed et al., Reference Ahmed, Mohammed, Yusuf, Ahmed and Huldt1988; Tété-Bénissan et al., Reference Tété-Bénissan, Doctorant, Banla, Balogou, Aklikokou and Gbeassor2018), thus suggesting a risk of transmission of T. gondii to pregnant women.
Toxoplasmosis in pregnancy: the Nigerian picture
It is certain that toxoplasmosis research and management priority is given relatively little attention compared to other parasitic diseases such as malaria. The little attention it has received is as a result of the risk toxoplasmosis poses in congenital infection. In Nigeria, the situation is worse as very few studies have addressed toxoplasmosis during pregnancy. Despite this obvious neglect, the Nigerian current situation on toxoplasmosis during pregnancy seems better than that of parasitic diseases such as schistosomiasis (Salawu and Odaibo, Reference Salawu and Odaibo2013, Reference Salawu and Odaibo2014). This is understandable as the status of the latter in congenital infection is yet to be fully ascertained. The diagnostic problem associated with toxoplasmosis could be responsible for the relative lack of data in Nigeria. Most of the studies employed the use of ELISA serological assay to determine anti-T. gondii immunoglobulin G (IgG) which involves some technicality and power supply.
There are currently 17 reported studies on maternal toxoplasmosis in five geopolitical zones of Nigeria. From the first report in 1992 (Onadeko et al., Reference Onadeko, Joynson and Payne1992) (Table 2), five other studies have been reported in the southwestern part of Nigeria. The south-south and the north-central regions have only reported two studies each on toxoplasmosis during pregnancy while three and four studies have been linked to the north-east and north-west regions of Nigeria respectively. Currently, there is no report on toxoplasmosis during pregnancy in the southeastern part of Nigeria. This is, however, not appropriate considering the demand for meat and probable environmental conditions of the region which could favour transmission of the causal agent of the disease.
Table 2. Seroprevalence studies on maternal toxoplasmosis in Nigeria

Generally, the seroprevalence of maternal toxoplasmosis is higher in the southern than in the northern part of Nigeria (Fig. 2). This discrepancy in occurrence may be due to the differences in the meat consumption level in the regions. The southerners are known to consume more meat than the northerners and they also relish some meat types such as pork and chicken which are natural hosts of T. gondii thus increasing their exposure to T. gondii. Another probable explanation is the environmental conditions that favour the development of unsporulated oocysts of the parasite. The extreme temperature and decrease relative humidity of the north (Eludoyin et al., Reference Eludoyin, Adelekan, Webster and Eludoyin2014) can make the unsporulated oocysts of T. gondii to be less environmentally resistant (Meerburg and Kijlstra, Reference Meerburg and Kijlstra2009) thereby decreasing its infectivity. The seroprevalence of T. gondii in Northern Nigeria is, however, high enough to merit adequate intervention in the region. The significant occurrence of toxoplasmosis in the region has been attributed to the people's love for pets, especially cats which are the definitive host of the parasite (Nasir et al., Reference Nasir, Aderinsayo, Mele and Aliyu2015). A significant relationship between the transmission of toxoplasmosis and contact with domestic cats has been widely reported (Lin et al., Reference Lin, Liao, Liao, Chen, Kuo and He2008; Zemene et al., Reference Zemene, Yewhalaw, Abera, Belay, Samuel and Zeynudin2012).

Fig. 2. Mean seroprevalence of toxoplasmosis during pregnancy in geopolitical zones of Nigeria. Note: SW, south-west; SS, south-south; NC, north-central; NE, north-east; NW, north-west.
There has been a decline in the incidence and prevalence of maternal toxoplasmosis in Nigeria since the early 1990s (Fig. 3). However, this decline is not per se as a result of a good alert system or management policy in pregnant women. It is more likely to be associated with the peculiarity of the locations and the study population.
