INTRODUCTION
Understanding complex interactions between the environment and parasites is an important component in determining which factors are vital for maintaining viable populations within ecosystems, particularly under the influence of climate change. Trematodes are potentially susceptible to climatic conditions both during the free-living stages of their life cycle and also whilst developing within ectothermic molluscan hosts. Temperature is an important factor influencing the life of organisms (Precht et al. Reference Precht, Laudien, Havsteen, Precht, Christophersen, Hensel and Larcher1973) and is intrinsically linked to climate change. Evaluating trematode thermal biology is therefore an important step in furthering our understanding of parasite population dynamics under a changing climate.
In order to develop a predictive framework of trematode responses to elevated temperatures resulting from climate change, a large-scale analysis of the available empirical information is needed. In a pioneering study Poulin (Reference Poulin2006) analysed the effects of temperature on cercarial emergence from molluscan hosts with experimental data from the scientific literature. Using the Q 10 value, a common measure of temperature-driven reaction rates, over a 10 °C range at a focal temperature of ≈20 °C cercarial output showed a mean 8-fold increase, which was far higher than the 2- to 3-fold increases normally observed with physiological processes. Poulin concluded that the small increases in temperature envisaged under climate change will promote the proliferation of infective stages in many ecosystems.
This interpretation has proved particularly influential, having been incorporated into many subsequent studies associated with climate change e.g. Marcogliese (Reference Marcogliese2008); Mas-Coma et al. (Reference Mas-Coma, Valero and Bargues2009). However, subsequent investigations suggest that temperature may have a more complex effect on cercarial biology. Morley et al. (Reference Morley, Adam and Lewis2010) undertook a detailed examination of temperature effects on cercarial emergence of the mid-latitude species Echinoparyphium recurvatum. Although elevated emergence, as measured by Q 10, was recorded in the low temperature range of 10–20 °C (≈15 °C), at higher ranges emergence initially stabilized between 15 and 25 °C (≈20 °C) before declining at higher temperatures of 20–29 °C (≈25 °C). High Q 10 values in the low temperature range are associated the minimum emergence temperature threshold (METT), a point at which emergence rates decrease to almost zero, and appears to be 2–3 °C higher than the minimum development temperature threshold (MDTT) where intramolluscan development ceases (Pflüger, Reference Pflüger1980; Pflüger et al. Reference Pflüger, Roushdy and El Emam1984).
Similarly, cercarial emergence has been found to show distinct interspecific and intraspecific variations under field conditions (Koprivnikar and Poulin, Reference Koprivnikar and Poulin2009a), whilst a meta-analysis of cercarial survival and metabolism showed that temperature did not exert any substantial disproportionate effect and many species demonstrated zones of thermostability over normal temperature ranges with intraspecific variations being shown by strains in both laboratory and natural species (Morley, Reference Morley2011a).
The emergence of cercariae is not an isolated activity but is dependent on the rate of cercarial development within the molluscan host (Asch, Reference Asch1972). For elevated levels of emergence to be sustainable over prolonged periods, which is a potential implication of the findings of Poulin (Reference Poulin2006), responses of cercariae to temperature must also have comparable changes in development rates above those normally encountered for physiological processes. The effects of temperature on intramolluscan trematode development have been extensively studied but never comparatively analysed. It is therefore timely to assess this aspect of their thermal biology. Similarly, recent advances in cercarial thermodynamics suggest that a more detailed re-analysis of temperature effects on emergence are required, to assess the relationship between cercarial development and emergence. In particular, 2 important aspects of emergence biology, namely the METT and its influence on Q 10 values and acclimation of mollusc-trematode associations to regimes of temperature have not been previously assessed.
Cercarial emergence at, or close, to the METT is generally very low with values of less than 10 individuals over a 24-h period being not uncommon. High Q 10 values are therefore inevitable if measurements taken close to the METT are compared with results 10 °C higher, in the optimum temperature range, where diurnal emergence may often exceed 1000 individuals. Such elevated Q 10 results are not only common for biological activities measured at low temperature ranges of existence (Newell, Reference Newell and Wieser1973) but also appear to have little relevance to climate change in situations where summer temperatures above the normal optimum may occur. Biological optima will reflect typical latitudinal water temperatures. For trematode species from low latitudes (⩽35°) optimum temperature conditions typically appear to exist from 20 to 30 °C (≈25 °C) with METT occurring around 15 °C e.g. Anderson and May (1979), Mills (Reference Mills1980), Pflüger et al. (Reference Pflüger, Roushdy and El Emam1984). On the other hand, mid-latitude species (36–60°) have lower optima generally from 15 to 25 °C (≈20 °C), with METT around 10 °C or less e.g. Lyholt and Buchmann (Reference Lyholt and Buchmann1996), Morley et al. (Reference Morley, Adam and Lewis2007, Reference Morley, Adam and Lewis2010), Thieltges and Rick (Reference Thieltges and Rick2006), although species from high mountain altitudes, low ocean depths, or other extreme environments will be obvious exceptions to these generalizations. The use of a single focal temperature range for all species such as ≈20 °C (used by Poulin, Reference Poulin2006), will inevitably include measurements of species at both low temperature ranges, encompassing the METT, as well as those over optimum temperature ranges and may provide a distorted view of temperature effects at a global level. However, analyses undertaken on multiple-step 10 °C ranges, as utilized by Morley et al. (Reference Morley, Adam and Lewis2010), allow a more detailed evaluation of temperature effects, including the influence of the METT, on cercarial emergence.
