Published online by Cambridge University Press: 19 April 2005
The salivary glands are the organs of osmoregulation in ticks and, as such, are critical to the biological success of ticks both during the extended period off the host and also during the feeding period on the host. Absorption of water vapour from unsaturated air into hygroscopic fluid produced by the salivary glands permit the tick to remain hydrated and viable during the many months between blood-meals. When feeding, the tick is able to return about 70% of the fluid and ion content of the blood-meal into the host by salivation into the feeding site. This saliva also contains many bioactive protein and lipid components that aid acquisition of the blood-meal. The salivary glands are the site of pathogen development and the saliva the route of transmission. The importance of the multifunctional salivary glands to tick survival and vector competency makes the glands a potential target for intervention. Here we review the cell biology of tick salivary glands and discuss the application of new approaches such as expressed sequence tag projects and RNA interference to this important area in the field of tick and tick-borne pathogen research.
Almost two millennia ago, the wonders of tick osmoregulation and excretion were commented upon by Pliny the Elder (A.D. 23–79) in his 37 volume Historia Naturalis when he wrote, “a tick simply filled to bursting point with its victim's blood and then died because it had no anus” (from Hillyard, 1996). A millennium and a half later, the Reverend Dr Thomas Moufet (1553–1604) also noted in his Insectorum sive Minimorum Animalium Theatrum that “[Ricinus] is filled with food abundantly and yet there is no passage for any excrement”. Quite correctly, these ancient natural historians observed the tremendous increase in body size of feeding female ticks and then suddenly these engorged ticks would detach and fall to the ground, barely able to move with their enormous rounded bodies and unwilling to reattach. However, the conclusions of Pliny and Moufet were incorrect that this apparent onset of tick ill-health was due to an inability to excrete caused by the lack of anus or that these inactive engorged females would die as a result. Indeed, if Pliny and Moufet had continued their observations on these engorged female ticks for a few weeks, they would have seen that the females (most likely Ixodes ricinus) would deposit several thousand eggs before dying thus completing the life cycle. Ticks do possess an anus and excrete a small amount of nitrogenous waste (mainly guanine). However, whilst feeding on the host ticks are actively excreting about 70% of their imbibed water and ions, but Pliny, Moufet or other observers would not see this hidden process since the tick returns the excess water and ions back into the host via its mouthparts. In ticks, it is the salivary glands that are the organs of osmoregulation not the Malpighian tubules (Fig. 1).

Fig. 1. Schematic of a feeding adult female tick (Amblyomma americanum) illustrating the relative position and size of the internal organs. The racemose-like structure of the salivary gland show the small Type I acini that are involved in off-host osmoregulation and the larger, more posterior, Type II and III acini that are involved in on-host osmoregulation, are the site of synthesis and secretion of protein and lipid factors (anticoagulants, immunosuppressants etc.) and are the site of pathogen development and replication. A male tick is depicted on the underside of the female in copula, a process that takes about 48 h as the spermatophore is inserted into the female genital pore by the male's mouthparts and involves copious salivation from the male-specific type IV acini (Feldman-Muhsam, Borut & Saliternik-Givant, 1970). The boxes indicate the composition of the main ions (units millimolar equivalents) in the various compartments as they are imbibed from the blood into the gut lumen, pass from the gut into the haemolymph and, finally, through the salivary gland back into the host in the saliva (data from Hsu & Sauer, 1975).
Studies of tick salivary glands are of major importance in tick biology because of their extraordinary physiology, their role in pathogen transmission and secretion of potentially useful secretory products. The earliest work focused on structure, histology and the striking growth and reorganization of the salivary glands in ixodid females during feeding (Till, 1961; Chinery, 1965; Binnington, 1978). From these studies it was shown that salivary glands occupy the antero-lateral one-third to one-half of the tick's haemocoel with an abundance of oxygen supplying trachea. Three acini types, I, II and III, attached to a main and branching ducts in females and four (I–IV) in males, comprise the salivary glands of ixodid ticks. Two acini types (A and B or I and II) make up the salivary glands in argasid ticks (Coons & Roshdy, 1981). With the discovery of the salivary glands as organs of fluid excretion (Howell, 1966; Gregson, 1967; Tatchell, 1967) research was accelerated on understanding the control and mechanism of fluid secretion (Tatchell, 1969; Kaufman & Phillips, 1973). This research confirmed control by nerves and dopamine as the principal neurotransmitter at the neuroeffector junction controlling fluid secretion. Hypotheses were developed to reconcile the structural changes in the salivary glands with the tick's capacity to excrete copious quantities of excess fluid during tick feeding; however, these hypotheses remain to be verified (Fawcett, Binnington & Voight, 1986). Other studies by Rudolph & Knulle (1974) identified the salivary glands as the source of a hyperosmotic secretion that facilitates absorption of water from unsaturated air during protracted periods off the host by unfed ticks. It was postulated that the type I acinus is associated with secreting the hyperosmotic secretion. With increasing awareness of the significance of the ixodid tick's capacity to remain attached and feeding on a host for relatively long periods, work has intensified on identifying factors in tick saliva that counter the haemostatic, immune and inflammatory responses of the host directed at the attached and feeding tick. This aspect of salivary gland biology aims to identify secretory proteins that may be targeted and used as vaccines for immunological control of ticks and tick-borne pathogens or possibly as molecules for designing novel pharmaceutical agents (see the accompanying chapter by Valenzuela in this Supplement). Our purpose here is to provide a brief overview of salivary gland structure and an overview of its physiology with an eye toward seeing how this information contributes to understanding the tick's unique success as an ectoparasite and its ability to be an efficient vector of pathogens.
The anatomy and morphogenesis of tick salivary glands during tick feeding has been previously reviewed (Sauer et al. 1995; Coons & Alberti, 1999). An abbreviated description is included here to provide an overview of the tissue's complexity. The paired salivary glands are acinar, lying anterolaterally on both sides of the tick's hemocoel (Fig. 1). Paired salivary ducts enter a shallow tube, the salivarium, lying above the pharynx. The salivarium empties into the buccal cavity where outward-flowing saliva alternates with inward-moving host tissue fluid. The alternating sucking and salivation cycles have been elegantly monitored electronically by Kemp & Tatchell (1971).
