INTRODUCTION
The successful transmission of trematodes from one host to another is a fundamental process in their life histories. Almost all species use a mollusc as first intermediate host, in which asexual reproduction takes place resulting in the production of cercariae. Cercariae emerge from the mollusc host and have a brief free-living life span, limited by the depletion of an endogenous non-renewable glycogen store to a few hours or days (Ginetsinskaya, Reference Ginetsinskaya1988).
Cercariae represent a crucial phase in the parasite's life cycle when the majority of species must disperse, locate, identify, and infect a suitable target host. Throughout this period they are exposed to a range of biotic and abiotic factors that can significantly influence the duration of survival and consequently change the dynamics of the parasite population. Temperature is probably the most important abiotic climatic factor that can affect trematodes during this important phase of the transmission cycle. It directly affects cercarial metabolism and activity with the rate of glycogen utilization varying with the temperature of the surrounding water medium, increasing at higher temperatures due to more intense parasite movement and hence resulting in elevated mortality (Ginetsinskaya, Reference Ginetsinskaya1960).
There is increasing concern regarding the impact of climate change on the occurrence of parasitic diseases. Climate is known to modulate the extent and intensity of parasitism, affecting the distribution and survival rate of intermediate hosts and vectors, as well as directly influencing the reproduction and maturation rate of the parasites themselves (Mas-Coma et al. Reference Mas-Coma, Valero and Bargues2009). As average temperatures are predicted to rise under climate change, and warmer conditions may be more favourable for parasite transmission, understanding the thermobiology of parasites has become an important goal to allow accurate predictions on the way parasitism may change in the future. However, few comparative studies on the physiological response of different parasite species to temperature changes have been undertaken, particularly for trematodes that are influenced by temperature over a number of stages in their life cycles.
Poulin (Reference Poulin2006) undertook the one major comparative evaluation of trematodes examining thermal effects on cercarial emergence using the Q 10 value, a measure of temperature-controlled reaction rates, at a temperature range of approximately 15–25°C (≈20°C). After analysing experimental data compiled from the scientific literature, Poulin (Reference Poulin2006) concluded that the overall temperature-mediated increases in cercarial output were much more substantial than those expected from basic physiological processes with species from lower latitudes demonstrating more pronounced temperature-driven increases in cercarial output than those from higher latitudes. Nevertheless, Q 10 values at ≈25°C (20–30°C) were generally lower than those found at ≈20°C suggesting that elevated cercarial output was not maintained with continually rising temperatures.
However, not all studies on cercarial emergence correlate with this interpretation. More recent work has suggested a dissimilar impact of temperature changes resulting from global warming. For example, Morley et al. (Reference Morley, Adam and Lewis2007, Reference Morley, Adam and Lewis2010) found that the emergence, survival, and infectivity of Echinoparyphium recurvatum cercariae remained largely unchanged over a temperature range of 17–25°C, which corresponded to typical summer temperatures found in many freshwater habitats in temperate regions. Whilst Koprivnikar and Poulin (Reference Koprivnikar and Poulin2009) found substantial interspecific and intraspecific differences in cercarial emergence from isolated marine habitats with differing summer temperatures.
Indeed, there are many reasons to believe that trematodes as a group have a complex and variable relationship with temperature. For example, some trematode species have peak metabolic activity that did not correlate with that found in their molluscan hosts but instead reflected the body temperature of their individual definitive hosts (Vernberg, Reference Vernberg1963). Furthermore the thermal environment of the molluscan host had little or no influence on the thermal metabolic acclimation pattern of trematode larvae with each species having a distinct pattern of acclimation (Vernberg and Vernberg, Reference Vernberg and Vernberg1965). The ability of emerged cercariae to acclimate to extremely high temperatures has also been demonstrated, although the level of tolerance varied between species. Acclimation for 3 h at 29°C before exposure to 43°C increased the thermal tolerance of cercariae whilst maintenance at 4°C for a similar time-interval significantly reduced heat resistance (Gammermaister, Reference Gammermaister1977).
At more realistic environmental temperatures intriguing thermal responses of cercariae have also been noted. Young et al. (Reference Young, Bundy and Taylor1984) found that survival of the tropical marine cercariae Cercaria caribbea LXXI over a range of temperatures demonstrated a distinct zone of thermostability, which corresponded with normal environmental temperatures in the sampled habitat. However, conventional wisdom with regards to cercarial survival, although acknowledging optimum conditions exists for individual species (Pietrock and Marcogliese, Reference Pietrock and Marcogliese2003), has never widely considered thermostable plateau's, despite their importance for parasite transmission under climate change and their widespread occurrence in other kinds of ectotherms e.g. Wieser (Reference Wieser and Wieser1973).
Young et al. (Reference Young, Bundy and Taylor1984) further demonstrated an empirical relationship between mean expected life span and the half-life of the population (t 0·5) and that the reciprocal of t 0·5 is a good index of glycogen utilization. On this basis the common measures of temperature effects on reaction rates, Q 10 and Arrhenius activation energy (E* or μ), for thermal changes in t 0·5 can be determined, providing an index of temperature responses on cercarial metabolism. The Q 10 is the factor by which a reaction velocity is increased for a rise of 10°C (Prosser, Reference Prosser1973). Higher Q 10 values are often obtained at low experimental temperatures whilst lower values are encountered at high temperatures (Newell, Reference Newell and Wieser1973). The Arrhenius critical incremental energy of activation (E*) is considered the most realistic measure of temperature-driven reaction rates and represents the energy which molecules in their initial state must acquire before they can participate in a chemical reaction. A physiological process depends on a catenary series of reactions, each with its characteristic critical thermal increment. At its simplest level, the rate of the entire process is governed by the slowest reaction in the series, and this is the master reaction. Therefore the E* value for a complex physiological activity is the value of its limiting or pacemaker step and generally ranges from 1–25 Kcal./mole (Hoar, Reference Hoar1983).