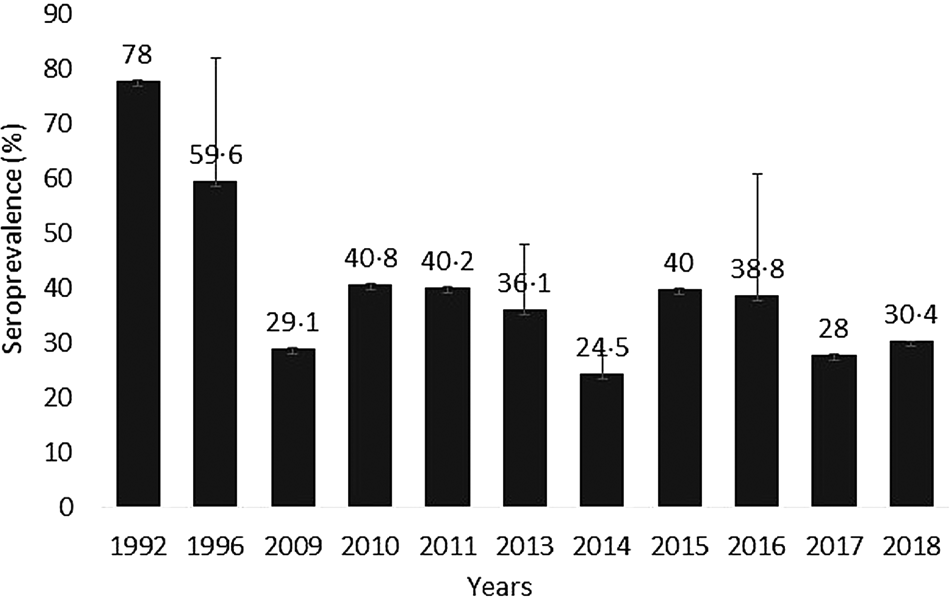
Fig. 3. Incidence of T. gondii maternal infection in Nigeria (1992–2018).
Congenital toxoplasmosis and adverse pregnancy outcomes
The general overview
Congenital toxoplasmosis, with the global annual incidence of 190 100 cases (Torgerson and Mastroiacovo, Reference Torgerson and Mastroiacovo2013), is a major health problem resulting in severe burdens for those affected from foetus to adulthood (Carlier et al., Reference Carlier, Truyens, Deloron and Peyron2012). Maternal transmission is a rare occurrence if T. gondii infection occurs before pregnancy (Silveira et al., Reference Silveira, Ferreira, Muccioli, Nussenblatt and Belfort2003) but the risk becomes increased in immunocompromised women (Montoya and Remington, Reference Montoya and Remington2008). It is important to state that not all acute maternal infection will result in congenital disease (Carlier et al., Reference Carlier, Truyens, Deloron and Peyron2012). Other factors besides the mother's immunity that influence the likelihood of vertical transmission from mothers to their foetuses include the genotype of the mother, gestational age at the time of infection, the genetic make-up of the parasite and its virulence (Carlier et al., Reference Carlier, Truyens, Deloron and Peyron2012; Halonen and Weiss, Reference Halonen and Weiss2013).
Congenital infection due to vertical transmission of toxoplasmosis is generally lower in the early gestational age i.e. first trimester (10–15%) compared to that found in the third trimester (60–90%). However, a more severe disease occurs during infection in the first trimester (Hernández-Cortazar et al., Reference Hernández-Cortazar, Acosta-Viana, Ortega-Pacheco and Guzman-Marin2015). The adverse pregnancy outcomes associated with T. gondii infection during the first trimester include abortion, stillbirth and premature birth (Chaudhry et al., Reference Chaudhry, Gad and Koren2014). If the pregnancy is successfully maintained, neonatal deformation as a result of infection may cause a wide range of clinical morbidities such as blindness, heart and brain defects, neurological damage, chorioretinitis, mental retardation and death (Chaudhry et al., Reference Chaudhry, Gad and Koren2014). Abortion is usually a rare occurrence if maternal infection occurs in the third trimester. Congenital infection in newborns infected in the last trimester usually goes unnoticed at birth but chorioretinitis develops in the child later in life (Carlier et al., Reference Carlier, Truyens, Deloron and Peyron2012).
As earlier mentioned, pre-pregnancy exposure to T. gondii poses little or no risk to the unborn child even in the case of re-infection during pregnancy, if the mother is infected with the same strain. However, few studies have reported possibilities of reactivation of the disease during pregnancy, sometimes from different and more strains, which could result in adverse pregnancy outcomes (Garweg et al., Reference Garweg, Scherrer, Wallon, Kodjikian and Peyron2005; Carlier et al., Reference Carlier, Truyens, Deloron and Peyron2012). This reactivation is often known to be initiated by the cystic form of the parasite (Borges et al., Reference Borges, Silva, Brito, Teixeira and Roberts2019). In summary, the majority of available evidence suggests that previous maternal infection confers protection against vertical transmission of T. gondii but alteration in systemic immunity as a result of hormonal changes in some pregnancies can lead to toxoplasmosis activation which can result in transplacental transmission of the T. gondii to the growing foetus (Opsteegh et al., Reference Opsteegh, Kortbeek, Havelaar and van der Giessen2015).