Each of the acini types in both ixodid and argasid ticks consist of multiple cell types (Fig. 2). The type I acinus is without a valve and directly attached to the main duct via a short acinar duct and short distances along the two posterior branches of the main duct The agranular acinus type I in ixodid ticks consists of a single central cell, multiple pyramidal cells, a constrictor cell and peritubular cells surrounding the short acinar duct (Krolak, Ownby & Sauer, 1982). The cells do not change substantially in size or appearance and the number of cells remain constant during tick feeding (Barker et al. 1984). The pyramidal cells are marked by tortuous plasma membrane infoldings with closely associated mitochondria that prompted some early investigators to propose that acinus type I performed a major role in fluid excretion during tick feeding (Kirkland, 1971). It is now generally believed that the principal function of the type I acinus is secretion of a hyperosmotic fluid for use in the mechanism of water vapour uptake in unfed ticks (see below).

Fig. 2. Arrangement of the three alveoli types of the ixodid tick female salivary gland (adapted from Binnington, 1978). The alveolus types are distinguished by differential shading. Type I alveoli attach directly to the main salivary duct and to some principal branches of that duct. A diagrammatic section of a type I alveolus with the cell types is shown above (adapted from Barker et al. 1984). Type II alveoli are more abundant in the proximal section and type III are more abundant in the distal section of the large branches. A schematic representation of the structure of the type III alveolus and cell types from an unfed tick is depicted below (from Fawcett et al. 1986, with permission) The types II and IV alveoli have a similar organization to type III alveoli but different cell types. Type IV alveoli are found only in males. Type II and especially type III undergo remarkable and complex cytological transformation to meet the tick's changing physiological requirements (including fluid transport) during tick feeding (see Fawcett et al. 1986). Nerve supply and tracheoles to the gland are not shown. (Artwork by Kerry Stricker, Oklahoma State University.) (Reproduced from Sauer et al. (1996) with permission from CABI International.)
Elegant ultrastructural and light microscopic descriptions of granular acini in salivary glands of both argasid and ixodid ticks have been published (Roshdy & Coons, 1975; Binnington, 1978; Megaw & Beadle, 1979; Coons & Roshdy, 1981; Walker, Fletcher & Gill, 1985; Fawcett et al. 1986; Gill & Walker, 1987). The type II acinus in ixodid ticks is composed of granular ‘a’, ‘b’, ‘c1–c4’ based upon morphology and reactions to specific stains. The type II acinus also contains agranular ablumenal interstitial cells and a single adlumenal interstitial cell. The acinus has a cuticular-lined acinar duct with valve and surrounding neck cells. The acinus increases greatly in size during feeding even though by the end of feeding most of the granules in granular cells are depleted. The overall organization of the type III acinus is similar to type II but with only three granular cell types, ‘d’, ‘e’ and ‘f’. The type III acinus also increases greatly in size during tick feeding and the ‘f’ cell undergoes extensive transformation with increasing plasma membranes with associated mitochondria. The transformed ‘f’ cell and proliferating ablumenal interstitial cells are believed to be responsible for secreting the bulk of the fluid across the salivary glands to facilitate concentration of the blood-meal. In support of this, the ‘f’ cells in males, which imbibe much smaller blood-meals than females, do not increase in size comparable to females (Coons & Lamoreaux, 1986). The single adlumenal interstitial cell in ixodid type II and III acini (Krolak et al. 1982) is thought to function as a myoepithelial-like cell during tick feeding. During blood-meal concentration, fluid accumulates in an expanding acinar lumen and is expelled by contraction of the adlumenal cell winding in web-like fashion around the apical side of cells in acini II and III.
Salivary gland structure, development and degeneration in ixodid larvae and nymphs have been reviewed in Sauer et al. (1995). In general, acini types and cells are similar to those found in adults, except for the absence of type IV in larval ticks (Till, 1961; Chinery, 1965). A recent study of tick salivary gland development revealed that glands and ducts could be identified in 23 day-old embryo Ixodes ricinus and that gland development was complete in 26 day old embryos (Jasik & Buczek, 2004). Future studies on the development of embryonic salivary glands may give insight into the temporal and tissue movement of pathogens known to be efficiently transmitted transovarially (e.g. Babesia spp., Rickettsia rickettsii) and pathogens poorly transmitted transovarially (e.g. Borrelia burgodorferi).
The salivary glands of argasid ticks consist of only two types of acini, A and B (Roshdy & Coons, 1975) or I and II (Coons & Roshdy, 1981; El Shoura, 1985). Type I (A) is similar in location and structure to the type I acinus of ixodid ticks. The type II (B) is granular, consisting of cell types ‘a’, ‘b’ and ‘c’. A fourth type ‘d’ granular cell has been identified in the salivary glands of Ornithodoros savignyi (Mans et al. 2004). The acinar lumen empties into a chitinous duct lacking the complex valvular structure of ixodid acini types II and III. Interstitial cells are present that develop complex, branched canaliculi with increased plasma membranes and mitochondria but not to the same extent as that seen in type III acini of ixodid ticks. The salivary glands of argasid ticks, in contrast to ixodid ticks, are surrounded by a myo-epithelial sheath.
The salivary glands of ixodid females increase 25-fold in mass and protein content during tick feeding with acini types II and III exhibiting remarkable morphological change. Much of the granular material seen in salivary glands of unfed female ixodid ticks is absent from the salivary glands by the end of 7–14 days of feeding prior to repletion. Of note is the almost complete transformation of the type III acinus to a tissue with characteristics of a fluid transporting epithelium. The ixodid male salivary gland also increases in mass and protein content and exhibits expression of new genes during tick feeding but not to the same extent as that seen in females (Oaks et al. 1991; Bior, Essenberg & Sauer, 2002).
Upon completion of the blood-meal, the ixodid female tick drops to the ground, begins the slow regulated digestion of the blood-meal, vitellogenesis, oocyte maturation, oviposits and then dies. Within a few days of detachment, the glands degenerate with the appearance of notable autophagic whorls (Harris & Kaufman, 1981). This degeneration is neither necrotic nor pathological, but rather is a sequential and highly regulated physiological process of programmed cell death. Ecdysteroid treatment of glands in vitro or infusion into whole ticks causes gland degeneration (Harris & Kaufman, 1985). Haemolymph levels of ecdysteroids increase dramatically in the days immediately following detachment (Kaufman, 1991). An ecdysteroid-binding activity has been demonstrated in tick salivary glands of fed ticks (Mao, McBlain & Kaufman, 1995; Mao & Kaufman, 1998, 1999). Further, an ecdysteroid receptor (Guo et al. 1997) and retinoid X receptor (Guo et al. 1998) have been cloned and shown to be expressed in the salivary glands of Amblyomma amercianum. Integumental tissue synthesizes ecdysteroid in response to a protein factor from the synganglion via an increase in cAMP levels (Lomas, Turner & Rees, 1997). A protein factor from the male gonad stimulates gland degeneration in virgin females (Lomas & Kaufman, 1992) and may act by targeting the synganglion to release the ecdysteriodogenic protein which subsequently acts at the integument. Recently, a combination of two recombinant proteins (termed ‘voraxin’) from an A. hebraeum male gonad cDNA library were shown to stimulate salivary gland degeneration in vivo (Weiss & Kaufman, 2004), though it awaits further investigation to establish if voraxin stimulates ecdysteroid synthesis by the integument. Taken together, these results suggest a model for the stimulus of tick salivary gland degeneration by which a competent ecdysteroid receptor complex in the gland responds to rising ecdysteroid levels at completion of the blood-meal acquistion and this increased ecdysteroid sythesisis is stimulated in some manner by a protein factor from the male gonad.