The study by Young et al. (Reference Young, Bundy and Taylor1984) represented an important advance on our understanding and interpretation of the effects of temperature on cercariae. Unfortunately this pioneering work was never subsequently taken up and built upon by the scientific community. It therefore still remains to be determined whether thermostability of cercariae is a widespread phenomenon. This could have important implications for evaluating the impact of temperature changes under global warming on the efficiency of trematode transmission.
The aim of the present study was to (1) establish the comparative survival/metabolic response of cercariae to changing temperature from compiled experimental data using the methodology of Young et al. (Reference Young, Bundy and Taylor1984), and (2) determine the extent of cercarial thermostability across a range of species whilst placing these results in the context of global climate change. Data will be evaluated using the common measures of temperature-driven reaction rates (Q 10 and E*).
MATERIALS AND METHODS
Cercarial survival data were obtained from the scientific literature on experimental studies undertaken at different temperatures. A large number of studies of this kind exist; however, in order to effectively determine survival trends over increasing temperatures and identify evidence of thermostability only those investigations that utilized at least 5 temperature readings were used. This produced a more modest number of 16 studies undertaken on 13 species (Table 1). Two species, Schistosoma mansoni and Echinoparyphium recurvatum, had multiple investigations undertaken on them, allowing comparisons for strain variations. Three laboratory strains of S. mansoni had been studied in sufficient detail. These strains were ‘Puerto Rico 1’ (Lawson and Wilson, Reference Lawson and Wilson1980) maintained at the University of York, UK, and derived from a culture kept at the National Institute of Medical Research, UK, which originated from material collected in Puerto Rico during the early 1950s; ‘Puerto Rico 2’ (Sirag and James, Reference Sirag and James1982) maintained at the London School of Tropical Medicine and Hygiene, UK, originating from material collected in Puerto Rico during the mid-1960s; and ‘Tanzania’ (Purnell, Reference Purnell1966) maintained at the East African Institute for Medical Research, Tanzania, originating from material collected in Tanzania during the mid-1960s. Studies with 2 geographical strains of E. recurvatum also existed that utilized naturally infected snails collected from 2 sites in southeast England during the late 1980s- ‘Harting Pond’ (McCarthy, Reference McCarthy1999) and ‘Bushy Park’ (Morley et al. Reference Morley, Adam and Lewis2007).
Table 1. Characteristics of the cercarial species used in the analysis
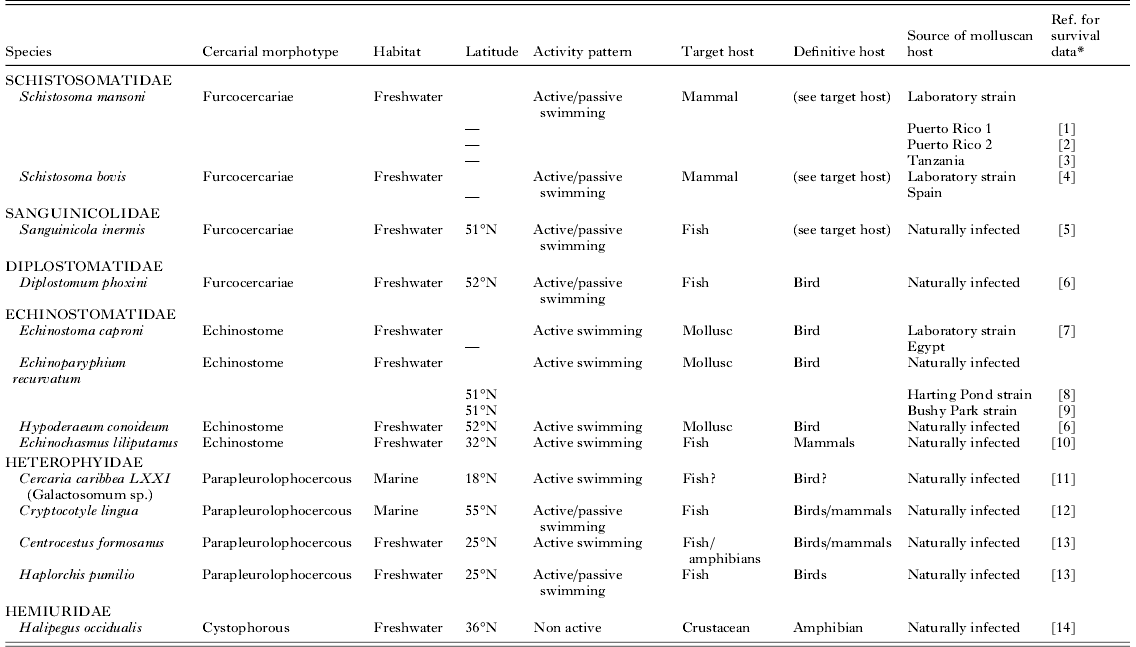
* References [1] Lawson and Wilson (Reference Lawson and Wilson1980), [2] Sirag and James (Reference Sirag and James1982), [3] Purnell (Reference Purnell1966), [4] Ramajo-Martin and Simon-Martin (Reference Ramajo-Martin and Simon-Martin1984), [5] Lee (Reference Lee1990), [6] Harris (Reference Harris1986), [7] Evans (Reference Evans1985), [8] McCarthy (Reference McCarthy1999), [9] Morley et al. (Reference Morley, Adam and Lewis2007), [10] Xiang et al. (Reference Xiang, Cheng Tai, Guo Ren, Tian Ping, Dabing and Wei Duo2000), [11] Young et al. (Reference Young, Bundy and Taylor1984), [12] Möller (Reference Möller1978), [13] Lo and Lee (Reference Lo and Lee1996), [14] Shostack and Esch (Reference Shostak and Esch1990).