Acclimation, which is an adaptation to a single factor in controlled experiments, can be divided into 3 phases (1) an initial phase of immediate responses, (2) a stabilization phase, and (3) the new stable condition. During the first 2 phases a rise in activity can occur, known as an overshoot, which must be allowed to decline before proceeding with measurements (Precht et al. Reference Precht, Laudien, Havsteen, Precht, Christophersen, Hensel and Larcher1973). Poulin (Reference Poulin2006) considered that differences across individual studies, such as acclimation, may cause some variation in the quality of data, but these differences were unlikely to bias the results. On the contrary, acclimation is the cornerstone of studies in thermal biology. Under normal circumstances the acclimation temperature (AT) needs to be the same as the experimental temperature (ET) and animals should be maintained at the AT for a sufficient period until disturbances caused by introducing organisms to new conditions have died down (Precht et al. Reference Precht, Laudien, Havsteen, Precht, Christophersen, Hensel and Larcher1973). The emergence of cercariae is a volatile activity, highly sensitive to abrupt changes in environmental conditions. Changes in temperature, illumination, and water condition can stimulate emergence (Kendall, Reference Kendall and Taylor1964). Indeed, this phenomenon has been exploited for the rapid harvesting of large numbers of viable cercariae for experimental purposes and is a widely used technique (Stirewalt, Reference Stirewalt1981; Frandsen and Christensen, Reference Frandsen and Christensen1984; Ginetsinskaya, Reference Ginetsinskaya1988). Measurements of emergence taken whilst animals are not acclimated to experimental conditions are consequently likely to produce false positive results at any given constant temperature.
Therefore, in order to determine the influence of elevated temperatures under global climate change, reliable analysis of the thermal biology of key components of trematode transmission is essential. The aim of the present study is to analyse, using the Q 10 value, the effects of temperature on cercarial development and emergence over a wide temperature range using 10 °C incremental increases. Such analysis will clarify whether or not acclimation and the METT influence cercarial output, and also whether any elevated emergence is complemented by related increases in thermal rates of development.
MATERIALS AND METHODS
Source of data
Data on cercarial development and emergence were obtained from the scientific literature on laboratory studies undertaken at different constant temperatures. For emergence data, the sources included by Poulin (Reference Poulin2006) were used as a starting point. Of these Pflüger (Reference Pflüger1980) and Fingerut et al. (Reference Fingerut, Zimmer and Zimmer2003) were not suitable for Q 10 analysis as emergence had been measured over too narrow a temperature range of 5 °C or less. Careful searching of databases revealed an additional 25 sources. In total 32 studies on development and 55 on emergence were used for analysis.
Cercarial development
For development sources which provided duration times from the initial miracidial infection to the first occurrence of emerged cercariae were analysed. Very few previous studies have focused on the effects on development rates of individual intramolluscan stages but both Al-Habbib and Al-Zako (Reference Al-Habbib and Al-Zako1981) and Al-Habbib and Grainger (Reference Al-Habbib and Grainger1983) found that each stage had a generally comparable rate over different temperature ranges, except at temperatures below the METT where cercariogenesis ceases. We therefore considered that the sources used for analysis were suitable for determining the thermal rates of cercarial development.
In the present study, the MDTT was defined as the point where all cercarial development in the molluscan host ceases, but as this was infrequently determined a value of ‘less than’ the lowest temperature reading was recorded. In some cases development was found not to occur at a very low temperature and therefore the MDTT was defined to occur in a range from this point up to the first recorded development temperature.
Cercarial emergence
For emergence, data on cercarial output typically ranged from 1 h to 24 h. Although rates of cercarial emergence are not constant over a 24-h period, this did not affect estimates of Q 10 because for any given species the same procedures were applied for cercarial counts at any given temperature, thus allowing a relative measure such as the Q 10 value to be established without bias (Poulin, Reference Poulin2006). In addition, there is a further risk that those studies measuring output for only 1 h (n = 6) may introduce atypical estimates into the analysis. However, the Q 10 values of these studies were found to fall within the range of values generated by the majority of other longer duration studies and therefore their inclusion in this analysis was not considered to introduce any bias. The METT for each study was determined as the point when the number of emerging cercariae fell to a level just above zero, and this was typically taken to be when values were less than 20 individuals per day. In many examples measurements of 20–50 individuals suggested that this occurred slightly below the lowest temperature reading taken and therefore a value of ‘less than or equal to’ this lowest reading was recorded. Where the METT was clearly well below the lowest temperature a value of ‘less than’ this recording was taken. Occasionally emergence was determined not to occur at a very low temperature and therefore the METT was defined to occur in a range from this point up to the lowest recorded emergence temperature.
