INTRODUCTION
Take away a cornerstone and any edifice will collapse or, at best, start to erode in a way that may make the edifice unsafe. The edifice that we consider here is the understanding of patterns of host specificity of trematodes of tropical marine fishes of Queensland, essentially the Great Barrier Reef (GBR). Much more than in parasitological fields where only one or a handful of species are considered, the broad-scale analysis of host specificity in a rich system such as this is reliant on the accurate recognition of hundreds of species of fish and trematodes. If the idea of identification is extended to the correct allocation of the individual worms from each individual fish examined, then tens of thousands of identification decisions are involved in reaching an accurate understanding of such a system. It is easy for such decisions to be mistaken, inaccurate or incomplete, and thus for our understanding of host specificity to be inaccurate. As knowledge of the fauna develops we may add new host/parasite combinations to the data-set and we may reconsider existing ones. Reconsideration can take the form of changes in the concept of the species (splitting or synonymy), changes in the understanding of prevalence and intensity that may affect our understanding of the significance of a given host/parasite combination, and the recognition and rooting out of spurious records. Progress in all these processes together comprises the systematic cornerstone of analysis of host specificity. A key observation is that the conceptual understanding that is emerging for this system is leading to an iterative feedback into our understanding of the reliability of the underlying data. Thus, here we analyse both the ‘cornerstone’, the data-set of records of GBR fish trematodes, and the ‘edifice’ of the understanding of host specificity that is built around it.
The host range of a parasite, its host specificity, is a key aspect of its biology (Poulin and Mouillot, Reference Poulin and Mouillot2003, Reference Poulin and Mouillot2005a, Reference Poulin and Mouillotb). Understanding of host range has implications for the nature of transmission, physiology, the understanding of disease, and major implications for the understanding of evolutionary processes such as co-speciation (Paterson and Banks, Reference Paterson and Banks2001) and host acquisition. Despite this central importance, there is a surprising shortage of quantitative data on the host specificity of many groups of parasites. One such group is the trematodes of fishes. These parasites are generally regarded as being quite variable in their host specificity. Some fish trematode species, for example the derogenid Derogenes varicus (Müller, 1784), have exceptionally low host specificity (Gibson et al. Reference Gibson, Bray and Harris2005) whilst other species are seemingly specific to individual species of fishes. The distribution of the different types of specificity has, however, not been analysed for a major data set. Here we analyse the host specificity of trematodes of fishes of the Great Barrier Reef and adjacent tropical Queensland waters to test the prediction that: ‘The majority of trematodes of coral reef fishes (in this case GBR fishes) are strongly host specific in their distributions’. This region is rich in trematodes (290 fully identified species in our analysis) and in fishes (243 fish species reported infected so far). Most of these records were made by the present authors and our collaborators. The advantage of this is that whatever taxonomic biases we may have (lumping or splitting) we are likely to have been relatively consistent in applying the bias. Here we analyse these data to explore the distribution of trematode species, genera and families relative to fish species, genera, families and orders.
In our consideration of the nature of the patterns of host specificity that we describe we rely on concepts first developed by Euzet and Combes (Reference Euzet and Combes1980). The first concept relates to their introduction of the descriptors ‘oioxenous’, ‘stenoxenous’ and ‘euryxenous’, referring respectively to specificity to individual host species, to closely related host species, and to host species only related by ecophysiological similarity. The second concept is in the explanation of host specificity. Euzet and Combes (Reference Euzet and Combes1980) invoked two filters to successful infection – encounter (whether the parasite can reach the host) and compatibility (whether it can establish in a host once it reaches it). We also explore the host specificity of trematodes of GBR fishes using a quantitative index recently developed by Poulin and Mouillot (Reference Poulin and Mouillot2003). This index incorporates information about taxonomic differences in the host range of parasite species in order to quantify relative specificity. In the light of the goals of the present volume we return periodically to the question of the reliability of the data that we analyse.
MATERIALS AND METHODS
Data-set compilation
We analysed published data and considered only records from marine, tropical waters of Queensland, mainly from Heron Island (23°29′S, 151°55′E) in the south and Lizard Island (14°40′S, 145°28′E) in the north up to a cut-off date of November 2010. Fish classification is based on that in Fishbase (Froese and Pauly, Reference Froese and Pauly2010). Trematode classification is as recognised in the current literature. All records of parasite family, genus and species and host order, family, genus and species were entered into an EXCEL spreadsheet. A master spreadsheet of unique host-parasite combinations was derived from this. This was in turn examined by pivot table analysis to explore a range of distributions. This spreadsheet and the references from which it is sourced is made available for use by other workers as Supplementary data (see supplementary material at Cambridge Journals On-line – http://journals.cambridge.org.PAR).