Although some variations in experimental protocol existed between the 16 studies they were not considered, in general, to be sufficiently influential to alter the trend of changing survival over a given temperature range. The typical study consisted of isolating recently emerged cercariae from molluscs acclimated to each temperature. Cercariae, either individually or in small groups, were then placed into containers of dechlorinated tap water and survival observed every few hours until all had died.
For the purposes of this investigation only data showing the time to 50% survival (t 0·5) were used, following the study of Young et al. (Reference Young, Bundy and Taylor1984) as a template which demonstrated that the reciprocal of the median survival time (1/t 0·5) in a cercarial population is a useful, though simplistic, measure of the rate of glycogen utilization and can thus be used as an index of temperature effects on the metabolism of cercariae. For comparative purposes the t 0·5 for each species over the studied temperature range was extracted from the original source reference and transformed to give a glycogen utilization rate index as follows-
To determine whether changing temperature substantially altered the rate of survival/metabolism of cercariae, Q 10 values were calculated using the original t 0·5 data for a range of temperatures that approximately encompassed temperature increases of roughly 10°C as follows: 5–15°C (≈10°C), 10–20°C (≈15°C), 15–25°C (≈20°C), 20–30°C (≈25°C), and 25–35°C (≈30°C). The Q 10 was calculated using the following form of the van't Hoff equation (Randall et al. Reference Randall, Burggren and French2001):
where n 1 and n 2 are (t 0·5−1)1 and (t 0·5−1)2 at temperatures t 1 and t 2 respectively. Q 10 values ranging between 2 and 3 are typically the norm and are indicative of a doubling or tripling of physiological rates per 10°C increase in temperature (Prosser, Reference Prosser1973; Randall et al. Reference Randall, Burggren and French2001). A value between 1 and 2 indicates little change, whereas less than 1 indicates a reduced rate. In general, Q 10 values of approximately 2–3 are usually encountered by ectotherms over the normal environmental temperature range of the organism.
The data were then subsequently analysed to determine the critical incremental energy of activation (E* or μ) over the same range of temperatures using the following form of the Arrhenius equation (Prosser, Reference Prosser1973):

where K 1 and K 2 are (t 0·5 –1)1 and (t 0·5−1)2 at absolute temperatures T 1 and T 2, and R is the gas constant (1·98 cal/mole). For many enzymatic and biological processes in living organisms E* values usually range from 1–25 Kcal./mole. Normal activation energy is approximately 10 Kcal./mole with many respiratory metabolic processes having values typically of 11 or 16 Kcal./mole (Crozier, Reference Crozier1924; Brandts, Reference Brandts and Rose1967; Hoar, Reference Hoar1983). Significant differences between Q 10 or E* values at each temperature range were analysed using Student's t-test.
A zone of thermostability in cercarial survival/metabolism was determined to occur where values of Log t 0·5−1 demonstrate a relative plateau over a temperature range that were different from values found at temperatures above and below. This was considered to arise when values changed by less than 0·10 over a 5°C range.
The potential influence of cercarial size on survival/metabolism over the temperature ranges was also assessed. There is a linear relationship between size of cercariae and respiration, smaller cercariae respiring at a higher level (Vernberg and Hunter, Reference Vernberg and Hunter1959). Cercarial size was calculated using the methodology of Poulin and Latham (Reference Poulin and Latham2003). As the tail of the cercariae and its glycogen reserves can play a large part in the survival of the organism (Ginetsinskaya, Reference Ginetsinskaya1960) measurements of both the cercarial bodies and tails were used. Data were obtained primarily from unrelated published sources of morphological descriptions in parasitology journals. Individual morphological information was not available for each of the multiple strains of S. mansoni and E. recurvatum used in this study and consequently data for these two species were derived from single sources.
Measurements of cercarial size recorded from the literature were either means or midpoints of ranges based on the examinations of the lengths and widths (μm) of both bodies and tails of numerous individuals. As trematodes are flat, the best measure of their size is their surface area (Poulin and Latham, Reference Poulin and Latham2003). This was obtained for both bodies and tails using the formula for the surface area of an ellipse (Poulin and Latham, Reference Poulin and Latham2003):
where L and W are the length and width of either bodies or tails respectively. For tails which bifurcate, measurements of the tail trunk and furcae were separately calculated before being combined to give a single measure of tail size.
All variables were log-transformed before use and analysed using Spearman's rank correlation coefficient with cercarial bodies, tails, or a combination of the two to give a total size, compared with Q 10 or E* values. Analysis was undertaken at ≈15°C, ≈20°C, ≈25°C, and ≈30°C using SPSS computer program.
RESULTS
Changes in temperature resulted in alterations in the survival/metabolism of cercariae. The nature and extent of modifications in cercarial responses to temperature varied between species. Nevertheless some general trends are apparent. A relatively ‘linear’ relationship of increasing cercarial mortality and glycogen utilization rate with temperature was demonstrated by Echinostoma caproni, Diplostomum phoxini, Hypoderaeum conoideum, Halipegus occidualis, and Schistosoma mansoni ‘Puerto Rico 1’ strain (Figs 1 and 4A). In contrast, a number of species have a ‘curved’ relationship of the glycogen utilization rate with elevated mortalities at low temperatures, survival rates increasing as the temperature is raised before peaking and subsequently declining at higher temperatures. These species include Sanguinicola inermis, Centrocestus formosanus, Haplorchis pumilio, and Cryptocotyle lingua (Fig. 2). Examination of the various attributes of these two groups (Table 1) revealed no apparent correlation between cercarial morphotype, activity pattern or host utilization for those demonstrating a ‘linear’ temperature relationship. In contrast, all species having a ‘curved’ temperature relationship had fish as the target host whilst 3 of the 4 species were of the parapleurolophocercous morphotype (Table 1).