Congenital toxoplasmosis in Nigeria and adverse outcomes
Despite the growing body of evidence on potential adverse pregnancy outcomes associated with T. gondii maternal infection, available data on the subject are extremely scanty in Nigeria. A case report correlated an occurrence of cyst of T. gondii in the brain of a 17-month old child (found by using computed tomography scan) with Toxoplasma IgG (Amadi et al., Reference Amadi, Ndu, Chinawa, Jean and Obidi2015). The mother's medical history during pregnancy revealed premature birth and the visible signs of toxoplasmosis in the child such as microcephaly and retarded growth indicated that the child might have acquired a congenital infection. A retrospective epidemiological study in the northern part of Nigeria also identified abortion (41.6%), stillbirth (61.5%) and neonatal death (62.5%) as common adverse events associated with maternal T. gondii infection (Alayande et al., Reference Alayande, Edungbola, Fabiyi and Awosan2013). Oboro et al. (Reference Oboro, Obunge and Wariso2016) reported 25% previous stillbirth or miscarriage occurrence in pregnant women positive to anti-Toxoplasma specific antibodies in Port Harcourt in the south-south region of Nigeria. In Lagos, a southwestern city, 23.8, 15.4 and 29.4% were reported for miscarriage, stillbirth and ocular problem in pregnant women who were seropositive to T. gondii antibodies (Deji-Agboola et al., Reference Deji-Agboola, Busari, Osinupebi and Amoo2011) respectively. In Gombe, another northern state, spontaneous abortion was as high as 60% in T. gondii-infected pregnant women while stillbirth and placental retentions were 6.8 and 10.4%, respectively (Ballah et al., Reference Ballah, Maikai, Magaji, Shuaibu, El-Nafaty, Sambo, Auwal, Faruk and Suleiman2017). These studies suggest that although seroprevalence is higher in the south than in the north, adverse events associated with maternal infection seem to be more serious in the north than in the south. There might therefore be other factors that could be responsible for increased adverse events in the north in addition to maternal T. gondii infection during pregnancy. The influence of other reproductive health associated pathogens, low level of education and poor medical practices are suggested.
Toxoplasma gondii transmission and immune interplay during pregnancy
Trophoblast cells are the main barriers for preventing T. gondii infection of foetus during pregnancy. However, the mechanisms that underline successful fetal infection despite these effective barriers are still not fully understood. It has been hypothesized that the switch from T helper (Th)-1 which is the normal immune response pathway associated with Toxoplasma infection to the (Th)-2 pathway as a result of the richness of placental microenvironment in interleukin 10 (IL-10) could promote infection of placental tissue (Barbosa et al., Reference Barbosa, Silva, Costa, Mineo and Ferro2008). The role of interferon-γ (IFN-γ), which is the major cytokine for (Th)-1 immune response in T. gondii infection-associated immune effectors during pregnancy in congenital toxoplasmosis, has also been described (Pfaff et al., Reference Pfaff, Abou-Bacar, Letscher-Bru, Villard, Senegas, Mousli and Candolfi2007). For example in an experimental mouse model, IFN-γ production in response to T. gondii infection-induced abortion in pregnant mice but not in pregnant mice where IFN-γ producing gene was knocked out (Shiono et al., Reference Shiono, Mun, He, Nakazaki, Fang, Furuya, Aosai and Yano2007). Further to this, an in vitro study showed that IFN-γ upregulates the expression of certain molecule known as intercellular adhesion molecule (ICAM)-1 adhesin that lines the surface of trophoblasts, thus, enhancing the adhesion of infected monocytes (Pfaff et al., Reference Pfaff, Abou-Bacar, Letscher-Bru, Villard, Senegas, Mousli and Candolfi2007). Placental inflammation leads to overexpression of ICAM-1 (Juliano et al., Reference Juliano, Blotta and Altemani2006) and eventual transepithelial transportation of the parasites (Barragan et al., Reference Barragan, Brossier and Sibley2005).
The unique ability of placental cells to select human leucocyte antigen-G which may modulate the local maternal immune response to favour infection tolerance could compensate for the deleterious effect that T. gondii infection may induce during pregnancy (Hunt et al., Reference Hunt, Petroff, McIntire and Ober2005).
