Introduction
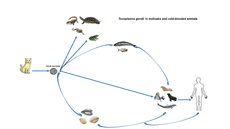
Toxoplasma gondii (T. gondii) is an obligate, intracellular, zoonotic, protozoan parasite that infects nearly all warm-blooded animals including humans, livestock, and some species of marine mammals as intermediate hosts and it is estimated that about 30% of the human population is affected by this parasite. In intermediate hosts, T. gondii multiplies by asexual reproduction leading to intracellular cysts in muscles and other organs. However, in felids as final hosts, sexual reproduction leads to discharge oocysts in their feces (Tenter et al., Reference Tenter, Heckeroth and Weiss2000; Dubey et al., Reference Dubey, Sundar, Hill, Velmurugan, Bandini, Kwok, Majumdar and Su2008; Taghadosi et al., Reference Taghadosi, Kojouri and Taheri2010; Aguirre et al., Reference Aguirre, Longcore, Barbieri, Dabritz, Hill, Klein, Lepczyk, Lilly, McLeod and Milcarsky2019). In immunocompetent humans, T. gondii infection is usually asymptomatic but may result in influenza-like symptoms. Although T. gondii can be serious, particularly for pregnant women, immunosuppressed individuals, or congenitally infected offspring (Tenter et al., Reference Tenter, Heckeroth and Weiss2000). Among immunocompromised individuals, T. gondii infection is common in late acquired immunodeficiency syndrome (AIDS), in which 25% of patients develop toxoplasmic encephalitis (Jones et al., Reference Jones, Kruszon-Moran, Wilson, McQuillan, Navin and McAuley2001; Aspinall et al., Reference Aspinall, Marlee, Hyde and Sims2002). If T. gondii infection occurs during pregnancy, it can cause miscarriage or congenital anomalies that affect the brain, eyes, or other parts of the fetus (Kim et al., Reference Kim, Kang, Kang, Sohn, Jean, Park, Kim and Kim2009). Disseminated toxoplasmosis and mental disorders can occur years after exposure to the parasite, and eye diseases have significant lifelong effects on a person's quality of life (Havelaar et al., Reference Havelaar, Haagsma, Mangen, Kemmeren, Verhoef, Vijgen, Wilson, Friesema, Kortbeek and van Duynhoven2012). The main routes of transmission of the infection are the contact with soil contaminated with T. gondii oocysts excreted from infected felids, ingesting meat containing encysted bradyzoites, consumption of water and vegetables containing sporulated oocysts, passing the parasite from the mother to the fetus through the placenta, blood transfusions, and organ transplants (Dubey and Beattie, Reference Dubey and Beattie1988; Taghadosi et al., Reference Taghadosi, Kojouri and Taheri2010; Koutsoumanis et al., Reference Koutsoumanis, Allende, Alvarez-Ordóñez, Bolton, Bover-Cid, Chemaly, Davies, De Cesare, Herman, Hilbert, Lindqvist, Nauta, Peixe, Ru, Simmons, Skandamis, Suffredini, Cacciò, Chalmers, Deplazes, Devleesschauwer, Innes, Romig, van der Giessen, Hempen, Van der Stede and Robertson2018).