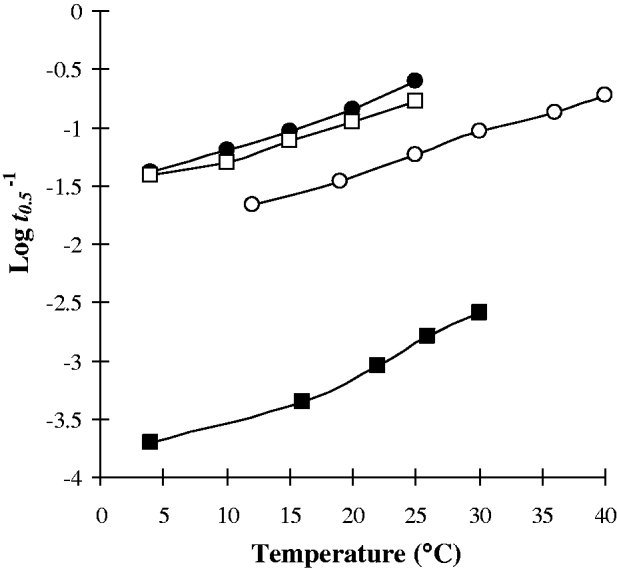
Fig. 1. Cercarial species demonstrating a ‘linear’ relationship of the glycogen utilization index (Log t 0·5−1) over temperature (○, E. caproni; ●, D. phoxini; □, H. conoideum; ▪, H. occidualis).
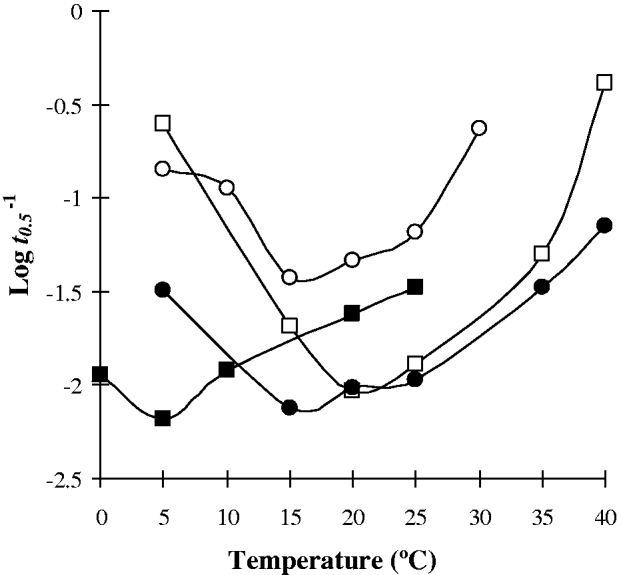
Fig. 2. Cercarial species demonstrating a ‘curved’ relationship of the glycogen utilization index (Log t 0·5−1) over temperature (○, S. inermis; ●, C. formosanus; □, H. pumilio; ▪, C. lingua).
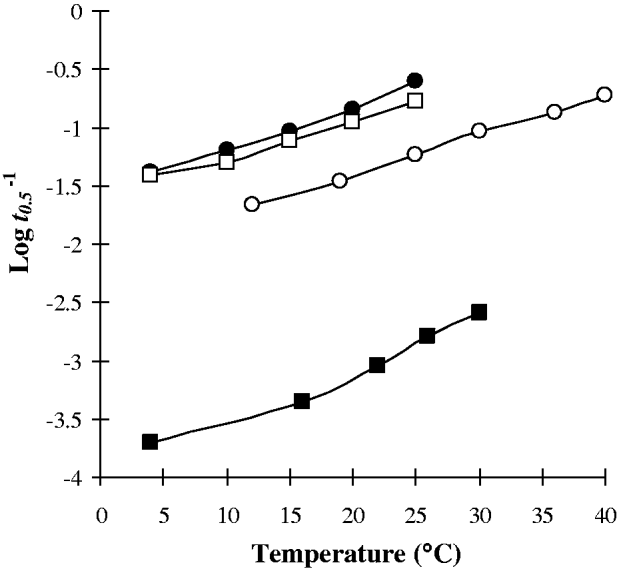
A third cercarial group also showed a reduced survival and increased glycogen utilization rate with rising temperature but had a thermostable zone at some point over the studied temperature range. Thermostability occurs with Schistosoma bovis, S. mansoni (‘Puerto Rico 2’ strain), C. caribbea LXXI, Echinochasmus lilputanus, Echinoparyphium recurvatum (‘Harting Pond’ and ‘Bushy Park’ strains), as well as with C. formosanus from the ‘curved’ relationship group (Figs 2 and 3). Thus 7 of the 16 studies examined had cercariae that demonstrated some degree of thermostability. There appeared to be no obvious relationship between the attributes of these species (Table 1) and their ability to demonstrate thermostability.

Fig. 3. Cercarial species demonstrating a zone of thermostability of the glycogen utilization rate index (Log t 0·5−1) over temperature (▲, S. bovis; ○, C. caribbea LXXI; ▪, E. liliputanus).
In addition one further study on S. mansoni (‘Tanzania’ strain) showed a variable but relatively stable glycogen utilization rate over the temperature range 18–27°C (Fig. 4A). No precise thermostable zone could be attributed to this study; nevertheless the variations may be associated with the age of this work and the relatively unsophisticated techniques possibly used in comparison to the more recent studies.