The degree of acclimation of molluscan hosts in each emergence study was determined from details within the respective individual Materials and Methods section. For some studies after being placed in the ET, a number of consecutive daily measurements were taken and therefore to provide acclimated data, readings on the first 2 days were discarded, constituting the acclimating period, and the remaining days’ data were used for calculating Q 10 values. Two days, or 2 diurnal emergence cycles, were considered to be a minimum period at any given temperature for which cercariae could confidently be considered to have been acclimated and was derived from our own personal experiences of studying cercarial emergence as well as analysing studies from the literature which presented daily output values over extended periods e.g. Lyholt and Buchmann (Reference Lyholt and Buchmann1996). Each study was categorized into one of the following groups. (1) Total-molluscs were experimentally infected with miracidia and maintained at a constant ET throughout both developmental and emergence periods but without any form of stimulation to induce emergence. (2) ST-experimentally infected molluscs were exposed to an abrupt change in conditions such as temperature, light, and water changes to stimulate cercarial emergence. (3) Duration in ‘days’-experimental or naturally infected molluscs were acclimated to the ET for a variable but specified number of days. (4) Gradual-infected molluscs were placed in ET and emergence measured over 5 or more days, including both pre-and post-acclimation periods; emergence was given as a mean value over the entire period with a greater number of measurements being taken from the post-acclimation phase. (5) None-experimental or naturally infected molluscs were placed in the ET and measurements of emergence taken without any acclimation period. Emergence data were subsequently categorized from these groups into either ‘Acclimated’ (including ‘Total’, ‘Gradual’ and those molluscs acclimated for 2 or more days) or ‘Non-acclimated’ (including ‘None’, ‘ST’ and those molluscs acclimated for less than 2 days).
Snail size was determined by Morley et al. (Reference Morley, Adam and Lewis2010) as a potential influential variable on temperature-determined emergence. However, as few studies in the present analysis have included data on molluscan size, a large-scale analysis was not possible. Nevertheless, a small number of studies of both development and emergence examined temperature effects within different sized molluscs and therefore these were included as separate studies for analysis. Similarly strain-specific difference may also affect the thermal responses of cercariae (Morley, Reference Morley2011a) and consequently each strain of the same species was also analysed as a separate study.
Analysis of data
The thermodynamic relationship of cercarial development and emergence was determined using the Q 10 value. This was calculated using the original data of development duration and emergence output from each source over a range of temperatures that approximately encompassed increases of roughly 10 °C to provide ‘low’, ‘optimum’ and ‘high’ temperature ranges as follows: 10–20 °C (≈ 15 °C), 15–25 °C (≈20 °C), 20–30 °C (≈25 °C) for mid-latitude species, and 15–25 °C (≈20 °C), 20–30 °C (≈25 °C), 25–35 °C (≈30 °C) for low-latitude species. At low and high ranges measurements encompassing precise 10 °C ranges were not always recorded, particularly in the low range associated with the METT. But all values were within 1 to 2 °C of this range and such small variations are unlikely to substantially change the Q 10 value generated. Similarly some studies did not measure values at exact 5 °C increments associated with these ranges and therefore where necessary data were extrapolated from measurements above and below these temperature readings to give an appropriate value. The Q 10 was calculated using the following form of the van't Hoff equation (Randall et al. Reference Randall, Burggren and French2001):
where n 1 and n 2 are development/emergence data at temperatures t 1 and t 2 respectively. Q 10 values ranging between 2 and 3 are typically the norm and are indicative of a doubling or tripling of physiological rates per 10 °C increase in temperature (Prosser, Reference Prosser1973; Randall et al. Reference Randall, Burggren and French2001). A value between 1 and 2 indicates little change, whereas a value less than 1 indicates a reduced rate. In general, Q 10 values of approximately 2–3 are usually encountered by ectotherms over the normal environmental temperature range of the organism.
Where data did not easily fit into 10 °C increments the analysis was supplemented with the critical incremental energy of activation (E* or μ). This is an alternative measure of temperature-driven reaction rates and represents the energy which molecules in their initial state must acquire before they can participate in a chemical reaction and can be considered a limiting or pacemaker step for complex physiological activity (Hoar, Reference Hoar1983). E* was determined using the following form of the Arrhenius equation (Prosser, Reference Prosser1973):
 $$E^* = \displaystyle{{ - 2.3R(Log{\kern 1pt} K_2 - Log{\kern 1pt} K_1 )} \over {\displaystyle{1 \over {T_2}} - \displaystyle{1 \over {T_1}}}} $$
$$E^* = \displaystyle{{ - 2.3R(Log{\kern 1pt} K_2 - Log{\kern 1pt} K_1 )} \over {\displaystyle{1 \over {T_2}} - \displaystyle{1 \over {T_1}}}} $$where K 1 and K 2 are development/emergence data at absolute temperatures T 1 and T 2, and R is the gas constant (1·98 cal/mole). For many enzymatic and biological processes in living organisms E* values usually range from 1 to 25 Kcal/mole. Normal activation energy is approximately 10 Kcal/mole with many respiratory metabolic processes having values typically of 11 or 16 Kcal/mole (Crozier, Reference Crozier1924; Brandts, Reference Brandts and Rose1967; Hoar, Reference Hoar1983).
All Q 10 values were log10 transformed and the significant differences between variables were analysed with Student's t-test using the SPSS computer package.
RESULTS
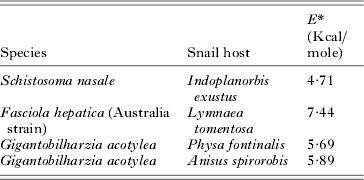
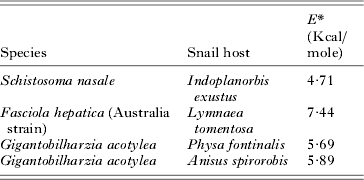
Temperature had a complex effect on cercarial development and emergence. There were only small changes in development rates over increasing temperature for both low and mid-latitude species (Tables 1 and 2, Fig. 1). Mean Q 10 values gradually declined with elevated levels of temperature ranging from approximately 3-fold at low temperature ranges for both latitudinal groups, to just over 2-fold at optimum ranges, then declining to thermostable values of 1–2 at high temperature ranges (Fig. 1). However, there were no significant differences in Q 10 values between any temperature range for either low or mid-latitude species (t ⩽ 2·027, P ⩾ 0·056), with the majority of values being substantially insignificant. A small number of species demonstrated thermostable development over wide temperature ranges of 20 °C with E* values of less than 8 Kcal/mole (Table 3).