Host specificity index
The host specificity index S TD developed by Poulin and Mouillot (Reference Poulin and Mouillot2003) was calculated using the TaxoBiodiv2 software (http://www.otago.ac.nz/zoology/downloads/poulin/) Poulin and Mouillot (Reference Poulin and Mouillot2005a). This index was used to calculate the average taxonomic distance among fish infected by a particular trematode species. We were unable to calculate the S TD* index of Poulin and Mouillot (Reference Poulin and Mouillot2005a) as accurate prevalence data were not available for all of the trematode taxa examined. The host taxonomic hierarchy used to calculate S TD included the categories phylum, class, order, family, genus and species and was based on the current classification recognized by Fishbase and the Integrated Taxonomic Information System (http://www.itis.gov/). Using these six taxonomic levels, the highest value the S TD index can take is 5 (when all hosts belong to different classes). An S TD value of 1 means that all of the host species for a given parasite species are congeners and a value of 0 means that there is only 1 host known for a given parasite species.
Results
The data-set
Records of identified trematode species from fully identified fish species are given in the Supplementary data (see supplementary material at Cambridge Journals On-line – http://journals.cambridge.org.PAR). There are 658 unique host-parasite combinations for 290 trematode species, 152 genera and 28 families. These are distributed among 8 orders, 38 families, 117 genera and 243 species of fishes. Table 1 summarises numbers of species reported so far from each trematode family. Coverage of the fauna is uneven. Several significant families (e.g. Didymozoidae, Haploporidae, Opecoelidae, Monorchiidae and Zoogonidae) remain incompletely studied based on the unreported material in our collection that has yet to be described formally. Some others (e.g. Acanthocolpidae, Aporocotylidae, Cryptogonimidae and Lepocreadiidae) are much better known as these families have been the focus of a number of intensive studies recently (Bray et al. Reference Bray, Cribb and Barker1993c, Reference Bray, Cribb, Waeschenbach and Littlewood2007; Bray and Cribb, Reference Bray and Cribb1998, Reference Bray and Cribb2007; Nolan and Cribb, Reference Nolan and Cribb2006a; Miller and Cribb, Reference Miller and Cribb2007a).
Table 1. Numbers of species of fully identified trematodes from 28 families reported from tropical Queensland fishes.

Trematode species
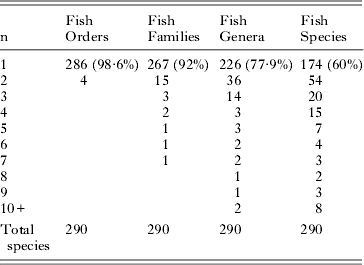
The reported host specificity of trematode species in the system is summarised in Table 2. Of the 290 species, only 4 (approximately 1·4%) have been reported from more than one order of fishes. These are: Bivesiculidae – Paucivitellosus fragilis Coil, Reid and Kuntz, 1965; Hemiuridae: Ectenurus trachuri (Yamaguti, 1934) and Tubulovesicula angusticauda (Nicoll, 1915); and Lepocreadiidae: Lepotrema clavatum Ozaki, 1932. At lower taxonomic levels, 92% of species are known from only one family, 77·9% from only one genus, and 60% from only one species of fish. Species that have been reported from fewer than five species account for 93% of all species. Thus, overall, host specificity of these fish trematode species is high. Table 3 shows the recorded host family distribution of the 23 species that have been reported from more than one fish family. The species include representatives of 10 trematode families and 21 genera.
Table 2. Numbers of the 290 trematode species reported from the Great Barrier Reef here and their distributions among their fish host orders, families, genera and species. The ‘n’ on the left refers to the number of orders, families, genera or species a given species of trematode has been reported (e.g. 15 trematode species have been reported from 2 different fish families).
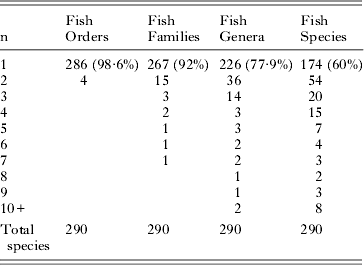
Table 3. The host family distribution of the twenty-three ‘euryxenous’ trematode species reported from GBR fishes.