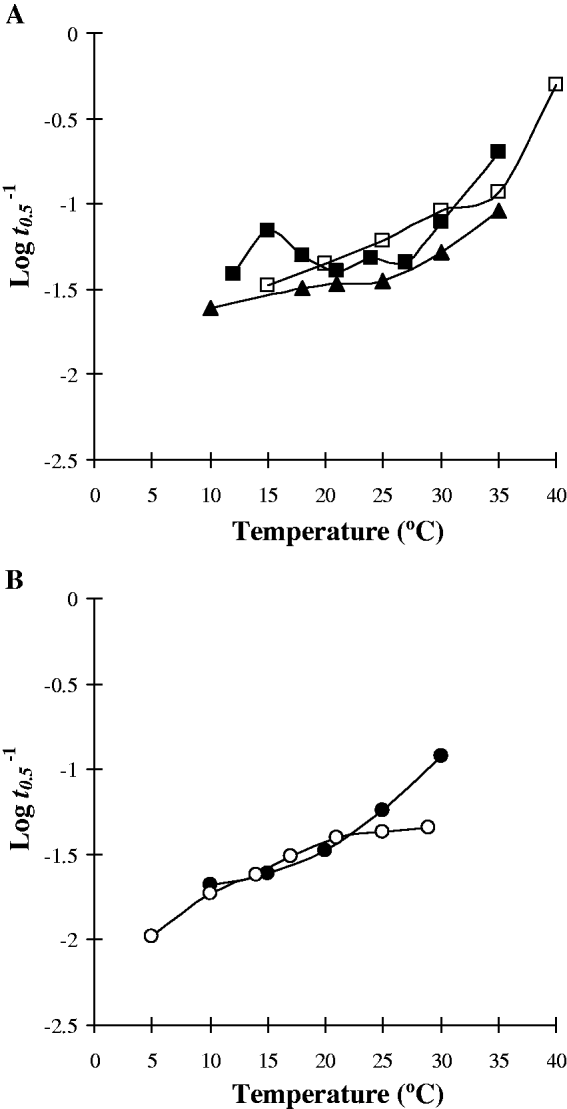
Fig. 4. Strains of the same cercarial species demonstrating different changes in the glycogen utilization rate index (Log t 0·5−1) over temperature. (A) S. mansoni (□, ‘Puerto Rico 1’; ▲, ‘Puerto Rico 2’; ▪, ‘Tanzania’). (B) E. recurvatum (●, ‘Harting Pond’; ○, ‘Bushy Park’).
Examination of the cercarial glycogen utilization rates of the 3 laboratory strains of S. mansoni and the 2 natural strains of E. recurvatum revealed many marked differences (Fig. 4). Each of the strains of S. mansoni demonstrated an individualistic response to increasing temperature (Fig. 4A) as previously described. The 2 strains of E. recurvatum showed similar increases in mortality and glycogen utilization rate with temperature in the range 10–21°C but diverged markedly at higher temperatures (Fig. 4B). Although both strains demonstrated thermostability they occurred at different temperature ranges. ‘Harting Pond’ strain showed greater stability at lower temperatures, being stable between 10 and 15°C. In contrast, ‘Bushy Park’ strain had stability at higher temperatures with a thermostable zone at 20–25°C.
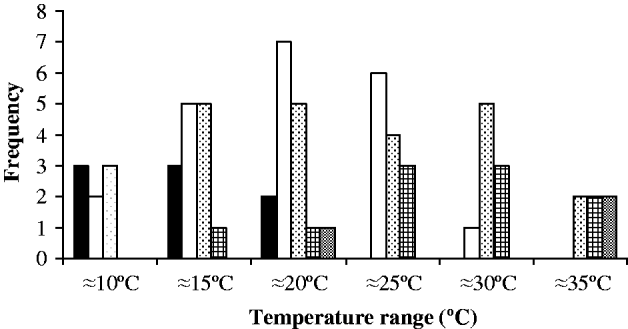
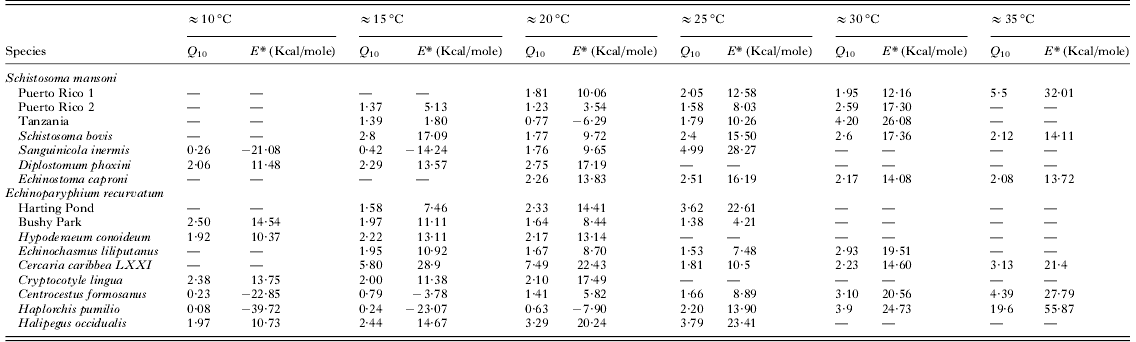
Values of Q 10 and E* over 10°C ranges for each cercarial species are shown in Table 2. and demonstrate no major variations over the majority of temperature ranges. There were no significant differences in any of the Q 10 values compared between ranges ≈15°C and ≈30°C. At extreme temperature ranges some differences were apparent (two tailed t-test, ≈10°C−≈30°C t=−3·232, P=0·006; ≈35°C−≈15°C t=−2·305, P=0·033, ≈35°C−≈20°C t=−2·272, P=0·034) although the limited numbers of species available for analysis at these temperature ranges makes these differences not particularly robust. The frequency of Q 10 values is shown in Fig. 5 and demonstrates that the majority of species at ≈20°C and ≈25°C had values of 1–2, indicative of relatively stable physiological rates. Nevertheless, between ≈15°C and ≈30°C many other species had Q 10 values of 2–3, representing typical normal increases in rates over 10°C ranges. Higher Q 10 values only became more increasingly frequent at ≈25°C and above. Values of E* generally mirror changes in Q 10 over the different temperature ranges (Table 2). There were no significant differences between E* values at each of the temperature ranges between ≈10°C and ≈25°C. However, significant differences occurred between values at ≈15°C or ≈20°C and high temperature ranges of ≈30°C or above (two tailed t-test t⩽ −2·150, P ⩽0·043) as well as values between ≈25°C and ≈35°C (two tailed t-test t=−2·633, P=0·017).