Fig. 1. Mean Q 10 values of cercarial development over different temperature ranges of mid-latitude (white bars) and low-latitude (black bars) species.
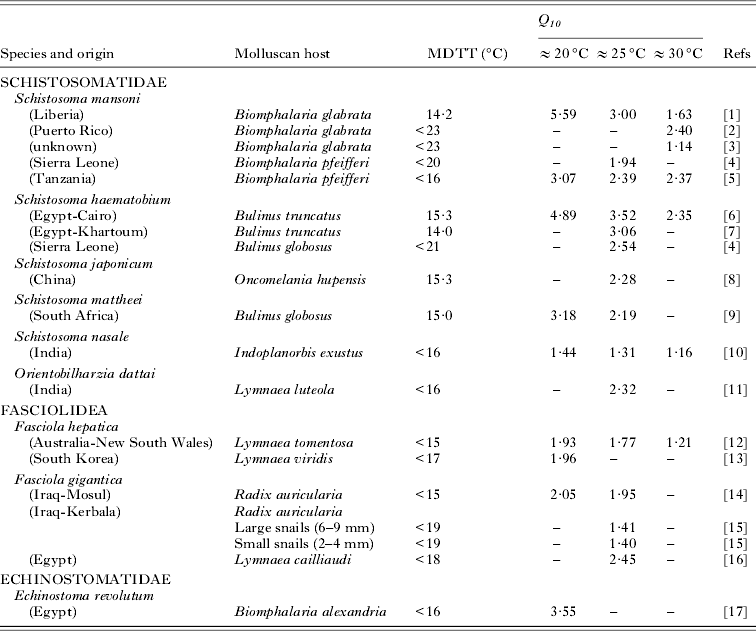
Table 1. Characteristics, the minimum development temperature threshold (MDTT), and Q 10 values of cercarial development for each low-latitude species over different temperature ranges
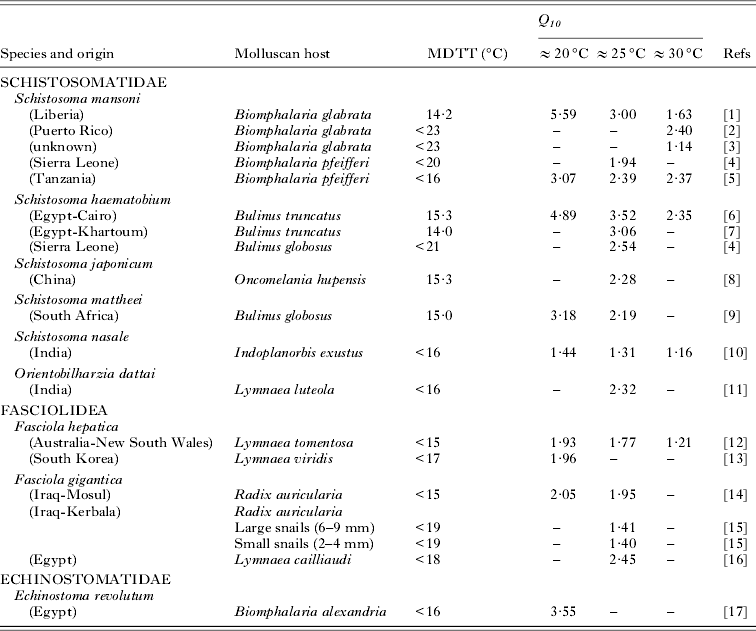
References- [1] Pfluger (Reference Pflüger1980), [2] Stirewalt (Reference Stirewalt1954), [3] Wagner and Moore (Reference Wagner and Moore1959), [4] Gordon et al. (Reference Gordon, Davey and Peaston1934), [5] Foster (Reference Foster1964), [6] Pfluger et al. (Reference Pflüger, Roushdy and El Emam1984), [7] Blankespoor et al. (Reference Blankespoor, Babiker and Blankespoor1989), [8] Yang et al. (Reference Yang, Utzinger, Sun, Hong, Vounatsou, Tanner and Zhou2007), [9] Frank (Reference Frank1966), [10] Biswas & Subramanian (Reference Biswas and Subramanian1988), [11] Dutt and Srivastava (Reference Dutt and Srivastava1962), [12] Boray (Reference Boray1963), [13] Lee et al. (Reference Lee, Cho and Lee1995), [14] Al-Habbib and Al-Zako (Reference Al-Habbib and Al-Zako1981), [15] Al-Jibouri et al. (Reference Al-Jibouri, Al-Mayah and Hassan2011), [16] Shalaby et al. (Reference Shalaby, Hassan, Soliman and Sherif2004), [17] Moravec et al. (Reference Moravec, Barus, Rysavy and Yousif1974).