Though the endocrine stimulus for tick salivary gland has been delineated in some detail, the actual mechanism and machinery in this cell death is unknown. L'Amoreaux, Junaid & Trevidi (2003) reported significant DNA fragmentation in degenerating salivary glands of female Dermacentor variablis using the in situ TUNEL technique. In Ixodes ricinus salivary degeneration, we observed limited TUNEL staining either in glands in vivo or cultured glands treated with ecdysteroid or several pro-apoptotic agents, though DNA laddering was obvious and degeneration was accompanied by increased caspase-3-like activity (B. Stewart & A. S. Bowman, unpublished observations). Interestingly, the type I acini in I. ricinus did not exhibit any morphology of programmed cell death throughout the 12 days post-host observation period, during which period the cellular material of the type II and III acini was completely removed. This indicates that in I. ricinus the type I acini are likely to remain functional for water vapour uptake during the egg laying period (>40 days).
Considerable effort has been devoted to studying control of salivary gland secretion and yet many questions remain. The salivary glands are innervated with no evidence of control by hormones (Sauer, Essenberg & Bowman, 2000). Nerves from the synganglion impinge upon salivary gland cells with differing opinions on the number originating from the synganglion (Megaw, 1977; Binnington & Kemp, 1980; Kaufman & Harris, 1983; Fawcett et al. 1986). The nerves travel posteriorly along the main salivary duct and divide into lobular nerves and enter at the base of each acinus and terminate at synapses on glandular cells near the lumen. Three types of synaptic vesicles have been identified in the axoplasm of nerve endings in the salivary glands, large vesicles with granular content of moderate density that may contain peptides, small homogeneously dense vesicles that are thought to contain catecholamines and non-granular vesicles (Fawcett et al. 1986).
The neurotransmitter dopamine stimulates fluid secretion by isolated salivary glands maintained in culture medium and in vivo when injected into the haemocoel of partially-fed female ticks (Kaufman, 1976; McSwain, Essenberg & Sauer, 1992). A dopamine, D1-like receptor has been found in the salivary glands of female lone star ticks linked to activation of adenylate cyclase (Schmidt, Essenberg & Sauer, 1981, 1982). GTP and non-hydrolyzable Gpp(NH)p stimulate adenylate cyclase in a washed particulate fraction of the salivary gland indicating a G protein-coupled dopamine receptor (Sauer et al. 1986). Adenylate cyclase, Na+,K+-ATPase activity and fluid secretion rates increase with increased tick feeding and are highest in salivary glands of mating, rapidly feeding females (reviewed by Sauer & Essenberg, 1984). Cyclic AMP also stimulates fluid secretion by isolated salivary glands maintained in culture medium (Needham & Sauer, 1975, 1979). Dopamine has been identified in the salivary glands and salivary gland nerves of Boophilus microplus and A. hebraeum (Binnington & Stone, 1977; Kaufman & Harris, 1983). Finally, phenylethylamines stimulate adenylate cyclase and fluid secretion in the rank order of dopamine>noradrenaline>adrenaline>isoproterenol (Schmidt et al. 1982; Kaufman & Wong, 1983). The increase in cAMP stimulates a salivary gland protein kinase that has been partially purified from salivary glands of A. americanum (Mane et al. 1985; Mane, Sauer & Essenberg, 1988). Genes for catalytic subunit isoforms of cAMP-dependent protein kinase have been cloned and sequenced (Palmer et al. 1999). Incubation of salivary glands with dopamine or cAMP and theophylline (inhibitor of phosphodiesterase) increased the phosphorylation of at least 12 salivary gland proteins. The function and cellular location of the phosphoproteins are unknown (McSwain, Essenberg & Sauer, 1985; McSwain et al. 1987). Overall, fluid secretion is controlled via a cAMP-dependent protein phosphorylation cascade following salivary gland stimulation by dopamine released from nerve endings.
Other receptors, however, and/or other pathways appear to be involved in controlling secretion. Dopamine antagonists spiperone, pimozide and haloperidol potentiate the stimulatory effect of dopamine without having a stimulatory effect on their own (Wong & Kaufman, 1981). The potentiating effect of spiperone is abolished by sulpiride. The authors postulated two possibilities to explain the results: (a) spiperone allosterically affects the dopamine receptor to increase secretion or (b) spiperone inhibits an inhibitory pathway that responds to dopamine. γ-Aminobutyric acid (GABA) also potentiates the effect of dopamine on stimulating fluid secretion (Lindsay & Kaufman, 1986). GABA antagonists, picrotoxin and biculline block GABA- and spiperone-induced potentiation of fluid secretion. GABA-induced potentiation of secretion by dopamine was also blocked by sulpiride. It was speculated that sulpiride and spiperone may interact with the GABA receptor. Salivary gland fluid secretion is also stimulated by ergot alkaloids (Kaufman & Wong, 1983). Ergot alkaloid stimulation is inhibited by sulpiride which is ineffective in reducing stimulation by dopamine. This was an unexpected result because ergot alkaloids interact with 5-hydroxytryptamine (5-HT) receptors but 5-HT has no effect on stimulating fluid secretion by isolated salivary glands (Needham & Sauer, 1975; Kaufman, 1977). Clearly more work is required to fully understand all pathways involved in controlling fluid secretion. Overall, fluid secretion is controlled via a cAMP-dependent protein phosphorylation cascade following salivary gland stimulation by dopamine released from nerve endings. A major task remaining is to verify the cellular location and mechanism of fluid secretion in the salivary glands.