The viability of Toxoplasma oocysts varies according to environmental conditions. The oocyst can remain infectious in areas with high and low humidity as well as in hot and dry regions (Walker et al., Reference Walker, Nokes and Jennings1992). Sporulated T. gondii oocysts can remain infectious in soil for at least 18 months (Esmerini et al., Reference Esmerini, Gennari and Pena2010). Freshwater runoff and drainage canals can transport cat feces containing oocysts in the soil to coastal waters through rivers and streams; the oocysts can contaminate freshwater and marine fauna (Fayer et al., Reference Fayer, Dubey and Lindsay2004). The oocysts can survive for at least 6 months in seawater. In aquatic environments, bivalve shellfish such as mussels, oysters, clams, and cockles are filter feeders that have the unique ability to concentrate and retain suspended particles, including T. gondii oocysts from the environment after filtering a large volume of water (Putignani et al., Reference Putignani, Mancinelli, Del Chierico, Menichella, Adlerstein, Angelici, Marangi, Berrilli, Caffara and di Regalbono2011; Coupe et al., Reference Coupe, Howe, Burrows, Sine, Pita, Velathanthiri, Vallée, Hayman, Shapiro and Roe2018). Experimental studies have shown that oocysts may remain viable for up to 85 days in oysters (Lindsay et al., Reference Lindsay, Collins, Mitchell, Wetch, Rosypal, Flick, Zajac, Lindquist and Dubey2004). The specific small subunit ribosomal RNA of T. gondii could be detected for up to 21 days after exposure in artificially exposed mussels, but viable oocysts were detected for only 3 days (Arkush et al., Reference Arkush, Miller, Leutenegger, Gardner, Packham, Heckeroth, Tenter, Barr and Conrad2003). Consumption of waterborne pathogens leads to the storage of these pathogens in bivalves' tissues (Ribeiro et al., Reference Ribeiro, Santos, Brito, Maciel, Da Silva and Albuquerque2015). Massie et al. showed that T. gondii is not capable of infecting invertebrates and the parasite is unable to spread or survive in visceral organs such as muscles. Nevertheless, oocysts may be concentrated in organs such as the digestive tract and others that are in close contact with water containing the organism (Massie et al., Reference Massie, Ware, Villegas and Black2010). Bivalve shellfish are known as excellent beacons for monitoring the health of estuarine ecosystems (Marquis et al., Reference Marquis, Bishop, Record, Countway and Fernández Robledo2019) and can act as paratenic hosts for T. gondii oocysts (Cong et al., Reference Cong, Zhang, Yuan, Zou, Li and Liang2019) that in these hosts, the parasite lives without any developmental stage and is then transmitted to the next host. Bivalve shellfish is a source of infection for marine mammals when consumed as food (Cole et al., Reference Cole, Lindsay, Howe, Roderick, Dubey, Thomas and Baeten2000). In some areas, bivalves are preyed upon by sea otters and other marine mammals, so obtaining and concentrating T. gondii oocysts by bivalves plays an important role in the mechanism of transmission of this parasite from land to sea (Miller et al., Reference Miller, Miller, Conrad, James, Melli, Leutenegger, Dabritz, Packham, Paradies and Harris2008). The occurrence of toxoplasmosis in marine mammals raises concerns that cold-blooded marine animals could be potential sources of T. gondii infection (Omata et al., Reference Omata, Umeshita, Murao, Kano, Kamiya, Kudo, Masukata, Kobayashi, Maeda and Saito2005). Cole et al. in 2000 showed that marine mammals could become infected by consuming invertebrates. Besides, oysters can be a danger to public health because people like to eat oysters in nature. In some countries, mussels are very popular because of their taste and nutritional value. However, they may represent a danger to people given the oysters are eaten raw in most coastal areas of the world, such as China (Cong et al., Reference Cong, Zhang, Yuan, Zou, Li and Liang2019).
In the case of fish, a study in goldfish (Carassius auratus) showed that T. gondii tachyzoites could persist for up to 3 days after infection by intramuscular inoculation. This has also been confirmed by polymerase chain reaction (PCR) analysis of inoculated tissues and bioassay in mice with homogeneous tissues of infected fishes by intraperitoneal inoculation (Omata et al., Reference Omata, Umeshita, Murao, Kano, Kamiya, Kudo, Masukata, Kobayashi, Maeda and Saito2005). Given that experimentally the tachyzoites of T. gondii survive for more than 7 days in cell culture at 37°C, it does not appear that the infection of the organism in cold-blooded animals is epidemiologically significant under natural conditions (Omata et al., Reference Omata, Umeshita, Murao, Kano, Kamiya, Kudo, Masukata, Kobayashi, Maeda and Saito2005). Because oocysts-carrying fishes may be able to mechanically transmit viable parasites to larger predators at the top level of the food chain, including marine mammals, different routes of transmission should be considered. A new route for oocyst transmission involves the complex interaction of suspended bioparticles, biofilms, small invertebrates, and gastropods. Extracellular polymeric materials