Table 2. Characteristics, the minimum development temperature threshold (MDTT), and Q 10 values of cercarial development for each mid-latitude species over different temperature ranges

References- [1] Al-Habbib and Grainger (Reference Al-Habbib and Grainger1983), [2] Ollerenshaw (Reference Ollerenshaw1971), [3] Nice (Reference Nice1979), [4] Akramova et al. (Reference Akramova, Azimov and Shakarboev2010) [5] Watertor (Reference Watertor1968), [6] Waadu and Chappell (Reference Waadu and Chappell1991), [7] Harris (Reference Harris1986), [8] Lewis (Reference Lewis1976), [9] Dovgalev (Reference Dovgalev1987).
Table 3. Values of E* of cercarial species demonstrating developmental thermostability over the temperature range 15 to 35 °C
A precise MDTT was only rarely given in the studies but, when determined, it typically occurred between 14 and 16 °C for low latitude species and equal to or less than 10 °C for mid-latitude species (Tables 1 and 2). Some specific strain differences in thermal biology were apparent, particularly over optimum ranges for both mid- and low-latitude species (Tables 1 and 2). The influence of snail host species on the thermodynamics of cercarial development was less clear cut. Little or no differences were apparent in Q 10 values of the same species strain of Metagonimus yokagawai and Gigantobilharzia acotylea developing in different species. In contrast, the same strain of Diplostomum spathaceum demonstrated different Q 10 values when established in 2 Lymnaeid species (Table 2). The influence of snail host size was studied only in 1 strain of F. gigantica which did not substantially alter the thermal responses of this trematode (Table 1).
Cercarial emergence in both mid- and low-latitude species demonstrated a similar pattern of thermal responses, declining over the 3 temperature ranges of each latitudinal group with increasing temperature (Tables 4 and 5, Fig. 2a, b). From the 55 studies examined, only 2 exceptions to this trend occurred from the acclimated group. These were the low-latitude species Haplorchis pumilio (India strain), where a stable Q 10 value at optimum ranges substantially increased at the high temperature range (Table 4) and Maritrema novaezealandensis (Otago harbour 3), where an extremely elevated Q 10 value occurred in the optimum range, associated with a high METT, followed by an abrupt low Q 10 value at the high range (Table 5). These atypical results were excluded from the subsequent analysis and will be considered in detail in the Discussion.

Fig. 2. Mean Q 10 values of cercarial emergence over different temperature ranges (white bars- non-acclimated, black bars- acclimated). (a) Mid-latitude species. (b) Low-latitude species.
Table 4. Characteristics, the minimum emergence temperature threshold (METT), acclimation status, and Q 10 values of cercarial emergence for each low-latitude species over different temperature ranges

References- [1] Fried et al. (Reference Fried, Laterra and Kim2002), [2] Kuntz (Reference Kuntz1947), [3] Nojima and Sato (Reference Nojima and Sato1978), [4] Mangal (Reference Mangal2009), [5] Lo (Reference Lo1972), [6] Pfluger et al. (Reference Pflüger, Roushdy and El Emam1984), [7] Mao et al. Reference Mao, Li and Wu1949, [8] Gumble et al. (Reference Gumble, Otori, Ritchie and Hunter1957), [9] Frank (Reference Frank1966), [10] Al-Habbib and Al-Zako (Reference Al-Habbib and Al-Zako1981), [11] Al-Jibouri et al. (Reference Al-Jibouri, Al-Mayah and Hassan2011), [12] Soliman (Reference Soliman2009), [13] Shalaby et al. (Reference Shalaby, Hassan, Soliman and Sherif2004), [14] Lee et al. (Reference Lee, Cho and Lee1995), [15] Boray (Reference Boray1963), [16] Lo and Lee (Reference Lo and Lee1996), [17] Umadevi and Madhavi (Reference Umadevi and Madhavi1997), [18] Terhune et al. (Reference Terhune, Wise and Khoo2002), [19] Shostak and Esch (Reference Shostak and Esch1990).
Table 5. Characteristics, the minimum emergence temperature threshold (METT), acclimation status, and Q 10 values of cercarial emergence for each mid-latitude species over different temperature ranges (+, −Marine species)

References- [1] Al-Habbib and Grainger (Reference Al-Habbib and Grainger1983), [2] Nice (Reference Nice1979), [3] McCarthy (Reference McCarthy1989), [4] Morley et al. (Reference Morley, Adam and Lewis2010), [5] Šarovnová (Reference Šarounová2011), [6] Schmidt and Fried (Reference Schmidt and Fried1996), [7] Awi (Reference Awi1986), [8] Lyholt and Buchmann (Reference Lyholt and Buchmann1996), [9] Harris (Reference Harris1986), [10] Raisyte (1968), [11] Mouritsen and Jensen (Reference Mouritsen and Jensen1997), [12] Mouritsen (Reference Mouritsen2002), [13] Fredensborg et al. (Reference Fredensborg, Mouritsen and Poulin2005), [14] Koprivnikar and Poulin (Reference Koprivnikar and Poulin2009b), [15] Studer et al. (Reference Studer, Thieltges and Poulin2010), [16] Rees (Reference Rees1948), [17] Prinz et al. (Reference Prinz, Kelly, O'Riordan and Culloty2011), [18] Lowenberger and Rau (Reference Lowenberger and Rau1994), [19] Thieltges and Rick (Reference Thieltges and Rick2006), [20] Gayevskaya (Reference Gayevskaya1972), [21] Martin and Vazquez (Reference Martin and Vazquez1984), [22] Lewis (Reference Lewis1976), [23] Rojo-Vazquez and Simon-Martin (Reference Rojo-Vazquez and Simon-Martin1985), [24] Dovgalev (Reference Dovgalev1987).