Salivation in vivo can be studied by injection of a dopamine solution into the haemocoel of partially fed ticks recently removed from the host. Alternatively, a solution of pilocarpine, a cholinomimetic agent, can be applied to the surface of the tick through which it is rapidly absorbed and causes the release of dopamine from the salivary nerves resulting in salivation. In vitro studies of isolated tick salivary gland secretion allow researchers to control conditions more than the in vivo approach and have led to most advances in our understanding of tick salivary gland secretion. The original in vitro method was a modification of the Ramsay technique utilised for studying fluid secretion of insect Malpighian tubules. Tick salivary glands are placed in a small volume of medium under liquid paraffin in a Petri dish and the main salivary duct drawn from the medium into the liquid paraffin. Agonists (e.g. dopamine) or antagonists can be added to the media causing changes in the rate of fluid secretion by the gland expressed as a droplet at the end of the salivary duct that can be measured with a microscope and ocular micrometer and converted to the volume secreted (e.g. Needham & Sauer, 1975). The alternative in vitro method is the ligated duct gravimetric method in which the main salivary duct of isolated tick glands are occluded by ligation with a very fine strand of silk or hair, placed in media containing the agents of interest and changes in weight recorded on an analytical balance (e.g. Harris & Kaufman, 1985). The ligation method measures fluid uptake by the gland rather than true secretion but has the advantage of allowing several glands to be studied simultaneously.
Recently, we investigated the stimulation of fluid uptake in isolated Ixodes ricinus salivary glands by dopamine using the ligated duct technique (S. Whitelaw & A. S. Bowman, unpublished observations). As expected, dopamine caused an increase in gland weight (≡fluid uptake) (Fig. 3A). When the histology of these glands was examined, we observed that there was no increase in the acini diameter, but that the lumen of the type III acini of the dopamine-treated glands were greatly increased (Fig. 3B) with the cytoplasm of the cells forced back toward the basal lamina (Fig. 3C–D). It is believed that the adluminal interstitial cell in the type III acinus functions as a myoepithelial cell and contracts to eject the fluid from the expanded acinus into connecting ducts (Coons et al. 1994; L'Amoreaux, Needham & Coons, 1994). We observed no histological change in Type I to implicate these acini in the fluid secretion, as has previously been suggested by some workers (e.g. Kirkland, 1971). These studies clearly demonstrate that the type III acini are indeed the acini involved with bulk fluid movement in tick salivary glands as has been hypothesized for 30 years.

Fig. 3. Demonstration of dopamine-stimulated fluid uptake by partially-fed female Ixodes ricinus salivary glands and involvement of type III acini. (A) The main salivary duct of isolated glands were ligated and the glands incubated in media (control) or media supplemented with 10 μM dopamine. The change in weight was recorded on an analytical balance at 6 min intervals (Mean±S.E.M., n=4). Control glands were subjected to dopamine stimulation at 18 min (arrow) to check viability of these glands. (B) These ligated glands were then fixed (1% osmium tetroxide), processed, and sectioned (semi-thin, 0·5 μm), stained with toluidine blue. The diameter of the acini and the lumen were determined using Image-Pro Plus (Media Cybernetics). No difference was observed between treatments for the total acini size (P>0·05), but the acini lumen were greater (P<0·05) following dopamine treatment (Mean±S.E.M., n=30). (C) Type III acini in the ligated control glands show no or small lumens. A type II acini with its more prominent secretory granules is also visible in this section. (D) The type III acini of dopamine-stimulated glands show large fluid filled lumens with the acini cells pushed back towards the basal lamima. In both C and D, scale bar=55 μm.
Using the in vitro assay techniques, dopamine-stimulated secretion was shown to be inhibited by oubain (70%) and bafilomycin (25%) (McSwain et al. 1997), indicating a role for a Na+/K+-ATPase and V-ATPase in supplying the electromotive force in salivary gland secretion or modification. But what is known about the fluid (i.e. water) transport in tick salivary glands? Historically, transport across biological lipid membranes was thought to be by simple diffusion, primarily driven by osmotic forces generated by ion fluxes. Two lines of evidence suggested the existence of water channels: (1) certain cells exhibited very high water permeability almost approaching the diffusion of water in a solution, and (2) certain cells change their water permeability in response to stimuli (e.g. anti-/diuretic hormones). The landmark discovery of a water channel in human red blood cell membranes (Preston et al. 1992) changed our understanding of water transport physiology. The water channels were renamed aquaporins (AQPs) (for reviews see Borgnia et al. 1999; Verkman, 2002). There are 11 (AQP0–10) well-characterized mammalian AQPs with additional AQPs found in fish, plants, insects, yeast and bacteria. Based on sequence homology and permeability properties, AQPs are now subdivided into aquaporins that are water selective (AQP0, 1, 2, 4, 5, 6, 8 and 10) and the aquaglyceroporins (AQP3, 7 and 9) that are permeated by water, glycerol, urea and other non-electrolytes. The AQPs all have similar structures: 6 membrane-spanning domains, intracellular NH2- and COOH-termini and a pair of highly conserved motifs (aspargine-proline-alanine, NPA). The AQP folds on itself in the ‘hour glass model’ with the two NPA motifs forming the ‘water-pore’. The aquaglyceroporins are characterized by an aspartate after the 2nd NPA motif (NPARD). Many AQPs are inhibited by mercurial reagents due to a cysteine near the second NPA motif.
Mammalian salivary glands contain several AQP types (for review see Ishikawa & Ishida, 2000). AQP1, 3 and 5 have been immunolocalised in human salivary glands: AQP5 (apical membrane) and AQP3 (basolateral membrane) in the salivary gland acinar cells and AQP1 in the endothelial cells and red blood cells associated with the glands (Gresz et al. 2001). AQP5 is sensitive to Hg2+ mercury. Saliva from AQP5 null mice has less volume, higher osmolality; higher Na+, K+ and Cl− levels, but similar total protein and amylase content to wild type mice (Ma et al. 1999). AQP5 is shuttled from intracellular vesicles to the apical membrane within seconds in response to pilocarpine and adrenaline (Matsuzaki et al. 1999; Ishikawa & Ishida, 2000) in a process that is dependent on [Ca2+]i and [Ca2+]e. It should be noted that there are many similarities between the mouse and tick salivary gland: pilocarpine and adrenaline cause salivation in tick glands and this is dependent on Ca2+.
We investigated evidence of a role for an AQP in Ixodes ricinus salivary glands (S. Whitlelaw & A. S. Bowman, unpublished observations). Using the ligated salivary duct technique, the dopamine-stimulated secretion in I. ricinus salivary glands was completely inhibited by co-incubation with 10 μM HgCl2 (Fig. 4A), but there was no difference in the adenylate cyclase activity in these glands (data not shown) indicating the inhibition of secretion by Hg2+ was not due to non-specific toxicity. Further, incubation of the Hg2+-treated glands with 50 μM β-mercaptoethanol rescued their ability to secrete (Fig. 4B). Our results suggested that an Hg2+-sensitive AQP might be involved in tick salivary gland water transport.