are relatively abundant in the marine environment and are involved in the transmission of soil-derived pathogens, including T. gondii, by combining marine macroaggregates or adhering to biofilms on the surface of seaweeds or other benthic organisms (Wotton, Reference Wotton2004; Shapiro et al., Reference Shapiro, Krusor, Mazzillo, Conrad, Largier, Mazet and Silver2014). Most marine mammals feed on aquatic cold-blooded animals, and T. gondii does not multiply in cold-blooded animals (Bossart, Reference Bossart2011). T. gondii causes mortality in free-range marine mammals, including some endangered species (Cole et al., Reference Cole, Lindsay, Howe, Roderick, Dubey, Thomas and Baeten2000; Conrad et al., Reference Conrad, Miller, Kreuder, James, Mazet, Dabritz, Jessup, Gulland and Grigg2005; Di Guardo and Mazzariol, Reference Di Guardo and Mazzariol2013). Certain marine mammals (e.g. seals) serve as food animals for humans (Forbes et al., Reference Forbes, Measures and Gajadhar2009; Tryland et al., Reference Tryland, Nesbakken, Robertson, Grahek-Ogden and Lunestad2014; Reiling et al., Reference Reiling, Measures, Feng, Boone, Merks and Dixon2019). Therefore, fish can affect public health by infecting marine mammals. Also, the risk of T. gondii in fish, acting as carriers of oocysts on the body surface or transiently in the guts, is an occupational issue, and workers, especially pregnant women, should be aware of the risk of exposure to parasite during the primary production stages (fishing and harvesting) and while moving raw fish to produce fishery products and even some fish are eaten raw (Marino et al., Reference Marino, Giunta, Salvaggio, Castello, Alfonzetti, Barbagallo, Aparo, Scalzo, Reale and Buffolano2019). The meat and eggs of reptiles are considered a delicacy as well as their leather has attracted the attention of many consumers and has led to the breeding of species such as crocodiles (Magnino et al., Reference Magnino, Colin, Dei-Cas, Madsen, McLauchlin, Nöckler, Maradona, Tsigarida, Vanopdenbosch and Van Peteghem2009; Hoffman and Cawthorn, Reference Hoffman and Cawthorn2012). Considering the importance of mollusks (shellfish) and some cold-blooded animals as seafood and the absence of sufficient information on the prevalence of T. gondii in mollusks and cold-blooded animals worldwide, this article aims to help to better understand the potential of these animals to transmit toxoplasmosis to humans based on the available literature.
Methods
Study design
This systematic review was performed following the preferred reporting items for Systematic Reviews and Meta-Analysis statements (PRISMA) (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015). The methods of this systematic review have already been published in the PROSPERO database under the following number CRD42020203071.
Search strategy
We identified original articles on the prevalence of T. gondii in mollusks and cold-blooded animals worldwide published in the English language from inception to 1 August 2020. A comprehensive literature search was conducted in PubMed, ScienceDirect, ProQuest, Scopus, and Web of Science using the terms (with the Boolean term OR): ‘toxoplasmosis’ OR ‘Toxoplasma gondii’ OR ‘T. gondii’ and cold-blooded and mollusk search terms (with the Boolean term OR): ‘reptile’ OR ‘snake’ OR ‘fish’ OR ‘shellfish’ OR ‘bivalve shellfish’ OR ‘oyster’ OR ‘mussel’ OR ‘mollusk’ OR ‘snail’ OR ‘turtle’ OR ‘cold-blooded’. Toxoplasma search terms and cold-blooded and mollusk search terms were combined with the AND Boolean term. Also, the bibliographies of any eligible papers identified were studied for additional references.
Inclusion and exclusion criteria
We imported English language papers that have been formally published as journal articles and studies investigating the prevalence of T. gondii in mollusks and cold-blooded animals by microscopic techniques as well as molecular and serological methods. Narrative reviews, systematic reviews, experimental studies, and dissertations were excluded.
Study selection and data extraction
Two independent reviewers assessed titles and abstracts to obtain articles that met the inclusion criteria. If the title and abstract of the article could not be rejected with certainty by both researchers, the full text of the article was retrieved and assessed for eligibility. In this study, researchers did not have any disagreement and the coefficient of agreement (κ) was 100%. In the next step, two investigators extracted the data related to the retrieved papers and placed them in summary tables under the following headings: first author's last name, year of publication, place of study, samples, diagnostic methods, genes, type of antibody, cut-off, the total number of samples, number of Toxoplasma positive, and genotypes. The lists of extracted information are presented in Tables 1 and 2.
Table 1. Data characteristics of the included cross-sectional studies for the prevalence of T. gondii in cold-blooded animals

ELISA, enzyme-linked immunosorbent assay; MAT, modified agglutination test; IHAT, indirect hemagglutination test; PCR, polymerase chain reaction; IgM, immunoglobulin M; IgG, immunoglobulin G; n, number.