At low temperature ranges there were very high Q 10 values for both acclimated and non-acclimated species as measurements were analysed upwards from the METT which did not demonstrate any significant differences between acclimation status for either mid- or low-latitude species (t ⩽ 1·175, P ⩾ 0·257). The METT was typically found between 12 and 18 °C for low-latitude species and between 7 and 14 °C for mid-latitude species with a tendency for non-acclimated studies to have a higher METT (Tables 3 and 4). Over the optimum ranges of ≈20 °C for mid-latitude and ≈25 °C for low-latitude species, Q 10 values drastically declined. For acclimated species mean Q 10 values are thermostable being 1·01 for low-latitude species and 1·57 for mid-latitude species. However, non-acclimated species still demonstrate elevated mean Q 10 values over optimum ranges of 14·63 and 7·69 at low and mid-latitudes respectively (Fig. 2a, b). There is a significant difference between acclimated and non-acclimated Q 10 values at these optimum ranges for both low (t= − 2·722, P = 0·013) and mid-latitude species (t=2·381, P=0·026). At temperature ranges above the optimum there is a trend of declining mean Q 10 values. However, data on emergence at these temperatures are relatively few. Acclimated mid-latitude species have mean Q 10 values of 0·69 whilst low latitude species have a value of 0·55. In contrast, non-acclimated species have mean Q 10 values of 2·92 and 1·13 at low and mid-latitudes respectively (Fig. 2a, b). These acclimation differences in Q 10 values were significant for low-latitude species (t = 2·926, P = 0·015) but not for mid-latitude species (t = 0·083, P = 0·937).
There was a significant difference between Q 10 values in the low temperature range and optimum ranges for both mid-latitude (t = 4·291, P < 0·001) and low-latitude (t = − 2·167, P = 0·047) acclimated species. However, values at the high temperature range were only significantly different from the optimum range for low-latitude species (t = 2·981, P = 0·011). In contrast, non-acclimated species demonstrated significant differences only between low and optimum temperature ranges for mid-latitude species (t = 2·159, P = 0·045).
Strain-specific differences in emergence rates were difficult to determine due to variations in precise levels of acclimation between most species where multiple studies had been undertaken. Nevertheless, strains of Fasciola gigantica from Egypt and Iraq did show distinct differences in Q 10 values within the optimum ranges (Table 4). Differences in emergence due to snail size were investigated in only 2 studies (Table 4, 5) with conflicting results making further interpretation not possible.
Thermostability of cercarial emergence over wide temperature ranges was difficult to conclusively determine for many studies due to both the volatile nature of this biological activity and that only a few studies were able to investigate emergence over extensive ranges. Nevertheless, 2 studies showed that little variation in output occurred over a range of either 16 °C for Schistosoma mansoni (Brazil/Egypt/Puerto Rico mixed strain) or 20 °C for Fasciola hepatica (Australia strain) giving E* values that rose by 8·8 Kcal/mole or 7·59 Kcal/mole before declining by −4·57 Kcal/mole or −4·27 Kcal/mole respectively.
DISCUSSION
Temperature has a complex effect on cercarial development and emergence dependent on the specific temperature ranges, latitude of each species under investigation, and the degree of experimental acclimation. Freshwater studies have dominated the present analysis. However, because both freshwater and marine organisms are exposed to the same basic range of diurnal temperatures, capable of extending up to 10–11 °C or as little as 1–3 °C dependent on habitat they demonstrate comparable physiological responses to temperature changes (Vladimirova Reference Vladimirova2000, Dell et al. Reference Dell, Pawar and Savage2011). Therefore these results are equally applicable to both freshwater and marine environments.
The effects of temperature on cercarial development generally demonstrate typical doubling or tripling of rates over 10 °C increases. Elevated rates were only apparent in the low temperature ranges associated with measurements taken upwards from the MDTT, returning to normal biological activity over optimal temperature conditions, thereby demonstrating responses similar to those shown by many ectothermic animals (Newell, Reference Newell and Wieser1973; Precht et al. Reference Precht, Laudien, Havsteen, Precht, Christophersen, Hensel and Larcher1973). The MDTT was often not accurately determined, primarily due to the technical difficulties involved. Nevertheless, the MDTT clearly occurred at lower temperatures for mid-latitude species than in low-latitude species with some evidence to suggest differences between geographical strains of species e.g. Schistosoma haematobium. Furthermore, development rates were rarely determined in the vicinity of the MDTT, principally because the main indicator of development was the first occurrence of emerged cercariae, and the METT occurs at a higher temperature than the MDTT. Therefore Q 10 values for low temperature ranges do not demonstrate the same elevated values as found for emergence over similar ranges. Measurements at temperatures above optimal conditions were more restricted, due to the thermal limitations of the molluscan host, but appeared to show no decline in the rate of development, rather thermostability or a doubling of physiological activity was the norm. These results indicate that cercarial development is not excessively influenced by temperature and appears unlikely to support sustained levels of elevated emergence over prolonged periods, although this requires further work to conclusively determine. A small number of species or strains demonstrated thermostability over a wide temperature range of 20 °C. Thermostability over such an extensive temperature range has previously been demonstrated for the survival/metabolism of certain miracidial species (Morley, Reference Morley2012a) suggesting that at least some trematode species are exceptionally thermotolerant. This ability may be related to climatic conditions encountered within specific habitats as only the Australian strain of F. hepatica demonstrates such extensive thermotolerance in its cercarial development.