Fig. 4. Evidence for the role of a mercurial-sensitive aquaporin in fluid uptake by I. ricinus salivary glands. (A) Ligated salivary glands were incubated in either media (control), or media supplemented with 10 μM dopamine, or media supplemented with 10 μM dopamine and 10 μM mercuric chloride. Uptake of fluid was assessed gravimetrically, as described for Fig. 3. Mean±S.E.M., n=9. (B) Ligated glands were incubated in media containing 10 μM mercuric chloride for 10 min. Half the glands were transferred to media containing 10 μM dopamine with 10 μM mercuric chloride. The other half were transferred to media containing 10 μM dopamine with 10 μM mercuric chloride and 50 μM β-mercaptoethanol. Uptake of fluid was assessed gravimetrically, as described for Fig. 3. Mean±S.E.M., n=4.
In December 2003, results of an EST project (18400 ESTs) on the salivary glands of Rhipicephalus appendicluatus became available on the TIGR website. Two contigs (TC77 and TC1970) have high homology to aquaglyceroporins (AQP3, 7 & 9) from vertebrates at the protein level (e.g. TC77 is homologous to mouse AQP9 (35% identity, 53% similarity) and TC1970 is homologous to human AQP3 (36% identity, 55% similarity). However, though TC77 and TC1970 are similar, they are definitely different gene products with only 38% identity between each other. Topological analysis of TC77 and TC1970 reveals 6 transmembrane-spanning domains and intracellular NH2- and COOH-termini like all AQPs and the NAPRD sequence of the aquaglyceroporins. While TC77 contains two NPA motifs that form the water-pore, TC1970 contains one NPA motif, but has an isoleucine substitution for the alanine (i.e. NPI) in the first motif. This is an extremely exciting and unusual finding. Both TC77 and TC1970 possess a cysteine five amino acids upstream of the second NPA motif that is likely to lead to inhibition by Hg2+ and may explain our findings of the Hg2+-sensitivity in our secretion studies in I. ricinus (Fig. 4). Additionally, TC77 contains a phosphorylation sequence (P=0·997) within the COOH-terminus (serine residue # 274) that could possibly be involved in translocation, as in AQP2 translocation (serine residue # 256). Further investigation of aquaporins in tick salivary glands should prove fruitful in our attempts to better understand the movement of water and other non-polar solutes into the saliva.
Prostaglandins (PGs) are an important group of biologically active lipid molecules in mammals (Smith, 1992) and invertebrates (Stanley-Samuelson, 1994). Tick saliva and salivary glands contain unusually high amounts of PGE2 and PGF2α. PGs of the two-series i.e. PGE2, PGD2, PGF2α, PGI2 are derived from the polyunsaturated fatty acid, arachidonate (AA; 20[ratio ]4, n-6). AA constitutes approximately 8% of the fatty acids in salivary glands of partially-fed female A. americanum increasing from about 2% in unfed ticks (Shipley et al. 1993a) and increasing more than any other fatty acid (Shipley et al. 1993b). The increase in AA content in the salivary glands is not only a reflection of the AA in the blood meal because the amount in salivary glands is far higher than other internal tick tissues (Bowman et al. 1993). Most animals are capable of synthesizing AA from essential fatty acid linoleate by a series of desaturation and elongation reactions. However, female A. americanum have an extremely degenerate fatty acid synthesis capability lacking the ability to desaturate or elongate fatty acids with more than one double bond (Bowman et al. 1995a), though they do possess a stearoyl CoA desaturase gene (Luo et al. 1997). All fatty acids in the salivary glands with more than one double bond were shown to be sequestered from the host blood meal. Radio-labeled AA when fed to A. americanum via a capillary tube was incorporated only into phosphatidylcholine and phosphatidylethonolamine in a ratio that was different from other fatty acids (Bowman et al. 1995b).
AA is released by activity of an intracellular phospholipase A2 (PLA2). Treatment of isolated salivary glands with Ca2+ ionophore A23187 increased the level of free AA (Bowman et al. 1995c). A23187 increases free levels of intracellular Ca2+ and, typically, cytosolic PLA2s are stimulated by Ca2+. The increase in free AA was reduced in a dose-dependent manner by the PLA2 inhibitor oleyloxyethylphosphorylcholine indicating that the increased free AA was dependent upon the activity of PLA2 (Bowman et al. 1995c). Dopamine was also shown to increase free AA and its ability to do so was inhibited by verapamil suggesting that dopamine stimulates PLA2 through the opening of a voltage-dependent Ca2+ channel. One of the difficulties associated with studying PGs in tick salivary glands has been the problem of showing biosynthesis of PGs by isolated salivary glands despite inordinately high levels in saliva (Bowman et al. 1995d; Pedibhotla, Sauer & Stanley-Samuelson, 1997) and the high levels of AA in the salivary glands. However, PG synthesis was shown in whole ticks when dopamine-induced saliva collected from ticks fed [3H]-AA was shown to contain radiolabelled PGs (Bowman et al. 1995d). The attempts to demonstrate synthesis in vitro used a labeled substrate and standard incubation conditions employed in mammalian tissues. Robust synthesis by isolated salivary glands was demonstrated at high concentrations of exogenous AA (Aljamali et al. 2002) that previously had not been employed (Bowman et al. 1995d; Pedibhotla et al. 1997). The reasons why a high concentration is required are unknown but several possibilities exist to explain the results. Salivary glands have a very high capacity for the uptake and incorporation of AA into phospholipids by isolated salivary glands (Bowman et al. 1995d; Madden et al. 1996). This attribute is consistent with the tick's ability to efficiently sequester AA from the host bloodmeal. This esterification system can be overcome with high media levels of AA (>100 μM) leading to higher levels of free AA that could enter the PG synthase pathway. Another possibility is the PG synthase pathway in tick salivary glands has a low affinity for AA or it may preferentially use endogenous AA as it is released via PLA2 from the phospholipid fraction where it is stored.
As noted, the saliva of several tick species contains extremely high levels of PGs and much higher levels than that found in mammalian inflammatory exudates (Bowman, Dillwith & Sauer, 1996). PGs of the 2-series exhibit anti-haemostatic, vasodilatory, immuno-suppressive and anti-inflammatory activities but the exact functions of PGs in tick saliva are presumed and await further study (Bowman et al. 1996). In contrast, much is known about the physiology of PGE2 in tick salivary gland secretion (Sauer et al. 2000).