Fig. 5. Frequency distribution of Q 10 values over each studied 10°C temperature range. (Black columns: Q 10 values 0–1, White columns: Q 10 values 1–2, dotted column: Q 10 values 2–3, cross hatch column: Q 10 values 3–5, grey column: Q 10 values +5.)
Table 2. Values of Q 10 and E* over the six temperature ranges for each cercarial species
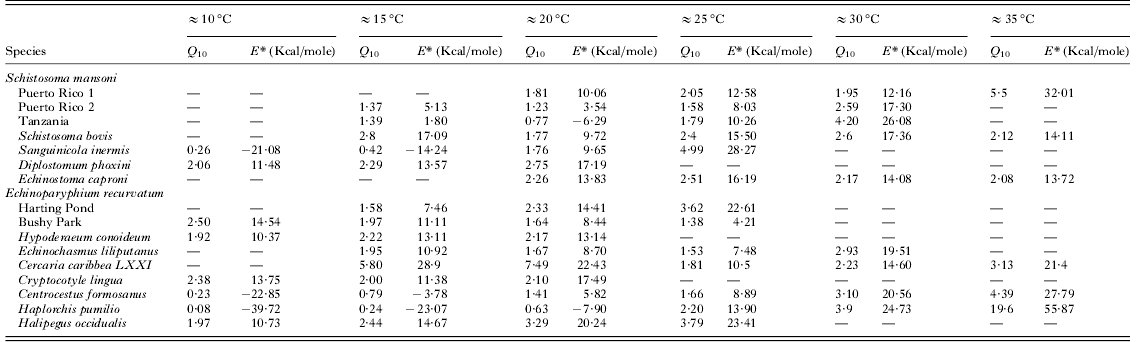
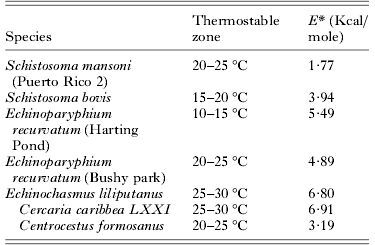
Those species that demonstrated thermostability had low E* values over their thermostable zones ranging from 1·77 to 6·91 Kcal./mole (Table 3). Strain differences were particularly apparent in these thermostable temperature ranges. Schistosoma mansoni (‘Puerto Rico 2’ strain) had an E* value of 1·77 Kcal./mole over 20–25°C (Table 3) compared with 10·51 Kcal./mole for ‘Puerto Rico 1’ and 3·59 Kcal./mole for ‘Tanzania’ strains over the same range. Similarly, whilst E. recurvatum (‘Harting Pond’ strain) demonstrated stability over 10–15°C and had E* values of 5·49 Kcal./mole the ‘Bushy Park’ strain demonstrated an activation energy of 10·38 Kcal./mole over the same temperature range. Whilst at 20–25°C the ‘Bushy Park’ strain was stable with E* values of 4·89 Kcal./mole ‘Harting pond’ showed activation energy of 19·49 Kcal./mole.
Table 3. Values of E* of cercarial species demonstrating thermostability over the relevant stable temperature range
Analysis of the influence of cercarial species size (data not shown) on thermodynamics indicated that there was no significant correlation between the size of cercarial bodies, tails, or whole cercariae and the Q 10 or E* values occurring at any of the temperature ranges, as measured with the Spearman's rank correlation coefficient.
DISCUSSION
Temperature has a substantial effect on cercariae. The present study has utilized species with diverse examples of morphotypes, target hosts, activity, and molluscan sources, allowing a wide range of trematode life histories to be evaluated. Although some caution must be taken when assessing species from laboratory cultures because they can become inbred, resulting in alterations in their functional biology in comparison to naturally infected individuals of the same species (Morley, Reference Morley2011). Nevertheless, it is clear from this study that widespread interspecific and intraspecific differences in the thermodynamics of cercarial survival/metabolism exist, making any specific focus on laboratory-cultured species redundant.
A number of patterns in the way that cercarial species respond to changes in temperature are apparent. Many species have a simple ‘linear’ relationship of increasing glycogen utilization rate and decreasing survival with rising temperature over the majority of the thermal range studied, accelerating rapidly only at very high temperatures. In contrast, a number of species show a ‘curved’ relationship, having high mortalities at low temperatures with increasing survival as the temperature is raised before declining again. A further group also demonstrates similar patterns to the above but also possesses a distinct zone of thermostability. Metabolic plateaus of this kind have been reported in a wide range of ectothermic animals with typical curves rising steeply in the lower temperature before flattening out. In this lower range Q 10 values are usually substantially above 2 whilst in the plateau range between 1 and 2 (Wieser, Reference Wieser and Wieser1973). In general, within the present study this thermostable zone appeared to extend over at least a 5°C increment with low activation energy values of less than 7 Kcal./mole. In some species the zone was bracketed both above and below with relatively modest increases in mortality creating a more extensive constancy. The presence of a thermostable zone suggests that an adaptive mechanism exists, allowing maintenance of a certain degree of metabolic homeostasis by some species at mean summer temperatures within the normal range expected to be encountered in aquatic habitats at these particular latitudes. Young et al. (Reference Young, Bundy and Taylor1984) speculated that there were 2 potential ways such mechanisms could operate. The first is a diminished enzyme-substrate affinity with increasing temperature, which could result from thermal molecular reorganization, effectively counteracting the thermal increase in the number of molecules exceeding the critical activation energy (E*) for the rate-limiting metabolic reaction. Alternatively, it could result from the interactions of 2 separate enzymatic systems, one increasing as the other decreases in activity with rising temperature.