Table 2. Data characteristics of the included cross-sectional studies for the prevalence of T. gondii in mollusks (shellfish)

H, hemolymph; G, gill; D, digestive gland; GIT, gastrointestinal tracts; IL, intervalvular liquid: DH, digestive gland and hemolymph; HG, hemolymph and gill; DG, digestive gland and gill; PCR, polymerase chain reaction; PCR–RFLP, restriction fragment length polymorphism; qPCR, quantitative PCR; FLAG, real-time PCR fluorescent amplicon generation assay; HRM, high-resolution melting analysis; n, number.
The quality of publications
In this review, the methodological quality of each of the selected publications was estimated based on the Newcastle-Ottawa Scale criteria (Stang, Reference Stang2010). This checklist includes three different categories, namely selection, comparability, and exposure. One of these cases is two options and can get two points in the evaluation. The selected publications were then classified as low quality (1–2), moderate quality (3–5), and high quality (6–7). The quality scores of different eligible studies are represented in online Supplementary Table S1.
Results
Study identification and selection
In the initial literature search, a total of 2534 records were identified; after removing duplicates and non-eligible papers based on title and abstract, 47 articles were selected for full-text screening. Of 47 studies, 18 articles were excluded because they were experimental design, two papers had irrelevant outcomes, one was a review article, and one was a dissertation. In addition, an article was added while checking references. Finally, 26 articles were reviewed. Cross-sectional articles published from 1976 to 2020 were included in the systematic review. Figure 1 briefly shows the search process in this systematic review article.

Fig. 1. Flow diagram of the study design process.
Prevalence of T. gondii in cold-blooded animals
Cold-blooded animals in which the prevalence of T. gondii has been studied include frogs and toad (Levine and Nye, Reference Levine and Nye1976), turtles (Feitosa et al., Reference Feitosa, Brasil, Parentoni, Vilela, Nety and Pena2017), crocodiles (Feitosa et al., Reference Feitosa, Brasil, Parentoni, Vilela, Nety and Pena2017; Ferreira et al., Reference Ferreira, de Macêdo-Júnior, Lopes, Silva, Ramos, Júnior, Vitaliano, Santiago, Santos and Mineo2020), snakes (Nasiri et al., Reference Nasiri, Teymurzadeh, Karimi and Nasiri2016; Anah and Al-Mayali, Reference Anah and Al-Mayali2018), and fish (Taghadosi et al., Reference Taghadosi, Kojouri and Taheri2010; Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014; Aakool and Abidali, Reference Aakool and Abidali2016; Marino et al., Reference Marino, Giunta, Salvaggio, Castello, Alfonzetti, Barbagallo, Aparo, Scalzo, Reale and Buffolano2019). One of these studies examined crocodiles and turtles (Feitosa et al., Reference Feitosa, Brasil, Parentoni, Vilela, Nety and Pena2017). Fish and oysters were also studied in one study (Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014). The included studies in our systematic review study were conducted in Iran (n = 3), followed by Brazil (n = 2), USA, Iraq, Italy, and China (n = 1).
A total number of 2988 samples of cold-blooded animals in nine studies were entered into the systematic review, including 146 serum samples (50 fish and 96 reptiles) were evaluated for anti-T. gondii IgG and IgM antibodies using different serologic tests [enzyme-linked immunosorbent assay (ELISA), modified agglutination test (MAT), and indirect hemagglutination test (IHAT)] out of which 17 cases (5 fish and 12 reptiles) were positive for anti-T. gondii IgG and IgM antibodies. In a study by Ferreira et al. (Reference Ferreira, de Macêdo-Júnior, Lopes, Silva, Ramos, Júnior, Vitaliano, Santiago, Santos and Mineo2020), the number of positive cases was calculated based on MAT and IHAT findings. The result of the MAT is included here. Also, 2659 samples (1268 fish and 441 pools from 1293 fish as well as 98 reptiles) of different tissues, such as brain, intestines, lung, heart, spleen, liver, kidney, digestive tract tissues, gill, and skin/muscles, were examined for the prevalence of T. gondii using different molecular tests (PCR, nested-PCR, real-time PCR, and digital PCR) out of which 111 cases (19 fish and 32 pools from fish as well as 60 reptiles) were positive for T. gondii. One study used a microscopic method to diagnose T. gondii in amphibian (frog and toad), and of the 183 samples examined, only one was positive (Levine and Nye, Reference Levine and Nye1976). In one study, the fishes were not tested separately, and each species was divided into groups of 3, 5, 6, 8 or 10 units according to the size and weight of the fish. From each fish, intestine, gills, and multiple aliquots of skin–skeletal muscles complex (maximum weight 10 g) were divided separately in asepsis (Marino et al., Reference Marino, Giunta, Salvaggio, Castello, Alfonzetti, Barbagallo, Aparo, Scalzo, Reale and Buffolano2019). The genes used in these studies were B1, GRA6, and 18S rRNA (Aakool and Abidali, Reference Aakool and Abidali2016; Nasiri et al., Reference Nasiri, Teymurzadeh, Karimi and Nasiri2016; Anah and Al-Mayali, Reference Anah and Al-Mayali2018). The characteristics of the included studies are depicted in Table 1.