Incubation of isolated salivary glands of partially-fed female A. americanum with the PLA2 inhibitor oleyloxyethylphosphorylcholine (OPC) reduced dopamine-induced fluid secretion and cAMP levels in the glands (Qian et al. 1997). Inhibition of secretion by OPC was reversed by PGE2 and its analogue, 17-phenyl-trinor PGE2. The results suggested that PGE2 has an autocrine or paracrine effect in modulating tick salivary gland secretion. This hypothesis was confirmed by identification of a PGE2-specific receptor in the plasma membrane fraction that exhibits a single, high affinity PGE2-binding site that is saturable, reversible, specific for PGE2 and coupled to a cholera toxin-sensitive guanine nucleotide regulatory protein (Qian et al. 1997). PGE2 does not stimulate adenylate cyclase activity in isolated salivary gland membranes (Qian et al. 1998) suggesting that the noted PGE2 effects on cAMP levels and fluid secretion are indirect, likely through its ability to mobilize intracellular Ca2+. PGE2 stimulates an increase in intracellular inostitol trisphosphate (IP3) and stimulates an efflux of Ca2+ from pre-labeled salivary gland acini (Qian et al. 1998). Protein secretion from dispersed salivary gland acini was shown to be specific for PGE2, as compared to PGF2α or the thromboxane analogue U-46619, in accordance with their binding affinities for the PGE2 receptor (Yuan et al. 2000). In mammals, PGE2 receptors are classified into four subtypes: EP1, EP2, EP3, EP4 (Coleman et al. 1990; Negishi, Sugimoto & Ichikawa, 1993). EP1 receptors mobilize Ca2+ and the other receptors affect adenylate cyclase (Coleman et al. 1990). It appears that the PGE2 receptor in tick salivary glands is EP1-like. The mammalian PGE2 EP1 receptor agonist 17-phenyl trinor PGE2 was as effective as PGE2 in stimulating secretion of anticoagulant protein by dispersed salivary glands (Yuan et al. 2000). This finding is consistent with the previous finding that 17-phenyl trinor PGE2 partially reverses the small inhibition of dopamine-stimulated salivary gland fluid secretion by PLA2 inhibitor OPC. PGE2 and the non-hydrolyzable analogue of GTP, GTPγS were shown to activate phospholipase C (PLC) directly in a membrane-enriched fraction of the salivary glands (Yuan et al. 2000). TMB-8, an inhibitor of IP3 receptors on the endoplasmic reticulum, inhibited PGE2-stimulated secretion of anticoagulant protein. Overall, the results support the hypothesis that PGE2 stimulates secretion of tick salivary gland protein via a phosphoinositide signaling pathway and mobilization of intracellular Ca2+ (Yuan et al. 2000).
A model was proposed whereby dopamine released at the neuro-effector junction binds to a dopamine, D1-like receptor on the salivary glands to effect fluid secretion (Sauer et al. 2000). The dopamine receptor is coupled to a G-protein to activate adenylate cyclase and increase cAMP. The increase in cAMP controls fluid secretion (Sauer et al. 1986). Dopamine also opens a voltage-gated Ca2+ channel allowing an influx of extracellular Ca2+ to stimulate a cytosolic PLA2 to liberate free AA. The free AA liberated is converted by the cyclooxygenase pathway to PGE2 and PGF2α and possibly small amounts of PGI2 (Aljamali et al. 2002). High levels of PGE2 and PGF2α are secreted into the host via saliva (Ribeiro et al. 1992; Aljamali et al. 2002). In addition, PGE2 interacts with an EP1-like receptor coupled to a G-protein to activate PLC and increase intracellular IP3 (Qian et al. 1997, 1998; Yuan et al. 2000). The increased Ca2+ mobilized by IP3 regulates exocytosis of anticoagulant protein from secretory vesicles into the saliva.
The mechanism for intracellular trafficking of secretory vesicles and exocytosis are complex; however, highly conserved SNARE complex proteins have been identified in all neuronal and non-neuronal secretory cells studied. SNARE terminology is based upon the requirement of N-ethylmaleimide [NEM-sensitive fusion protein (NSF)] and soluble NSF attachment proteins (SNAPs) involvement in the process. SNAREs are defined as the receptors for SNAPs. SNARE proteins associated with vesicles are referred to as v-SNAREs and those associated with the plasma membrane as t-SNAREs. NSF uses energy from ATP hydrolysis to dissociate SNARE complexes after membrane fusion, enabling SNARE proteins to be recycled for subsequent rounds of fusion (May, Whiteheart & Weis, 2001). GTP-binding proteins (e.g. Rab 3A) are localized to the cytosolic face of specific intracellular secretory vesicle membranes where they are thought to function in vesicle trafficking. Additional components exist that inhibit fusion of vesicle and plasma membranes until the cell receives an appropriate signal, typically a rise in intracellular Ca2+. A major Ca2+-signaling protein is synaptotagmin (Geppert et al. 1994). Proteins in the salivary glands of partially-fed female lone star ticks cross-react individually with antibodies to SNARE complex proteins synaptobrevin-2, syntaxin-1A, syntaxin-2 and SNAP-25, synaptotagmin, Rab 3A and nSec1 (Karim et al. 2002). Significantly, antibodies to these SNARE complex proteins inhibit PGE2-stimulated secretion of anticoagulant protein in permeabilized tick salivary glands (Karim et al. 2002). Further, employing the technique of double-stranded RNA interference to target and ‘knock-down’ the v-SNARE protein synaptobrevin, PGE2-stimulated protein secretion was inhibited in isolated A. americanum salivary glands (Karim et al. 2004). The results strongly suggest that SNARE and cell trafficking regulatory proteins are present and functioning in the process of PGE2-stimulated, Ca2+-regulated protein secretion in tick salivary glands. The control and mechanism of vesicle transport are likely important for pathogen transmission. Lyme disease spirochetes are believed to move across cells by a transcytotic mechanism, entering by endocytosis, exiting by exocytosis (Hechemy et al. 1992; Kurtti et al. 1994). It seems likely that pathogen delivery to the host may be facilitated by the tick's mechanisms of transport.