The occurrence of a zone of thermostablity demonstrated by those species in the present analysis would appear, in general, to correlate with expected temperatures over the seasonal transmission period in aquatic habitats and latitudes where such species are found. The only major exception would appear to be S. bovis, which demonstrated thermostability at a lower range of 15–20°C. This would, at first glance, appear to be an incongruous result for a species noted for infecting ruminants in subtropical Africa. However, this species was isolated from a distinct geographical strain in Salamanca province, Spain, a high altitude area on the west central Spanish plateau that experiences hot dry summers and cold winters. The molluscan intermediate host in this region is Planorbarius metidjensis. In general, this snail aestivates in the muddy banks of dried up water-courses during the hot summer months, interrupting the parasite's life cycle (Ramjo-Martin and Simon-Vincente, Reference Ramajo-Martin and Simon-Vicente1988; Yacoubi et al. Reference Yacoubi, Zekhnini, Rondelaud, Vignoles, Dreyfuss, Cabaret and Moukrim2007). Consequently, a transmission window exists only during the autumn and spring months when temperatures are cooler and thus S. bovis cercariae would appear to demonstrate thermostability at temperatures that most closely reflect climatic conditions during these seasons.
A number of cercarial species demonstrated increased mortality at low temperatures. As survival was reduced to only a few hours in many cases the complete exhaustion of the glycogen reserves from increased metabolism seems an unlikely reason for this pattern. Rather it is more probable that a reduced enzyme function is responsible, resulting in a decline in the amount of energy available to the organism for physiological processes such as metabolism and ionic and osmotic regulation. The fact that only certain cercariae experience apparent problems at such temperature extremes is concordant with the view that different metabolic enzyme systems exist in certain species.
The majority of values of Q 10 and E* over the temperature ranges show either stable of typical doubling to tripling of physiological rates with 10 °C increases, only at high temperatures are values substantially raised. This suggests that, in general, cercarial survival/metabolism is not greatly affected by temperature beyond what would normally be expected for an ectotherm and can be considered to be reasonably well adapted to the normal thermal range encountered by each species.
Nevertheless, geographical and laboratory strains of species have profound influences on the cercarial responses to temperature. Differences in experimental parameters between the studies, such as water hardness, seem unlikely to be responsible as these factors, although influencing the duration of survival, do not substantially alter the relationship with temperature (Morley et al. Reference Morley, Crane and Lewis2001). A marked difference in survival/metabolism of E. recurvatum cercariae from two different geographical populations was apparent. Although both populations demonstrated similar trends at lower temperatures, with ‘Harting Pond’ demonstrating greater stability between 10 and 15°C, they diverged substantially as the temperature rose above 25°C with the ‘Bushy Park’ strain remaining relatively stable at higher temperatures whilst the ‘Harting Pond’ strain demonstrated a sharp increase in mortality. Such differences may be associated with parasite acclimation and the summer water temperature of each habitat. At Harting, the site is a large cool pond used as a trout fishery where peaks of 20–21°C were recorded over the summer (McCarthy, Reference McCarthy1989). In contrast, at Bushy Park the sampling site was a slow-moving shallow stream between 2 ornamental ponds where summer water temperatures as high as 25–26°C occurred (N. J. Morley, unpublished observations). Similarly, the different laboratory strains of S. mansoni cercariae also had divergent patterns of survival/metabolism. ‘Puerto Rico 1’ strain (Lawson and Wilson, Reference Lawson and Wilson1980) shows a typical ‘linear’ response, whilst ‘Puerto Rico 2’ strain (Sirag and James, Reference Sirag and James1982) demonstrates a more gradual increase in mortality with a zone of thermostability between 20 and 25°C. The ‘Tanzania’ strain (Purnell, Reference Purnell1966), however, has a variable, but relatively stable, survival over increasing temperature, only accelerating as the temperature rises above 30°C, except at 15°C where an apparent experimental artefact caused an unexpected atypical elevated mortality which was not continued as the temperature rose.
To what extent these variations between the 3 strains of S. mansoni are due to conditions in the habitats from which they were originally sampled or are a product of long-term laboratory culture cannot be determined. Nevertheless, there is no question that intraspecific differences clearly exist and must be considered when evaluating the potential impact of climate change on trematode transmission viability.
What role may thermal acclimation play in these variations in cercarial survival? The time-span of metabolic responses to temperature can demonstrate virtual immediate compensation or can involve a longer-term gradual change in compensation (Walker and Barrett, Reference Walker and Barrett1983). If cercariae metabolize in a similar manner as typical ectotherms it would be expected that they possessed biochemical mechanisms that facilitated their ability to cope with diurnal and seasonal variations in ambient temperature, both whilst developing in the molluscan host as well as during their free-living stage. Immediate compensation would also be required to overcome the metabolic disruption caused by the drastic change in temperature experienced by those cercarial species that directly infect an endothermic host e.g. S. mansoni. Certainly there is some evidence of immediate compensation by bird trematode cercariae when exposed to high temperatures (Gammermaister, Reference Gammermaister1977), whilst thermal metabolic acclimation patterns appear to be distinctly different for each trematode species and likely reflect the thermal regime present in the definitive host (Vernberg and Vernberg, Reference Vernberg and Vernberg1965), indicative of long-term compensation. From the present study it is clear that geographical strains of E. recurvatum show survival/metabolic adaptation related to their individual habitat, which may be correlated with different thermal conditions encountered there. Certainly thermal adaptation of parasites is a concept little discussed in relation to climate change (Raffel et al. Reference Raffel, Rohr, Paull and Johnson2010). Metabolic theory suggests that smaller organisms should have a faster metabolism (West et al. Reference West, Woodruff and Brown2002), and therefore parasite acclimation responses may be more rapid than their hosts, potentially making ectothermic organisms suffering from thermal stress more susceptible to infection when temperatures become increasingly variable (Raffel et al. Reference Raffel, Rohr, Paull and Johnson2010).