Prevalence of T. gondii in mollusks (shellfish)
The prevalence of T. gondii in shellfish was investigated in 18 articles. As illustrated in Table 2, the identified studies were conducted in five countries distributed worldwide, including the USA (n = 5), Italy (n = 5), Brazil (n = 4), China (n = 3), and New Zealand (n = 1). A total number of 13 447 samples (8069 shellfish and 1327 pools from 5378 shellfish) were examined for the prevalence of T. gondii using different molecular tests [TaqMan, PCR, nested-PCR, semi-nested PCR, PCR–restriction fragment length polymorphism (PCR–RFLP), real-time PCR, endpoint PCR, real-time PCR fluorescent amplicon generation assay (FLAG), high-resolution melting analysis (HRM), simplex PCR screening, and multiplex genotyping assays] out of which 692 cases (634 shellfish and 58 pools) were positive for T. gondii. In several studies, specimens were used as pooled samples (Esmerini et al., Reference Esmerini, Gennari and Pena2010; Putignani et al., Reference Putignani, Mancinelli, Del Chierico, Menichella, Adlerstein, Angelici, Marangi, Berrilli, Caffara and di Regalbono2011; Ribeiro et al., Reference Ribeiro, Santos, Brito, Maciel, Da Silva and Albuquerque2015; Monteiro et al., Reference Monteiro, Rocha, Silva, Mesquita, Rosário, Ferreira, Honorio, Melo, Barros and Scofield2019; Tedde et al., Reference Tedde, Marangi, Papini, Salza, Normanno, Virgilio and Giangaspero2019; Silva et al., Reference Silva, Silva, Watanabe, Chaves Bezerra, Bezerra, Gomes, Freire, Santos, Carvalho Neta and Silva2020). The pools of several shellfish were formed based on the similarity of morphometric characteristics (Silva et al., Reference Silva, Silva, Watanabe, Chaves Bezerra, Bezerra, Gomes, Freire, Santos, Carvalho Neta and Silva2020). Each pool was analysed as an experimental sample. Then, using a digital scale, the maximum amount of sample for DNA extraction was weighed according to the manufacturer of the kit protocol (Tedde et al., Reference Tedde, Marangi, Papini, Salza, Normanno, Virgilio and Giangaspero2019). In these studies, the B1 gene of T. gondii as the target sequence was the most common gene for molecular assays (Miller et al., Reference Miller, Miller, Conrad, James, Melli, Leutenegger, Dabritz, Packham, Paradies and Harris2008; Esmerini et al., Reference Esmerini, Gennari and Pena2010; Putignani et al., Reference Putignani, Mancinelli, Del Chierico, Menichella, Adlerstein, Angelici, Marangi, Berrilli, Caffara and di Regalbono2011; Aksoy et al., Reference Aksoy, Marangi, Papini, Ozkoc, Delibas and Giangaspero2014; Marangi et al., Reference Marangi, Giangaspero, Lacasella, Lonigro and Gasser2015; Shapiro et al., Reference Shapiro, VanWormer, Aguilar and Conrad2015; Cong et al., Reference Cong, Zhang, Hou, Wang, Ma, Zhu and Chen2017, Reference Cong, Zhang, Yuan, Zou, Li and Liang2019; Monteiro et al., Reference Monteiro, Rocha, Silva, Mesquita, Rosário, Ferreira, Honorio, Melo, Barros and Scofield2019; Tedde et al., Reference Tedde, Marangi, Papini, Salza, Normanno, Virgilio and Giangaspero2019; Santoro et al., Reference Santoro, Viscardi, Boccia, Borriello, Lucibelli, Auriemma, Anastasio, Veneziano, Galiero and Baldi2020).