The ability of unfed ticks to survive prolonged periods without feeding is a hallmark of tick biology and an attribute that is assisted by the tick's ability to absorb water from unsaturated air (Needham & Teel, 1991). The lowest relative humidity (RH) at which water vapour uptake is possible in ticks and a few other arthropods is defined by the critical equilibrium humidity (CEH). In most unfed ticks the CEH is in the range of 85 to 90% RH. In a simple but elegant study, Rudolph & Knulle (1974) identified the mouth as the site of water vapour uptake and the salivary glands as a likely source of hygroscopic material secreted into the oral cavity. Fluid in the salivarium of re-hydrating A. amercianum females was shown to have a melting point of −10 to −12 °C, a solution whose water molecules would be in equilibrium at an RH of ≈90% (Sigal, Needham & Machin, 1991). These authors postulated that as ticks become dehydrated, the type I acini secrete salts from increasingly concentrated haemolymph onto the mouthparts until rehydration and subsequent re-ingestion is possible. The type I acinus is likely the source of the hygroscopic material. Megaw & Beadle (1979) compared the ultrastructural features of the ‘pyramidal’ cells to cells found in nasal salt glands of birds and reptiles. Type I acini are found in argasids and ixodids and in larvae, nymphs and adults all of which possess the ability to take water from unsaturated air. Rudolph & Knulle (1979) noted that heavy infestations of the protozoan Theileria annulata damaged the acini types II and III of Hyalomma anatolicum and left type I intact. Infected ticks were still capable of taking up water at 93% RH. More recently, the wholly solute-driven mechanism for explaining water vapour uptake was questioned by Gaede & Knulle (1997). The authors demonstrated that water from unsaturated air condenses on the hydrophilic cuticle on the hypostome of A. variegatum. The authors hypothesized that only a slightly hyperosmotic secretion from type I acini would be sufficient to change the water affinity at the adsorbing cuticle surface and release adsorbed water. The solution would then be drawn into the mouth by suction of the pharynx. The authors further suggested that another mechanism occurs in unfed ticks with a large water deficit. Here type I acini secrete ions which are removed from the haemolymph and produce hyperosmotic secretion (consistent with storage excretion as proposed by Needham & Teel, 1986). A crystalline substance remains and when the humidity increases above the critical equilibrium humidity, the substance dissolves and is ingested, providing another means for recovering lost water (Gaede & Knulle, 1997).
The salivary glands play a critical role in pathogen transmission at several levels. Factors in the saliva or salivary gland homogenate are known to greatly enhance pathogen transmission and establishment and are discussed in detail in the chapter in this Supplement by Nuttall & Labuda. For many pathogens the salivary gland is the site of development and replication, see the following chapters in this Supplement for details on specific pathogens: Anaplasma marginale (Kocan et al.); Theileria spp. (Bishop et al.); Babesia spp. (Bock et al.) and Borrelia burgdorferi (Piesman & Gern). For some pathogens (e.g. viruses), there appears to be little cell specificity either within the salivary glands or the tick body per se. Conversely, some protozoan pathogens have high specificity. For example, Theileria annulata only infects cell types e of type III acini – the mechanism or function of this high specificity is unknown, but elucidating the pathogen–gland ligands may well uncover effective transmission blocking targets. In this respect, research on tick salivary glands would greatly benefit from the development of methods for specific cell-type isolation or cell lines.
Recently, the application of genetic manipulation of tick-borne pathogens for the characterization of ligand-receptor interactions at the arthropod-pathogen interface was reported Pal et al. (2004) and powerfully demonstrated what is now possible in this field. The outer surface protein C (OspC) of Borrelia burgdorferi sensu stricto bound strongly to I. scapularis salivary gland both in an ELISA-like assay using gland homogenate and to whole glands. An OspC-deficient mutant B. burgdorferi did not bind to the salivary glands, but the OspC-deficient strain reconstituted to contain a plasmid encoding wild-type OspC did bind to salivary glands. It was shown in vivo that OspC was required for invasion of the salivary gland and subsequent transmission to mice. Further, Pal et al. (2004) demonstrated that IgG F(ab2) fragments to OspC were effective in blocking B. burgdorferi colonization of the salivary glands. Though not performed, one would anticipate that antibodies directed to the salivary gland ligand binding OspC would also effectively block entry of Borrelia into the glands. This study demonstrates both the intricacy and specificity of the interaction between the pathogen and the salivary gland and also the potential for interrupting these receptor–ligand interactions to block transmission once either receptor or ligand is identified.
Tick salivary glands have been studied by various groups worldwide for over 40 years reflecting the huge importance of this organ in the transmission of various pathogens to humans and domestic animals. With the information garnered over this period as a platform, what are the likely fruitful areas and future directions in tick salivary gland research? Undoubtedly, the great advances in molecular biology technologies will a have tremendous effect on our understanding of tick salivary gland physiology. There have been several expressed sequence tag (EST) projects on salivary glands of various tick species: Boophilus microplus (Crampton et al. 1998); Ambylomma americanum (Hill & Guitierrez, 2000; Bior et al. 2002); A. variegatum (Nene et al. 2002); Ixodes scapularis (Valenzuela et al. 2002). The largest publicly available salivary gland EST database is that for Rhipicephalus appendiculatus hosted on The Institiute for Genome Research's (TIGR) website (http://www.tigr.org). This project sequenced ~18500 ESTs derived from 4-day fed female salivary glands and yielded ~7350 unique transcripts. The primary reason for these salivary gland projects was driven by the interest in the secreted components of the salivary gland transcriptome that allow ticks to overcome host defence mechanisms and to obtain the prolonged blood-meal (see chapter by Valenzuela in this Supplement). However, these EST projects also yield important sequence information on genes involved in salivary gland cell biology. Now we have so much salivary gland sequence information available that we can already look forward to the ‘post-genomic era’ and the era of ‘functional genomics’. It is here where recently acquired sequence data and the information on salivary gland physiology and cell biology can be brought together to great synergistic benefit. The new sequence information can fill in gaps where anticipated components were implicated by previous functional assays. An example would be the finding of an aquaporin sequence in the R. appendiculatus EST project. A tick salivary gland aquaporin had been previously predicted in a model put forward by Sauer et al. (2000) and functional data had been acquired for a role of an aquaporin in fluid secretion by I. ricinus salivary glands (see above and Fig. 4A,B).