Host specificity index
The calculated range for the host specificity index S TD developed by Poulin and Mouillot (Reference Poulin and Mouillot2003) observed for all of the trematode species reported here was 0–3·67 and the mean 0·791. The low mean is the quantitative reflection of the generally oio- to stenoxenicity described qualitatively above. Table 1 also shows the range and mean S TD values calculated for each trematode family. The two highest figures returned, 3·5 and 3·67, were recorded for the hemiurids, Ectenurus trachuri and Tubulovesicula angusticauda. Key observations are that for most trematode families the mean S TD is around 1 or lower, which again reiterates, quantitatively, the general trend towards oio- to stenoxenicity in trematodes from tropical marine fishes of the GBR.
Oioxenicity
Oioxenous species, i.e. those infecting only a single host species, can be divided into three categories. The first category includes those species that infect a host species that has no close relatives. In this category we place Schistorchis zancli Hanson, 1953 which infects Zanclus cornutus, the only species in this family (Lo et al. Reference Lo, Morgan, Galzin and Cribb2001). Similarly, six species of the cryptogonimid genus Retrovarium Miller and Cribb, 2007 are in this category (Miller and Cribb, Reference Miller and Cribb2007a), as they are reported only from Symphorus nematophorus, which is the only species of its genus. The second category is those species that infect a single host species despite the presence in the system of other seemingly potential related hosts. In this category is Hurleytrematoides justinei McNamara and Cribb, Reference McNamara and Cribb2009 which has been found repeatedly in Canthigaster valentini but in none of 15 individuals of 3 other species of Canthigaster (McNamara and Cribb, Reference McNamara and Cribb2009). Similarly, numerous species of aporocotylids in lutjanids and siganids have been found in only single species despite significant numbers of sympatric congeners having been examined (Nolan and Cribb, Reference Nolan and Cribb2006a, Reference Nolan and Cribbb). For both these categories we do not expect our understanding of the nature of the specificity to change. The third category contains species known from only a single host species where we have either already collected but not published further host records, or there are further closely related host species yet to be examined in the system. For example, the aporocotylid Cardicola chaetodontis Yamaguti, 1970 is presently reported from just one species of Chaetodon on the GBR, but evidence suggests that it infects many more species.
Stenoxenicity
Stenoxenicity for trematode species is here pragmatically (and initially) defined as restriction to one family of fishes but infection of more than one species of that family. Of the 290 species in the data-set, 93 (32%) are stenoxenous by this definition. Examples of this form of specificity occur in all but six small or little studied trematode families. There are numerous examples of restriction to multiple congeners. For example, the lecithasterid Quadrifoliovarium maceria Chambers and Cribb, 2005 is known from three species of Naso (Chambers and Cribb, Reference Chambers and Cribb2006), the bucephalid Prosorhynchus robertsthomsoni Bott and Cribb, Reference Bott and Cribb2009 is known from three species of Cephalopholis (Bott and Cribb, Reference Bott and Cribb2009) and the faustulid Paradiscogaster quasimodo Bray, Cribb and Barker, Reference Barker, Cribb, Bray and Adlard1994 is known from four species of Chaetodon (Bray et al. Reference Bray, Cribb and Barker1994). Equally, there are numerous examples of distributions in multiple confamilial genera. Thus two species of Lepidapedoides (Lepocreadiidae) are both reported from two genera of caesionine lutjanids (Bray et al. Reference Bray, Cribb and Barker1996), the derogenid Derogenes pharyngicola Bray, Cribb and Barker, Reference Barker, Bray and Cribb1993 is known from two genera of pomacentrids (Bray et al. Reference Bray, Cribb and Barker1993c), and the haplosplanchnid Hymenocotta mulli Manter, 1961 is known from five genera of Mugilidae (Durio and Manter, Reference Durio and Manter1968a; Lester and Sewell, Reference Lester and Sewell1990; Cribb et al. Reference Cribb, Bray and Barker1994a, Reference Cribb, Bray, Littlewood, Pichelin, Herniou, Littlewood and Bray2001; Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003).