Intramolluscan trematode stages appear to be more resistant to high temperatures than their molluscan hosts (Vernberg, Reference Vernberg1969; Lee and Cheng, Reference Lee and Cheng1971), tending to have thermal optima that reflect the body temperature of their definitive hosts (Vernberg, Reference Vernberg1961). Therefore the responses of infected molluscs to above optimal temperatures are likely to be a limiting factor in determining the presence of trematodes under these conditions. In general, mortality rates of parasitized snails are higher under extreme elevated temperatures compared with uninfected molluscs (Stirewalt, Reference Stirewalt1954; Vernberg and Vernberg, Reference Vernberg and Vernberg1963; McDaniel, Reference McDaniel1969; Paull and Johnson, Reference Paull and Johnson2011) although the opposite has also been reported (Riel, Reference Riel1975) suggesting that differences in season, strain, habitat, or laboratory conditions may influence thermal tolerance.
The present study has shown that the thermal biology of cercarial emergence when studied over a wide temperature range and corrected for acclimation is not excessively influenced by temperature over typical optimal ranges. Nevertheless, at low temperature ranges very high Q 10 values occur whilst at high temperature ranges there is a tendency for Q 10 values to demonstrate a decline in emergence rates. However, 2 acclimated studies do not correspond to this pattern. The first, Haplorchis pumilio (India strain) a low-latitude species, demonstrates stability over the optimal range of ≈25 °C but at the higher range shows an elevated Q 10 value. This may be an experimental artefact as measurements of emergence at 25 °C were substantially lower than those found at 20 °C, 30 °C, and 35 °C, thereby generating the high Q 10 value. This study highlights the occasional difficulties in generating consistent results. The second study on the mid-latitude species Maritrema novaezealandis (Otago Bay 3) demonstrated an extremely high Q 10 value over an optimal range of ≈20 °C, followed by a more typical low Q 10 value of 0·75 at the high range of ≈25 °C. The study was undertaken at a particularly high standard and no deficiencies in experimental protocol are apparent. The elevated Q 10 value is therefore associated with an unusually high METT of 16 °C, more typical of low-latitude than mid-latitude species, and a much narrower than expected optimum range for emergence of less than 10 °C. Combined, these two factors generate this atypical Q 10 value at ≈20 °C. This result is clearly unusual when compared with other mid-latitude species and may indicate that either the species, or the habitat have contributed to its uncommon thermal biology, thereby highlighting the limitations of making large-scale comparisons of this kind.
Overall, at low temperature ranges both mid- and low-latitude species demonstrate high Q 10 values. Comparing the low cercarial output around the METT with those occurring 10 °C higher, in the optimum range, generates the elevated Q 10 values. Emergence is depressed at low temperatures because cercariogenesis by the trematode intramolluscan stages is reduced in order to produce either more daughter redia or secondary daughter sporocysts (Dinnik and Dinnik, Reference Dinnik and Dinnik1964; Hansen, Reference Hansen1975). This temperature range represents a transitional phase from little to no cercariogenesis at the METT to a maximum rate of cercariogenesis as the temperature rises into the optimum range. It is therefore the developmental priorities of these intramolluscan stages, influenced by temperature, that ultimately dictate the levels of cercarial emergence over this low temperature range.
Temperature at optimum ranges is shown to have only a limited or no effect on emergence in both acclimated low- and mid-latitude species. The majority of studies have shown stable, or a slight decline in Q 10 values at these temperature ranges. There is therefore no excessive effect of temperature on emergence at these ranges suggesting that emergence would not be directly affected by such thermal regimes under natural conditions.
At the high temperature ranges, emergence rates generally declined in most acclimated studies. Such a decline is unlikely to be due to a reduced capacity for cercariogenesis, as intramolluscan stages demonstrate no comparable fall in developmental rates and appear to be better adapted to higher rather than lower temperatures e.g. Vernberg (Reference Vernberg1961). A decrease in cercarial output is likely to be associated with a decline in the physiological status of the molluscan host. Mortality rates of infected molluscs are usually high at elevated temperatures e.g. Stirewalt (Reference Stirewalt1954), whilst cercarial emergence declines in a dying host (Pflüger et al. Reference Pflüger, Roushdy and El Emam1984; Karvonen et al. Reference Karvonen, Kirsi, Hudson and Valtonen2004). This reduction in cercarial output may be associated with reduced host activity as it approaches death (Boissier et al. Reference Boissier, Rivera and Mone2003), an important component of emergence rhythms (Anderson et al. Reference Anderson, Nowsielski and Croll1976), and a lower level of host feeding (Karvonen et al. Reference Karvonen, Kirsi, Hudson and Valtonen2004), a necessary factor for maintaining normal levels of cercarial production (Ataev, Reference Ataev1991).