Cercarial size in the present study was found to have no correlation with Q 10 or E* values obtained at any temperature range. However, these results with cercarial size can only be used as a crude indicator of the lack of correlation with temperature. This is because in ectotherms size is often reduced at higher rearing temperatures (Atkinson, Reference Atkinson1994), whilst size variations within species also occur as a result of platicity in development under different environmental conditions (Poulin and Latham, Reference Poulin and Latham2003). In the present investigation the majority of cercarial dimensions were not derived from the corresponding survival studies, as these data were not generally recorded, but were largely taken from the scientific literature of new species descriptions and life-cycle studies. As nothing is known about the potential effects of temperature on cercarial morphological characteristics, it still remains to be conclusively determined that cercarial size has no influence on the response of individuals to temperature change.
Latitude is another factor that may influence cercarial responses to temperature changes, as different thermal regimes occur at different latitudes. Poulin (Reference Poulin2006) found that species from lower latitudes demonstrated more pronounced temperature-driven increases in cercarial output than those from higher latitudes. Unfortunately it was not possible to analyse the effects of latitude on cercarial survival/metabolism due to the data set not being normally distributed, a large number of studies being undertaken on species from 51–55°N. Nevertheless, a cursory examination of the data suggests that tropical species do not show any tendency for elevated Q 10 or E* values in comparison to temperate species.
Changes in cercarial population densities are determined by both survival and emergence (Combes and Theron, Reference Combes and Theron1981). Therefore, when the results of the present study are considered in the context of results on the emergence of cercariae (Poulin, Reference Poulin2006; Morley et al. Reference Morley, Adam and Lewis2010) and general theories of ectothermic organisms responses to temperature (Wieser, Reference Wieser and Wieser1973; Newell, Reference Newell and Wieser1973) 3 likely scenarios for the impact of temperature, and hence climate change, on cercarial populations emerge. The first suggests that over the range 15–25°C (≈20°C) elevated levels of cercarial emergence (Poulin, Reference Poulin2006) with relatively stable parasite survival/metabolism (present study) will lead to a rise in the numbers of viable cercariae, which could increase the risk of higher levels of parasitism in the target host population. However at temperatures in the range 20–30°C (≈25°C), emergence will decline (Poulin, Reference Poulin2006) although many species will still retain stable or typical changes in survival metabolism (present study) resulting in smaller, but still viable, cercarial populations.
The second scenario considers that at temperatures regarded as low for any particular temperate to tropical species elevated levels of cercarial emergence will be encountered (Morley et al. Reference Morley, Adam and Lewis2010), a situation concordant with elevated Q 10 values recorded for many ectotherms at low experimental temperatures (Newell, Reference Newell and Wieser1973), accompanied by relatively typical survival/metabolism (present study). As temperatures approach those normally found in latitudes where the parasite occurs, emergence and survival/metabolism stabilizes creating transmission dynamics little influenced by temperature (Wieser, Reference Wieser and Wieser1973; Morley et al. Reference Morley, Adam and Lewis2007, Reference Morley, Adam and Lewis2010; present study).
The third scenario is a complex mixture of the first two, with widespread interspecific and intraspecific variations in responses to temperature (Koprivnikar and Poulin, Reference Koprivnikar and Poulin2009; present study) creating a situation of both relative stability for some species and increased variability for others. At present this third scenario appears to be the most probable one to occur under climate change.
Finally, the most important question raised from this study is how such interspecific and intraspecific variation in cercarial survival/metabolism thermodynamics may impact parasite transmission. Pechenik and Fried (Reference Pechenik and Fried1995) established for echinostomes that temperature had a similar effect on both t 0·5 and loss of infective capacity, suggesting that time to 50% cercarial mortality could act as a proxy indicator of infection potential. To what extent this work may be extrapolated to other trematode groups has yet to be fully determined. Certainly other factors not related to energy consumption can affect infectivity. Indeed, the low initial infectivity after emergence reported for some trematodes (Evans and Gordon, Reference Evans and Gordon1983; Whitfield et al. Reference Whitfield, Bartlett, Khammo and Clothier2003) may indicate that cercarial energy reserves do not become an influential factor on transmission until glycogen levels drop below a certain threshold as the cercariae age. Nevertheless, it is likely that a variable thermodynamical survival response of cercariae to changing temperature will influence transmission to some extent, particularly in relation to activity. A zone of thermostability demonstrated by some cercarial species and strains could ensure that parasite viability remains unchanged over a temperature rise of 2−4 °C, predicted to occur under the pressure of climate change.
The present study has demonstrated that temperature does not exert any substantial disproportionate effect on cercarial survival/metabolism. In fact, thermostability has been demonstrated to be widespread over temperatures typically encountered by many species. Nevertheless, interspecific and intraspecific differences in the response of cercariae to changing temperatures appear to be common, probably associated with specific temperature regimes encountered in the habitats from which they were sampled, and therefore unambiguous generalizations on the effects to survival/metabolism are problematic. Consequently, there is a very real possibility that establishing a globally encompassing framework for trematode transmission under climate change may not be possible.