An interesting case of stenoxenicity relates to a cryptogonimid, Mitotrema anthostomatum Manter, 1963, which has been found in just two serranids, Epinephelus fuscoguttatus and Cromileptes altivelis (Cribb et al. Reference Cribb, Bray, Barker and Adlard1996; Bott and Cribb, Reference Bott and Cribb2009). This host distribution is initially surprising because E. fuscoguttatus appears to be a ‘typical’ species of Epinephelus and large numbers of individuals of other species of the genus have never revealed infections of this species. The discrepancy was explained, however, when the phylogeny of the Serranidae was explored by molecular phylogenetics (Craig et al. Reference Craig, Pondella, Franck and Hafner2001; Craig and Hastings, Reference Craig and Hastings2007). It was shown that these two serranids are closely related and that Cromileptes is nested within Epinephelus so that C. altivelis is effectively a rather specialised species of Epinephelus. The phylogenetic relatedness of these species is evidently reflected by a combination of physiological and trophic similarity so that both are susceptible to infections with the same species of trematode which utilizes small fishes as second intermediate hosts (Cribb et al. Reference Cribb, Bray, Barker and Adlard1996). Thus, an anomalous specificity that was seemingly stenoxenic to family level, is convincingly interpreted as stenoxenic to intra-generic level.
Euryxenicity
As a starting point, euryxenicity is defined pragmatically here as distribution in more than one family of fishes. Of the 290 species in the data set, just 23 (8%) are euryxenous by this definition (Table 3). Careful examination of the species involved suggests that several distinct effects are in operation and that trematode family identity is important.
Nine of the 23 species with multiple host families are from the superfamily Hemiuroidea (Hemiuridae, Lecithasteridae and Sclerodistomidae). Although the identity of none of the species has been explored comprehensively with molecular approaches, it seems apparent that this superfamily is unique in that many species exhibit low host specificity. Notably, however, euryxenic specificity does not apply to all species in these families; many species show distinct specificity as, for example, the quadrifoliovariine lecithasterids of acanthurids (Chambers and Cribb, Reference Chambers and Cribb2006) for which molecular data are available. However, exceptionally low specificity has been reported elsewhere previously for the derogenid Derogenes varicus, several hemiurids such as Hemiurus communis Odhner, 1905, H. luehei Odhner, 1905 and Brachyphallus crenatus (Rudolphi, 1802) (Gibson and Bray, Reference Gibson and Bray1986) and the hirudinellid Hirudinella ventricosa (Pallas, 1774) (Gibson and Bray, Reference Gibson and Bray1977). All these cases are worthy of exploration by way of molecular methods, but we see no prospect that the pattern of numerous hemiuroids with exceptionally low host specificity will be overturned.
Despite the comparatively low specificity of the hemiuroid species in our data set, they certainly do not lack specificity. None has been found in more than 6 of the 38 families of fishes in the study. The host families tend to have recognisable ecological similarity. For example, the hemiurid Plerurus digitatus (Looss, 1899) has been reported from five families of large piscivores – Carangidae, Lutjanidae, Scombridae, Serranidae and Sphyraenidae (Bray et al. Reference Bray, Cribb and Barker1993a). The sclerodistomid Prosogonotrema bilabiatum Vigueras, 1940 is reported from caesionine and lutjanine lutjanids and from an ephippid (Manter, Reference Manter1969a; Lester and Sewell, Reference Lester and Sewell1990; Cribb et al. Reference Cribb, Bray, Littlewood, Pichelin, Herniou, Littlewood and Bray2001; Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003). The host species are all mid-water feeders and the ecological distribution is reinforced by the absence of this species from numerous demersal lutjanids. The species with the widest host family distribution, Thulinia microrchis Yamaguti, 1934, is known so far from six families – Chaetodontidae, Lethrinidae, Pomacanthidae, Pomacentridae, Serranidae and Siganidae (Manter, Reference Manter1969b; Lester and Sewell, Reference Lester and Sewell1990; Bray et al. Reference Bray, Cribb and Barker1993b; Barker et al. Reference Barker, Cribb, Bray and Adlard1994), which are not strongly linked by any obvious dietary similarity. Unpublished molecular evidence supports the conspecificity of T. microrchis from several of these.