Discussion
In this study, the current knowledge about the prevalence of T. gondii in mollusks (shellfish) and cold-blooded animals is systematically described. According to Tables 1 and 2, the highest and lowest prevalence rates of T. gondii in cold-blooded animals were related to the studies performed by Nasiri et al. as 80.88% (55/68) (Nasiri et al., Reference Nasiri, Teymurzadeh, Karimi and Nasiri2016) and Zhang et al. as 0.08% (1/1172) (Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014). Moreover, the highest and lowest prevalence rates of T. gondii in shellfish were observed in studies conducted by Staggs et al. as 46.34% (19/41) (Staggs et al., Reference Staggs, Keely, Ware, Schable, See, Gregorio, Zou, Su, Dubey and Villegas2015) and Zhang et al. as 0% (0/398) (Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014). Differences in prevalence in various regions are influenced by factors such as species, the size of the sample, method of detecting, the type of environment, and geographical location (Cong et al., Reference Cong, Zhang, Yuan, Zou, Li and Liang2019). The low levels of basic sanitation in the area and the presence of various species of wild and domestic cats may be predisposing factors to increase the incidence of parasites (Ribeiro et al., Reference Ribeiro, Santos, Brito, Maciel, Da Silva and Albuquerque2015). Another important factor is the type of tissue samples. The gills and digestive glands are the places where parasites are most often identified (Robertson, Reference Robertson2007; Leal and Franco, Reference Leal and Franco2008). Gills are ideal tissues for the detection of the parasite; oysters use a filter-feeding mechanism and can filter 5 L. Therefore, light particles such as T. gondii oocysts are retained in the gills (Ribeiro et al., Reference Ribeiro, Santos, Brito, Maciel, Da Silva and Albuquerque2015).
In most of the studies included in this systematic review, molecular methods have been used to investigate the prevalence of T. gondii in mollusks and cold-blooded animals (Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014). In the PCR method, the PCR type, the copy number of the target gene in the sample, and the inhibitors in the tissue samples are the main factors that may affect the detection limit. Various tests used in studies (nested-PCR, semi-nested PCR, real-time PCR, digital PCR, TaqMan, PCR–RFLP, endpoint PCR, FLAG, HRM, simplex PCR screening, and multiplex genotyping assays) and targets (B1, ITS1, GRA6, 18S rRNA, rep 529 gene, dhps, and SAG1) seem to be more sensitive than conventional PCR (Hurtado et al., Reference Hurtado, Aduriz, Moreno, Barandika and García-Pérez2001). Among them, the B1 gene, which has 35 copies in the genome, is the most widely used. The ITS-1, a non-coding spacer region, is used as a target for the development of a PCR method for the differentiation of species among apicomplexans such as Toxoplasma (Hurtado et al., Reference Hurtado, Aduriz, Moreno, Barandika and García-Pérez2001; Jauregui et al., Reference Jauregui, Higgins, Zarlenga, Dubey and Lunney2001). This region is conserved and the ITS-1 sequences showed 100% identity in 20 isolates of T. gondii (Homan et al., Reference Homan, Limper, Verlaan, Borst, Vercammen and van Knapen1997). The results of a study showed that the detection limit of the ITS-1 PCR assay was 500 fg of T. gondii DNA. This amount of DNA is present in 4.5 tachyzoites (Opsteegh et al., Reference Opsteegh, Langelaar, Sprong, den Hartog, De Craeye, Bokken, Ajzenberg, Kijlstra and van der Giessen2010). In addition, false-positive amplification occurs frequently using the B1 and ITS-1 primer sets. It is not known whether the dhps gene is single or multiple. In one study, this gene was able to detect 50 oocysts in the hemolymph (Coupe et al., Reference Coupe, Howe, Burrows, Sine, Pita, Velathanthiri, Vallée, Hayman, Shapiro and Roe2018). In comparison to dhps gene, the rep529 gene is purportedly the most sensitive (Su et al., Reference Su, Shwab, Zhou, Zhu and Dubey2010) because there are approximately 200–300 copies of this marker in the T. gondii genome (Homan et al., Reference Homan, Vercammen, De Braekeleer and Verschueren2000; Costa and Bretagne, Reference Costa and Bretagne2012).