It is possible to suppress or ‘knock-down’ the expression of selected genes in tick salivary glands both in vivo and in isolated glands by the technique of double-stranded RNA interference (dsRNAi). Whilst tick researchers do not have the luxury of gene knock-out strains of the Drosophila and C. elegans research communities, the dsRNAi technique does allow us to assess the importance of the gene in question in these ‘phenotypes’. The first studies using dsRNAi in tick salivary glands were on the secreted salivary components a histamine-binding protein (Aljamali et al. 2003) and an anticoagulant (Narasimhan et al. 2004), but the mechanism of PGE2-stimulated protein exocytosis has also been studied by dsRNAi knock-down of a synaptobrevin homologue (Karim et al. 2004). It is very likely that the dsRNAi knock-down approach will be extensively employed in tick salivary gland research in the future. As we look to the salivary gland as a potential ‘Achilles' heel’ for acaricide development, the importance, or otherwise, of postulated targets or the existence of unforeseen salvage pathways could be assessed by dsRNAi experiments. Likewise, development of potential vaccines able to block the binding and uptake of pathogens to the salivary gland can be tested by dsRNAi knock-down of the ligand-receptor.
It must be noted that despite the many tick salivary gland ESTs sequenced (probably approaching 40000 ESTs), there will still be a large requirement for standard gene hunting and identification studies for specific genes of interest. For example, throughout the available tick salivary gland EST databases there are extremely few G-protein-coupled receptors identified or transcripts that contain any of the characteristic motifs. Specifically, the dopamine receptor and PGE2 receptor still remain elusive. Potentially rare transcripts such as the receptors might not be expected to be sequenced in EST projects, but it is surprising that no prostaglandin synthase (or cyclooxygenase) gene has been identified in the EST projects when one considers the exceedingly high quantities of prostaglandin detected in the saliva or salivary gland homogenate. We have tried several approaches to clone and sequence the dopamine receptor and prostaglandin synthase without success. It is feasible that these gene sequences are already in the EST databases, but are so different from the expected sequences that the current bioinformatic programmes do not recognize them as such. This would explain the difficulty of homology cloning we have encountered. Whilst this is frustrating on one hand, it would indicate that such divergent genes might prove attractive acaracide targets, as well proving academically interesting.
Overall, the explosion of tick salivary gland transcript sequence information that has become available over the past 5 years can be combined with fundamental information about tick salivary gland physiology that has been gained over the past 40 years in a synergistic manner. The glands are vital to tick survival both on and off the host and are critical to pathogen development and transmission and, thus, present a target for acaracide development and pathogen transmission-blocking vaccines. The future for tick salivary gland research will now move into functional genomics utilizing the available sequence data and techniques such as dsRNAi to elucidate critical pathways and promising targets. It would appear that we are entering perhaps the most exciting time in tick salivary gland physiology in which tremendous advances are within our grasp.
This work was supported in part by the Leverhulme Trust (grant # F/00152/C to ASB) and the National Science Foundation (grant # IBN 9974299 to JRS). We also thank the Wellcome Trust for a Vacation Scholarship (grant to ASB) to fund Sandra Whitelaw's aquaporin work.
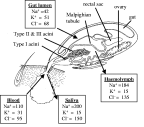
Fig. 1. Schematic of a feeding adult female tick (Amblyomma americanum) illustrating the relative position and size of the internal organs. The racemose-like structure of the salivary gland show the small Type I acini that are involved in off-host osmoregulation and the larger, more posterior, Type II and III acini that are involved in on-host osmoregulation, are the site of synthesis and secretion of protein and lipid factors (anticoagulants, immunosuppressants etc.) and are the site of pathogen development and replication. A male tick is depicted on the underside of the female in copula, a process that takes about 48 h as the spermatophore is inserted into the female genital pore by the male's mouthparts and involves copious salivation from the male-specific type IV acini (Feldman-Muhsam, Borut & Saliternik-Givant, 1970). The boxes indicate the composition of the main ions (units millimolar equivalents) in the various compartments as they are imbibed from the blood into the gut lumen, pass from the gut into the haemolymph and, finally, through the salivary gland back into the host in the saliva (data from Hsu & Sauer, 1975).

Fig. 2. Arrangement of the three alveoli types of the ixodid tick female salivary gland (adapted from Binnington, 1978). The alveolus types are distinguished by differential shading. Type I alveoli attach directly to the main salivary duct and to some principal branches of that duct. A diagrammatic section of a type I alveolus with the cell types is shown above (adapted from Barker et al. 1984). Type II alveoli are more abundant in the proximal section and type III are more abundant in the distal section of the large branches. A schematic representation of the structure of the type III alveolus and cell types from an unfed tick is depicted below (from Fawcett et al. 1986, with permission) The types II and IV alveoli have a similar organization to type III alveoli but different cell types. Type IV alveoli are found only in males. Type II and especially type III undergo remarkable and complex cytological transformation to meet the tick's changing physiological requirements (including fluid transport) during tick feeding (see Fawcett et al. 1986). Nerve supply and tracheoles to the gland are not shown. (Artwork by Kerry Stricker, Oklahoma State University.) (Reproduced from Sauer et al. (1996) with permission from CABI International.)

Fig. 3. Demonstration of dopamine-stimulated fluid uptake by partially-fed female Ixodes ricinus salivary glands and involvement of type III acini. (A) The main salivary duct of isolated glands were ligated and the glands incubated in media (control) or media supplemented with 10 μM dopamine. The change in weight was recorded on an analytical balance at 6 min intervals (Mean±S.E.M., n=4). Control glands were subjected to dopamine stimulation at 18 min (arrow) to check viability of these glands. (B) These ligated glands were then fixed (1% osmium tetroxide), processed, and sectioned (semi-thin, 0·5 μm), stained with toluidine blue. The diameter of the acini and the lumen were determined using Image-Pro Plus (Media Cybernetics). No difference was observed between treatments for the total acini size (P>0·05), but the acini lumen were greater (P<0·05) following dopamine treatment (Mean±S.E.M., n=30). (C) Type III acini in the ligated control glands show no or small lumens. A type II acini with its more prominent secretory granules is also visible in this section. (D) The type III acini of dopamine-stimulated glands show large fluid filled lumens with the acini cells pushed back towards the basal lamima. In both C and D, scale bar=55 μm.

Fig. 4. Evidence for the role of a mercurial-sensitive aquaporin in fluid uptake by I. ricinus salivary glands. (A) Ligated salivary glands were incubated in either media (control), or media supplemented with 10 μM dopamine, or media supplemented with 10 μM dopamine and 10 μM mercuric chloride. Uptake of fluid was assessed gravimetrically, as described for Fig. 3. Mean±S.E.M., n=9. (B) Ligated glands were incubated in media containing 10 μM mercuric chloride for 10 min. Half the glands were transferred to media containing 10 μM dopamine with 10 μM mercuric chloride. The other half were transferred to media containing 10 μM dopamine with 10 μM mercuric chloride and 50 μM β-mercaptoethanol. Uptake of fluid was assessed gravimetrically, as described for Fig. 3. Mean±S.E.M., n=4.