Five species of Lepocreadiidae are reported from more than one fish family. Of these, Hypocreadium patellare (Yamaguti, 1938) infects both monacanthids and balistids which are sister taxa, rendering this distribution plausible and effectively consistent with a stenoxenic rather than a euryxenic classification. Multitestis coradioni Bray, Cribb and Justine, Reference Bray, Cribb and Justine2010 was described from multiple specimens from a chaetodontid species and from a single specimen from a serranid, Epinephelus cyanopodus, which was considered an accidental host (Bray et al. Reference Bray, Cribb and Justine2010). We have examined 11 specimens of this serranid and hundreds of other serranids without finding more specimens. Similarly, Preptetos cannoni Barker, Bray and Cribb, Reference Barker, Bray and Cribb1993 is abundant in three siganid species but has also been seen as a single infection from a pomacentrid (Barker et al. Reference Barker, Bray and Cribb1993, Reference Barker, Cribb, Bray and Adlard1994; Bray et al. Reference Bray, Cribb and Barker1993b; Bray and Cribb, Reference Bray and Cribb1996). We have no basis to disregard the rare host-parasite combinations of Multitestis coradioni or Preptetos cannoni, but we suspect that they are of little biological importance in that the outlier hosts are infected so infrequently that it is reasonable to consider them accidental. In contrast, Preptetos xesuri (Yamaguti, 1940), predominantly a parasite of acanthurids, has been recovered from 2 of 6 specimens of the large pomacentrid Parma polylepis (Bray et al. Reference Bray, Cribb and Barker1993c) and Lepotrema clavatum Ozaki, 1932 has a remarkable distribution incorporating balistids, monacanthids, pomacentrids and (unpublished) blenniids. Thus, of the five superficially euryxenic lepocreadiids, one is effectively stenoxenic, two are stenoxenic except for exceptionally rare stragglers, and two are presently convincingly euryxenic.
Three species of Opecoelidae are reported from multiple host families and different circumstances apply to each. Pseudoplagioporus interruptus Durio and Manter, 1968 is a common parasite of several lethrinid species (Bray and Cribb, Reference Bray and Cribb1989). It was in addition reported from a haemulid by Durio and Manter (Reference Durio and Manter1968b). We have examined 102 haemulids and, although they are rich in opecoelids, we have never encountered this species; we suspect that Durio and Manter's (Reference Durio and Manter1968b) record was made in error or was an inconsequential accidental infection. Macvicaria heronensis Bray and Cribb, Reference Bray and Cribb1989 is common in lethrinids and has been recorded once in one of over 60 pomacentrid species examined. We thus interpret it as being effectively stenoxenic but with rare, probably accidental infections. Helicometra fasciata (Rudolphi, Reference Rudolphi1819) is far more complex. This species was first described from the Mediterranean Sea off Naples (Rudolphi, Reference Rudolphi1819) and although frequently reported elsewhere it is likely that several species are involved. On the GBR there are reports of forms that relate to this morphology from serranids and labrids and unreported material is known from several other families. Combined molecular and morphological work in progress suggests that two species are involved but that at least one of them does indeed have a wide host distribution. Enzyme electrophoretic studies in the Mediterranean have indicated that there are three cryptic species there (Reversat et al. Reference Reversat, Renaud and Maillard1989, Reference Reversat, Maillard and Silan1991), although other work has shown low and confusing levels of specificity in ‘H. fasciata’ (Paniagua et al. Reference Paniagua, Vilas, Sanmartin, Santamarina, Leiro and Ubeira1999).
Until recently the published literature indicated that two transversotrematid species from the region, Transversotrema haasi Witenberg, 1944 and T. licinum Manter, 1970, infect members of three and seven families respectively (Cribb et al. Reference Cribb, Bray and Barker1992). However, recent molecular studies (Hunter and Cribb, Reference Hunter and Cribb2010) have interpreted Transversotrema haasi as three new species (Hunter et al. Reference Hunter, Ingram, Adlard, Bray and Cribb2010). Two of these are stenoxenous in being restricted to a single family. The third, T. lacerta Hunter, Ingram, Adlard, Bray and Cribb, Reference Hunter, Ingram, Adlard, Bray and Cribb2010, infects members of two closely related families so that like Hypocreadium patellare of the Lepocreadiidae, it is effectively stenoxenous. Results for the “T. licinum” complex are not yet published but it may comprise as many as 15 species on the GBR, almost all with stenoxenous host specificity.
The bivesiculid Paucivitellosus fragilis Coil, Reid and Kuntz, 1965, the only euryxenic member of its family in this region, is exceptional in infecting blenniids and mugilids (Pearson, Reference Pearson1968; Lester and Sewell, Reference Lester and Sewell1990; Cribb et al. Reference Cribb, Bray and Barker1994a), which belong to separate orders of fishes. This host specificity has seemed plausible in the light of the ecological connection between these fishes – browsing on beach rock where infected first intermediate hosts occur (Pearson, Reference Pearson1968). However, Le Zotte (1954) commented that this family normally shows high host specificity so this unusual distribution warrants further attention.