Diagnosis and treatment hurdles
Diagnostic options
Generally, early detection of infection during pregnancy and administration of appropriate treatment has been shown both to reduce risk of transplacental transmission of T. gondii to the foetus and to mitigate eventual sequelae after the intrauterine infection has already been established (Stray-Pedersen, Reference Stray-Pedersen1992). It has always been challenging to diagnose congenital toxoplasmosis accurately because a combination of skills in epidemiological, clinical, laboratory and imaging analyses is required (Soares and Caldeira, Reference Soares and Caldeira2019). Because toxoplasmosis is not a prioritized disease in sub-Saharan African countries including Nigeria, health care providers are yet to define a systematic approach and procedures for administering proper diagnosis of the disease. In Nigeria and other places in the world, the first line of diagnosis is the detection of anti-Toxoplasma specific antibodies IgG and IgM which are serological markers of infection. Differentiating acute from chronic infection is one of the greatest challenges in the diagnosis of toxoplasmosis (Chaudhry et al., Reference Chaudhry, Gad and Koren2014). Although anti-Toxoplasma specific IgM antibodies clear out faster in the circulation than the IgG antibodies, they may remain detectable for years thus posing a difficulty in the diagnosis of congenital infection (Stray-Pedersen, Reference Stray-Pedersen1993). The absence of these two antibodies before or in the first trimester of pregnancy depicts no previous infection and this is very useful in identifying women at risk of maternal infection during pregnancy (Hedman et al., Reference Hedman, Lappalainen, Seppäiä and Mäkelä1989). The presence of only anti-Toxoplasma specific IgG antibodies but without IgM antibodies signifies that a chronic infection is diagnosed (Chaudhry et al., Reference Chaudhry, Gad and Koren2014). Interpretation of results, however, becomes difficult when serum tests positive for the two antibodies as the results might be due to either a low IgM titre from a previous infection or a recent infection (Jenum et al., Reference Jenum, Stray-Pedersen, Melby, Kapperud, Whitelaw, Eskild and Enj1998).
Recently, attention has been paid to development of point-of-care (POC) tests to detect Toxoplasma infection. This testing method offers the advantage of being able to detect both Toxoplasma-specific IgG and IgM simultaneously. Lateral flow immunochromatography-based Toxoplasma ICT IgG–IgM test has been employed to diagnose T. gondii infection in both sera and whole blood samples with sensitivity and specificity ranging from 96 to 100% (Begeman et al., Reference Begeman, Lykins, Zhou, Lai, Levigne, El Bissati, Boyer, Withers, Clouser, Noble, Rabiah, Swisher, Heydemann, Contopoulos-Ioannidis, Montoya, Maldonado, Ramirez, Press, Stillwaggon, Peyron and McLeod2017; Chapey et al., Reference Chapey, Wallon and Peyron2017; Lykins et al., Reference Lykins, Li, Levigne, Zhou, El Bissati and Clouser2018). The use of whole blood for POC diagnosis has especially been advocated in low-resource settings as it is less invasive and requires no sophisticated tools and electricity. POC testing can expand access to prenatal toxoplasmosis screening, and facilitate the screening of other congenital infections. This will improve the overall maternal and fetal health (Lykins et al., Reference Lykins, Li, Levigne, Zhou, El Bissati and Clouser2018).
Serologic methods are not reliable for the diagnosis of toxoplasmosis in HIV/AIDS or immunosuppressed patients due to a decline in specific antibodies production. Thus, for toxoplasmosis-suspected immunocompromised patients, it is recommended that other diagnostic methods be used to confirm the true infection status. These methods can include the use of polymerase chain reaction (PCR) for amplification of the parasite DNA, parasite isolation from blood or body fluids, and histologic examination of available tissues with T. gondii-specific stains (Montoya, Reference Montoya2002). PCR has been reported to have a sensitivity and specificity of about 100% when used to confirm the detection of infection in amniotic fluid samples (Hohfeld et al., Reference Hohfeld, Daffos, Costa, Thulliez, Forestier and Vidaud1994).