Both the MDTT and METT may not be fixed points for any particular species or strain. Sous (Reference Sous1992) found that under natural conditions the METT of the mid-latitude freshwater species Diplostomum chromatophorum changed throughout the summer season from 15 °C in July, gradually falling to 9 °C by October. Similarly Lyholt and Buchmann (1996) under controlled laboratory conditions were able to slowly acclimate D. spathaceum over a 6-week period to water temperatures down to 3 °C so that the METT decreased from 10 °C to temperatures as low as 4–6 °C. As emergence continued over the entire 42-day experiment it indicates that cercarial development was not interrupted and that the MDTT decreased in parallel to the METT. This variation in the METT according to environmental conditions would certainly explain differences in the low temperature emergence of S. mansoni where the METT was determined to be 16 °C in a Liberian strain (Pflüger, Reference Pflüger1980) but output of cercariae occurred at 12 °C in a strain of unknown origin (Fried et al. Reference Fried, Laterra and Kim2002).
Indeed, strain-specific differences have been found to be a characteristic of both development and emergence of cercariae and have also been demonstrated for their survival/metabolism (Morley, Reference Morley2011a). Such differences are probably associated with specific thermal regimes found in individual aquatic habitats (Morley, Reference Morley2011a). Nevertheless, some caution must be taken with the many studies in the present analysis that have been derived from laboratory strains which can become inbred, altering aspects of the functional biology (Morley, Reference Morley2011b). However, widespread interspecific and intra-specific differences in free-living or mollusc-associated trematodes are apparent under natural conditions suggesting that laboratory cultures may not respond to changes in environmental conditions beyond what would be expected due to normal variation.
Acclimation has been found to be a crucial element in interpreting emergence data in the present study. Its significance in thermal biology, in general, has been known for a long time and measurements of fully acclimated animals at an ET that corresponds to the AT are of particular importance, and the only way of determining whether variations in Q 10 values are either due to relevant biological aspects of the species or because of differences in the AT. Without acclimation inter- and intra-specific comparisons between species cannot realistically be undertaken at all (Precht et al. Reference Precht, Laudien, Havsteen, Precht, Christophersen, Hensel and Larcher1973). It is to be hoped that future studies will incorporate this important aspect of thermal biology into their experimental protocols.
Nevertheless, there may be a temptation to regard the non-acclimated emergence data of this study as being equivalent to cercarial output under abrupt thermal stress. This would be misguided. Most of this data represents the influence of a multitude of different stressors that the mollusc-trematode relationship has not acclimated to, such as light regime and intensity, water quality and quantity, and handling, which must be taken in to account. Furthermore, it is not possible to quantify temperature effects from these sources as they lack accompanying baseline data to compare the results against. Thus, studies on abrupt thermal stress require groups of properly acclimated animals whose responses can be directly compared against other acclimated animals not thermally stressed.
All studies in the present analysis have been undertaken at constant temperatures. Yet temperatures under natural conditions vary both diurnally and seasonally potentially questioning the validity of such experiments. Nevertheless, in a few cases cercarial development and emergence have been recorded under diurnal sinusoidal variations (Pflüger, Reference Pflüger1981; Al-Habbib and Grainger, Reference Al-Habbib and Grainger1983; Roushdy, Reference Roushdy1984). In general, these studies found that temperatures varying by 10 °C were not substantially different from constant temperature experiments undertaken at the median value between the two temperatures e.g. variations between 20 and 30 °C had a similar result to those undertaken at a constant 25 °C (Roushdy, Reference Roushdy1984), indicating the relevance of constant temperature experiments to natural conditions.
Unlike the results of Poulin (Reference Poulin2006) the present analysis has shown that constant temperature conditions have only a limited effect on cercarial development and emergence over optimum conditions and are concordant with previous studies on cercarial and miracidial survival/metabolism (Morley, Reference Morley2011a, Reference Morley2012a). These findings highlight the importance of considering a wide range of variables when conducting large-scale analyses. Certainly, one conceivable interpretation of this study suggests that temperature may have little or no direct effect at all on emergence. At low temperatures emergence is controlled by the degree of cercariogenesis within the intramolluscan stages, whilst at high temperatures the effects of thermal sensitivity on the health and longevity of molluscan hosts dictate the length and intensity of cercarial output. Consequently, variations in cercarial emergence over temperature ranges cannot be conclusively linked directly to any specific aspect of the biological activity of leaving the molluscan host. This suggests that any changes in cercarial population dynamics under climate change during summer conditions are unlikely to be associated with any direct effect of temperature on their functional biology. Nevertheless, an increase in average temperatures under a changing climate may lengthen the seasonal cercarial transmission window further into the spring and autumn, by maintaining the water temperature above the METT for longer, thereby extending the risk of parasitism to target hosts.
In conclusion, a greater understanding of the variations in cercarial populations under climate change are now more likely to be found in the field rather than in the laboratory. Cercarial numbers are known to vary both spatially and temporarily in aquatic ecosystems influenced by a range of factors, many of which are difficult to study in the laboratory, such as wind, water currents, and the specific geomorphology and biota of a habitat. However, direct measurement studies have been few, with cercariometry largely restricted to schistosomes (Aoki et al. Reference Aoki, Sata, Muhoho, Noda and Kimura2003, Morley, Reference Morley2012b). Yet this approach may provide a better insight into the influence of climate, particularly seasonal changes, for structuring the occurrence of cercariae within individual habitats, and the viability of the population as a risk to target hosts, than can be achieved by measuring cercarial output under the limitations of laboratory experiments. It would therefore be beneficial if future studies further explored this aspect of cercarial population dynamics.