The fellodistomid Proctoeces maculatus (Looss, 1901), the only euryxenic member of its family on the GBR, has been reported from labrids and sparids which, although both perciforms, are not otherwise especially closely related. Bray (Reference Bray1983) proposed sweeping synonymy in the genus Proctoeces on the basis that reported variation did not justify the recognition of multiple species. However, recent studies of this genus have indicated that several species occur, with rDNA sequences diverging between specimens from Queensland (Hall et al. Reference Hall, Cribb and Barker1999), the Gulf of Mexico (Olson et al. Reference Olson, Cribb, Tkach, Bray and Littlewood2003) and off Chile (Oliva et al. Reference Oliva, Valdivia, Cardenas, George-Nascimento, Gonzalez, Guinez and Cuello2010). Valdivia et al. (Reference Valdivia, Cardenas, Gonzalez, Jofré, George-Nascimento, Guiñez and Oliva2010) considered that there are at least three species in the genus and we will not be surprised if this genus proves to comprise more than one species in Queensland waters.
The monorchiid Paramonorcheides pseudocaranxi Dove and Cribb, 1998 has been reported from carangid and haemulid fishes; there is no ready explanation of this unusual distribution.
Thus, of the 22 superficially euryxenic GBR fish trematode species, 9 are hemiuroids which appear to have a genuine and unique propensity for euryxenicity, 2 are effectively stenoxenous because of the close relationship between their known host families, at least four have distributions that are stenoxenic except for inconsequentially rare infections, for one there is unpublished evidence of a complex of stenoxenous species and just six non-hemiuroids (‘Helicometra fasciata’, Lepotrema clavatum, Paramonorcheides pseudocaranxi, Paucivitellosus fragilis, Preptetos xesuri and Proctoeces maculatus) remain apparently euryxenic after more careful consideration.
Specificity of trematode genera and families
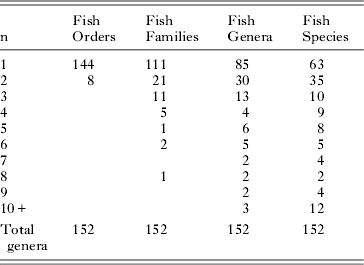
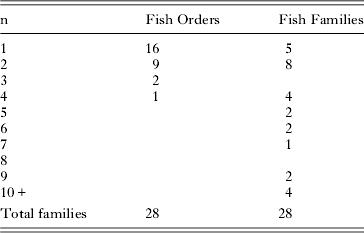
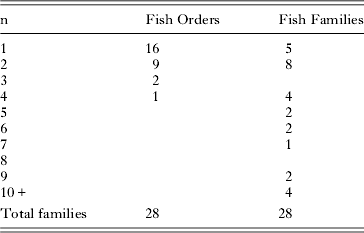
Table 4 summarises the distribution of numbers of trematode genera in numbers of fish orders, families, genera and species. The distributions again show relatively high specificity in that, in most categories, more than half the taxa are restricted (stenoxenic) to no more than two host taxa. Table 5 shows the distribution of trematode families in numbers of fish orders and families. At this level of distribution, restricted (stenoxenic) distributions have disappeared almost entirely. Just five trematode families are reported from only one fish family. These families (Didymozoidae, Gorgocephalidae, Haploporidae, Hirudinellidae and Microscaphidiidae) are either small (Gorgocephalidae n=2, Hirudinellidae n=1) or, in the case of the other three, have been little studied in these waters and certainly have broader family distributions than the current data set demonstrates. Overall at this level, trematode families are overwhelmingly euryxenic, infecting a broad range of families that share ecological similarity.
Table 4. Numbers of the 152 trematode genera reported from the Great Barrier Reef here and their distributions among their fish host orders, families, genera and species. The ‘n’ on the left refers to the number of orders, families, genera or species a given genus of trematode has been reported (e.g. 21 trematode genera have been reported from 2 different fish families).