Treatment and associated hurdles
In previous decades, little was known about the effectiveness of available treatment options for congenital toxoplasmosis. This deficiency was partly due to the fact that maternal infection could only be identified by screening and studies on treatment efficacy could only be conducted in places where antenatal screening for toxoplasmosis is a routine practice (Gilbert, Reference Gilbert2009). Also, the treatment efficacy of chemotherapy was linked to T. gondii short ‘therapeutic window' when treatment can be effective on tachyzoites. According to Gilbert (Reference Gilbert2009), ‘the window is limited by the duration of maternal parasitaemia, which probably ceases with the development of the maternal serological response'. Once in circulation, the therapeutic window of the parasite depends on how efficient the immune response of the foetus is able to potentiate the formation of cystic dormant bradyzoite which is resistant to antibiotics (Denkers and Gazzinelli, Reference Denkers and Gazzinelli1998). The maturation of fetal immunity is expected to be correlated with a shorter ‘window’ of tachyzoite replication and conversion to bradyzoite (Jamieson et al., Reference Jamieson, de Roubaix, Cortina-Borja, Tan, Mui and Cordell2008). The implication of these in treatment is that early administration of chemotherapy after seroconversion might undermine treatment effectiveness (The SYROCOT Study Group, Reference Thiebaut, Leproust, Chene and Gilbert2007). Although seroconversion in T. gondii plays an important role in treatment outcome depending on when it is administered, treatment failure could also be due to the invading parasite strain whether resistant or susceptible to drug treatment.
The correlation of window of therapy with lower treatment success by Gilbert (Reference Gilbert2009) has been largely underplayed giving more recent clinical evidence in different parts of the world. Several observational studies have confirmed that early maternal screening and treatment demonstrated significant impact in reducing T. gondii vertical transmission and prevented subsequent morbidities in babies and growing children (Li et al., Reference Li, Wei, Zhang, Peng and Lindsay2014). A significant reduction in maternal infection from 11 to 4% after amniotic fluid testing by PCR and subsequent treatment in France (Wallon et al., Reference Wallon, Peyron, Cornu, Vinault, Abrahamowicz, Kopp and Binquet2013), and a 6-fold lower risk of vertical transmission in Austria after antepartum treatment (Prusa et al., Reference Prusa, Kasper, Pollak, Gleiss, Waldhoer and Hayde2015) are important evidence to correlate prenatal treatment with lower risk of congenital infection. Unlike some developed countries that provide universal screening of pregnant women for congenital toxoplasmosis, such screening is uncommon in health service providers' centres in Nigeria.
It is important to note that only one clinical controlled drug trial study has been conducted so far for congenital toxoplasmosis. The study showed no significant difference in perinatal outcomes in spiramycin vs pyrimethamine + sulphadiazine groups (Mandelbrot et al., Reference Mandelbrot, Kieffer, Sitta, Laurichesse-Delmas, Winer, Mesnard, Berrebi, Le Bouar, Bory, Cordier, Ville, Perrotin, Jouannic, Biquard, d'Ercole, Houfflin-Debarge, Villena and Thiébaut2018). The lack of power due to premature termination of study before reaching the expected sample size was the major limitation of the study. The reason there have been no trials (until the recent well-structured trial) is that the lack of equipoise would render most trials unethical. Presently, treatment recommendations from experienced experts are advocated. A combination of pyrimethamine, sulphadiazine and folinic acid is the recommended treatment regimen for pregnant women who are infected with T. gondii after 18 weeks of pregnancy. The same treatment is recommended for those with already established fetal infection confirmed by amniotic fluid PCR positive result or ultrasound result revealing consistent fetal deformities characteristics of congenital toxoplasmosis (Remington et al., Reference Remington, McLeod, Thuilliez, Desmonts, Remington, Klein, Wilson and Baker2006). The basis for this recommended treatment strategy is particularly to eliminate fetal infection and prevent transmission in women especially in low resources countries where the diagnosis of amniotic fluid by PCR may not be feasible.
Conclusion
Considering the prevailing risk factors of toxoplasmosis in Nigeria, and the high prevalence level reported in many regions, it is evident that pregnant women are equally predisposed to the disease. However, the available reports in Nigeria on pregnant women showed that this disease is neglected. This is unsafe considering the deleterious impact of maternal toxoplasmosis on growing foetus. It is therefore important to gather more evidence on the prevalence of maternal toxoplasmosis in Nigeria, especially with the limited scopes of most of the currently available literature. Better designed baseline data of the disease occurrence in human, animals, food, soil and water are strongly recommended for an informed decision on control strategies.
Rational control of the disease requires a prompt and effective diagnosis, and early treatment during pregnancy. Prenatal treatment is likely to have a greater impact on the disease burden and its clinical manifestations than treatment after birth. A diagnostic option that will evade the common challenges in low resource countries such as Nigeria is recommended. Therefore, a POC diagnostic tool that will not require electricity and easy to perform can help for quick detection of T. gondii during pregnancy.
It is also important to state that owing to the significant burden of the disease on women and their foetuses, a comprehensive data-based information on maternal toxoplasmosis will facilitate the formation of a control plan that will subsequently be integrated into the public health policies of Nigeria.