Due to the technical limitations, the detection of the resistant form of the parasite (oocyst) in environmental samples is a scientific challenge. Although the nested-PCR method is very sensitive to detect T. gondii, because the oocyst has a very tough wall, it can interfere with DNA extraction. Therefore, it is difficult to detect oocysts in the environment (Ribeiro et al., Reference Ribeiro, Santos, Brito, Maciel, Da Silva and Albuquerque2015). In vitro encystation, grinding with glass beads, digestion with proteinase K, and the use of heat shocks are various techniques used to break the oocyst wall, but protocols were not standardized according to temperature and the number of freeze/thaw cycles (Dumètre and Dardé, Reference Dumètre and Dardé2003). The nested PCR method confirms the presence of T. gondii DNA, but does not differentiate between unsporulated and sporulated oocysts, the differentiation of these oocysts is important in disease transmission, as only sporulated T. gondii oocytes can be infectious (Dubey et al., Reference Dubey, Lindsay and Speer1998). To investigate this, a study used RT-PCR of the sporozoite-specific SporoSAG gene and detected T. gondii sporozoite mRNA in four of seven mussels (Coupe et al., Reference Coupe, Howe, Burrows, Sine, Pita, Velathanthiri, Vallée, Hayman, Shapiro and Roe2018). Although in this study it is possible to detect SporoSAG mRNA in sporulated but non-viable oocysts, it is not possible to claim with certainty that infectious oocysts were found in these mussels (Coupe et al., Reference Coupe, Howe, Burrows, Sine, Pita, Velathanthiri, Vallée, Hayman, Shapiro and Roe2018). In another study, nested PCR and FLAG were used. The results were consistent. Ease of use and speed, no need for post-reinforcement management for faster analysis, reduced risk of amplitude contamination and quantitative interpretation of results are some of the advantages typical of the real-time PCR technology that FLA demonstrates (Putignani et al., Reference Putignani, Mancinelli, Del Chierico, Menichella, Adlerstein, Angelici, Marangi, Berrilli, Caffara and di Regalbono2011). Some studies use the qPCR method. Despite the development of many qPCR techniques, some of them have limitations in the cost and performance of certain dyes in PCR. For example, TaqMan probes are expensive or dyes, which reduces reproducibility and/or analytical sensitivity (Bustin, Reference Bustin2002; Eischeid, Reference Eischeid2011). Dyes such as SyberGreen may inhibit PCR, but EvaGreen is more suitable for routine multiplex qPCR applications due to its less inhibitory effect and lack of dye redistribution (Mao et al., Reference Mao, Leung and Xin2007; Li et al., Reference Li, Chu, Liu, Jing, Liu and Hao2010; Eischeid, Reference Eischeid2011).
To improve the sensitivity of the assay, it is best to use a hemolymph sample instead of whole tissue homogenates, as the hemolymph is less viscous and probably contains less material that may inhibit DNA amplification by PCR (Shapiro et al., Reference Shapiro, VanWormer, Aguilar and Conrad2015). Some authors report detection limits of 100 oocysts in mussel tissues homogenate (Esmerini et al., Reference Esmerini, Gennari and Pena2010), five oocysts in mussel hemolymph (Shapiro et al., Reference Shapiro, VanWormer, Aguilar and Conrad2015) and even a single oocyst in mussel hemolymph (Staggs et al., Reference Staggs, Keely, Ware, Schable, See, Gregorio, Zou, Su, Dubey and Villegas2015). One study showed that the diagnosis of T. gondii in mussels that were experimentally infected using hemolymph and gill tissue was similar, while the detection of the parasite in the digestive gland was more sensitive in later depuration periods (Arkush et al., Reference Arkush, Miller, Leutenegger, Gardner, Packham, Heckeroth, Tenter, Barr and Conrad2003). Another diagnostic method is bioassay in mice, in which the amount of material orally administered to each mice and sporulated or viable positive samples is important. In one study, the sensitivity of isolation by bioassay was 103 oocytes and the sensitivity of detection by nested-PCR was 102 oocytes (Esmerini et al., Reference Esmerini, Gennari and Pena2010).