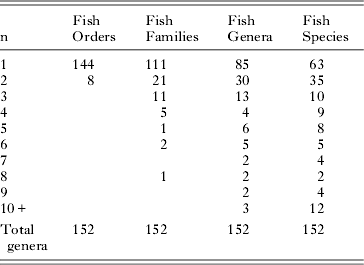
Table 5. Numbers of the 28 trematode families reported from the Great Barrier Reef here and their distributions among their fish host orders and families. The ‘n’ on the left refers to the number of orders and families a given family of trematode has been reported from (e.g. 9 trematode families have been reported from 2 different fish orders).
Discussion
Host specificity of species
The clear result of this analysis is that species of trematodes of fishes of the GBR and adjacent waters have high host specificity in that 60% are known from a single host species, 91% from no more than 4 host species, 78% from a single host genus, and 92% from a single host family. Despite this generally high (oio- to stenoxenous) host specificity, about 8% of the species are reported to infect more than one family of fishes. We see evidence, however, that some of the reports of euryxenous species are real, some have been made in error, some are euryxenous only if rare infections are included, and some are effectively stenoxenous because of the close relationship of the fish families infected. On this basis it is arguable that the proportion of oioxenous and stenoxenous species is actually higher than the 92% that we have calculated. However, further sampling may well reveal that some species presently categorised as oio- or stenoxenous will prove to be euryxenous. We therefore conclude that the figure of 92% oio/stenoxenicity is unlikely to change greatly as the fauna becomes better known, although the details of individual species may change dramatically. Perhaps most importantly, the figure for the proportion of steno- and euryxenicity will change depending on how these descriptors are defined. As we have seen, the possible recognition of accidental infections and host sharing at different taxonomic levels makes such judgements significantly subjective.
The summary of these observations completes an iterative loop in our understanding of the host specificity of species in this system. We now appreciate that oioxenicity or stenoxenicity are the typical patterns except for a subset of hemiuroids and a few isolated species from five trematode families. For the euryxenous hemiuroid taxa we can now contemplate the ecophysiological basis of the low specificity. For the six non-hemiuroids our conclusion is that we should doubt the records – mainly our own work! Studies of the Transversotrematidae in this system have shown that our initial morphology-based studies are capable of drastically underestimating species richness. We now suspect that inconsequentially rare samples, handling errors, or failure to recognise species level differences underpin all six of these cases, or at the very least, that these possibilities must be explored further before the euryxenicity can be accepted. Thus, the iteration discussed in this paper is manifested by the chain of data collection leading to pattern recognition and pattern discrepancy recognition. Recognition of pattern discrepancies stimulates further data collection. Two practical observations are germane here. First, the power of molecular approaches to resolve issues of the kind seen here are the still relatively new key to resolving these questions. Second, we admit freely that the extent to which the six species mentioned here are seemingly exceptional was not apparent to us prior to this analysis, and since the commencement of the writing of this paper we have started to actively explore the validity of some of these distributions.
Thus, a key conclusion of this analysis is that we think that no apparent case of low specificity should be accepted as definitive unless it has been tested by experimental or molecular methods. This view has been advanced previously for other systems (Miura et al. Reference Miura, Kuris, Torchin, Hechinger, Dunham and Chiba2005; Nolan and Cribb, Reference Nolan and Cribb2005; Poulin and Keeney, Reference Poulin and Keeney2010; Poulin and Leung, Reference Poulin and Leung2010). We predict that our current understanding of some species distributions (especially those of hemiuroid species) will survive such analysis but that some others will fail. It is also evident that extensive sampling is necessary if the true status of accidental infections (or mistaken records) is to be revealed.
Host specificity of supra-specific taxa
It is no surprise that above the parasite species level, various higher taxa should have progressively lower levels of host specificity. However, it is certainly striking how comprehensively the pattern has changed at the level of parasite family. Every family that has at least a moderate number of species and has been studied in any depth infects a range of unrelated fish families. This is the classic reflection of euryxenous specificity where the parasite taxa are seemingly tracking eco-physiological resources (Poulin, Reference Poulin2005) in the form of broad diets and broad gastrointestinal compatibility. The trematode family with the largest number of host families is the Lepocreadiidae. This distribution reflects in part the fact that this family is the most intensively studied of all on the GBR, but also the fact that marine lepocreadiid metacercariae infect a range of invertebrates and, occasionally, small vertebrates (Bray et al. Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009) or encyst on vegetation (Hassanine, Reference Hassanine2006) and are, therefore, susceptible to consumption by a wide range of fishes.