Given that production of shellfish has been considered as an industry worldwide and the consumption of contaminated raw shellfish may represent a considerable health threat, the importance of T. gondii in raw or undercooked seafood to serve as a method of parasite transmission should not be ignored. China is the world's largest producer and consumer of shellfish (Cong et al., Reference Cong, Zhang, Yuan, Zou, Li and Liang2019), and France, Spain, and Italy produce two-thirds of all European mussels (Tedde et al., Reference Tedde, Marangi, Papini, Salza, Normanno, Virgilio and Giangaspero2019). France produces >90% of cupped oysters (about 150 000 tons per year) and Spain <5000 tons as well as Italy produces <500 tons per year, which is not enough for internal consumption (Putignani et al., Reference Putignani, Mancinelli, Del Chierico, Menichella, Adlerstein, Angelici, Marangi, Berrilli, Caffara and di Regalbono2011). Oysters are cultured for the consumption of humans (Monteiro et al., Reference Monteiro, Rocha, Silva, Mesquita, Rosário, Ferreira, Honorio, Melo, Barros and Scofield2019). In some parts of the world, such as Brazil, oyster farming is a source of income for families (de Souza Sampaio et al., Reference de Souza Sampaio, Tagliaro, Schneider and Beasley2019).
Evaluation of the genotype is an important variable in studying the risk of contamination in cold-blooded animals and potential subsequent infection in humans, because infection with different clonal lineages of T. gondii offers very different clinical consequences. Type I strains are highly pathogenic in mice and are associated with acquired ocular disease and diffuse congenital toxoplasmosis. However, the detection of type I strains in cases of chronic infection reactivation in immunocompromised patients or placentas with non-congenital infection suggests that they may also be responsible for asymptomatic infections in healthy patients (Dardé et al., Reference Dardé, Ajzenberg and Smith2007). Type II strains are the most common strains in human diseases. These strains are responsible for many cases of asymptomatic toxoplasmosis in Europe and are also pathogenic for immature fetuses and immunocompromised patients (Dardé et al., Reference Dardé, Ajzenberg and Smith2007). Besides the three main clonal lineages of T. gondii, atypical and recombinant strains have been identified (Dardé, Reference Dardé2008). The distribution of T. gondii genotypes varies in different geographical areas (Lehmann et al., Reference Lehmann, Marcet, Graham, Dahl and Dubey2006); in North America and Europe, three distinct lineages (types I–III), in South America, very diverse with few lineages, in China, seven genotypes (Zhang et al., Reference Zhang, Yang, Wang, Tao, Xu, Yan, Song and Li2014). In America, new genotypes X and A have been identified in sea otters (Sundar et al., Reference Sundar, Cole, Thomas, Majumdar, Dubey and Su2008). Also, the T. gondii genotypes isolated from aquatic animals may be consistent with those isolated from terrestrial animals. Given that there is limited information on T. gondii genotypes from oysters, fish, and marine mammals in the world, further studies on T. gondii genotypes in aquatic animals are needed to provide stronger evidence for T. gondii transmission between aquatic animals and other animals, including humans. However, this risk factor was not specifically investigated in most studies; accordingly, this is considered as a basic gap. Also, a useful method for assessing the risk of infection in the consumer is to determine the parasitic load and parasite genotype, and the occurrence of T. gondii and the severity of the infection can be affected by the parasite form, dose, genotype, and immune status of the host (Santoro et al., Reference Santoro, Viscardi, Boccia, Borriello, Lucibelli, Auriemma, Anastasio, Veneziano, Galiero and Baldi2020). There were several limitations in the present systematic review as follows: (1) a limited number of studies have examined the prevalence of T. gondii in mollusks and cold-blooded animals; (2) the use of English articles due to lack of fluency in other languages; (3) the use of various diagnostic methods without equal specificities and sensitivities; and (4) lack of access to published information on the prevalence of T. gondii in mollusks and cold-blooded animals from many parts of the world.
Conclusion
Toxoplasma gondii can cause infection ranging from asymptomatic or mild infection in immunocompetent people to miscarriage or serious consequences for the fetus in pregnancy, and fatal encephalitis in AIDS patients. Due to the occurrence of T. gondii in shellfish and cold-blooded animals, the consumption of these animals as raw or undercooked in some parts of the world can be a serious public health concern. Therefore, people need to be aware of the risk of acquiring T. gondii through eating raw seafood, especially fishes and bivalve mollusks. Finally, many questions remain to be answered in future investigations and it is required to carry out further studies to obtain more accurate details regarding the prevalence of T. gondii in mollusks and cold-blooded animals.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000433.
Acknowledgements
This article is an approved plan from the Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran (number: 8317).
Author contribution
A.D. conceived and designed the study, and S.S. critically revised the manuscript. T.N. searched the literature, extracted the data and wrote the manuscript. All authors have read and approved the final manuscript.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The code of ethics of this plan is (IR. MAZUMS.REC.1399.616).