For this system, the relationship between the number of families of fishes a trematode family is known to infect is broadly linear with the number of species that is known for it (Fig. 1). Points below the trend line imply that a family is unusually concentrated in certain fish families and above the line implies lower host specificity than is typical. By far the most concentrated significant family is the Cryptogonimidae which has so far been found in only three fish families. The concentration is all the more remarkable when it is realised that just one species (Mitotrema anthostomatum) is known from serranids and that all the other 34 species are from lutjanids and haemulids which are closely related (Miller and Cribb, Reference Miller and Cribb2007b). This result is certainly not related to under-reporting of cryptogonimids from other families of fishes; we are not aware of any unreported specimens from further families of fishes from this region although certainly many other families are known as hosts elsewhere. Cryptogonimids are transmitted as metacercariae in the flesh of other fishes (Cribb et al. Reference Cribb, Bray, Olson and Littlewood2003). The strikingly restricted host range implies that a compatibility filter must inhibit cryptogonimids from developing in and radiating in the other piscivorous families such as carangids, muraenids, scombrids, serranids, sphyraenids, synodontids, and others, that we have examined in the region. In dramatic contrast, bucephalids, which are also transmitted as metacercariae in the flesh of fishes, are so far known from apogonids, blenniids, carangids, labrids, muraenids, scombrids, serranids, sphyraenids and synodontids (not all yet published). Perhaps most strangely, bucephalids are entirely absent from haemulids and lutjanids in our collections. There is thus an almost perfect but unexplained mutual exclusion of bucephalids and cryptogonimids in the piscivores in our data; the sole exception is the presence of Mitotrema anthostomatum which occurs with species of Prosorhynchus in two species of serranids.

Fig. 1. Number of trematode species per family (x axis) and number of fish families in which they occur. Cryptogonimidae is the bottom-right most point.

Fig. 2. Frequency distribution of S TD values for the 290 species of trematodes identified from tropical Queensland fishes.
Encounter and compatibility
With the exception of the Aporocotylidae and the Transversotrematidae, it can be assumed that all trematodes of fishes of this region are transmitted in the diet of their hosts. There is a huge literature that explores the nature of the diet of coral reef fishes. There are carnivores, herbivores, planktivores, detritivores, omnivores and almost every possible combination of these. Specialised and generalised diets are associated with extraordinary morphological (Westneat and Wainwright, Reference Westneat and Wainwright1989), behavioural (Saeki et al. Reference Saeki, Sakai, Hashimoto and Gushima2005) and neurological specialisations (Almany et al. Reference Almany, Peacock, Syms, McCormick and Jones2007). Further, it is established that fish diets can change ontogenetically (Kolasinski et al. Reference Kolasinski, Frouin, Sallon, Rogers, Bruggemann and Potier2009; Cole Reference Cole2010), seasonally (Letourneur et al. Reference Letourneur, Galzin and HarmelinVivien1997) and geographically (Saeki et al. Reference Saeki, Sakai, Hashimoto and Gushima2005). Against such an exceptionally complex background, and in the almost complete absence of data on the precise identity of the animals infected with trematode metacercariae in these waters, it is difficult to draw precise conclusions about the relative roles of encounter and compatibility. Yet, some important generalised conclusions are still possible. First, the general pattern of euryxenicity of trematode families leads us to conclude that broad dietary habits define an important component of the encounter filter. Thus, herbivores are susceptible to infection with combinations of enenterids, gorgocephalids, gyliauchenids, haploporids, haplosplanchnids and microscaphidiids (perhaps all from the ingestion of metacercariae encysted on vegetation) and lecithasterids and lepocreadiids, probably from the ingestion of small invertebrates associated with vegetation (either incidentally or intentionally). Invertebrate predators are subject to infection especially by gorgoderids, lecithasterids, lepocreadiids, monorchiids and opecoelids. Piscivores are primarily subject to infection by acanthocolpids, bucephalids, cryptogonimids, didymozoids, hemiurids and opecoelids. As discussed earlier, such broad patterns break down in the face of some striking absences. We have already noted the near mutual exclusion of bucephalids and cryptogonimids in piscivores. Among herbivores, we find gyliauchenids in acanthurids, pomacanthids, pomacentrids, siganids and zanclids – but never in the heavily herbivorous kyphosids. Kyphosids have, instead, enenterids and gorgocephalids.
A striking feature of the importance of diet is that, where one species or group of species adopts a diet different from that of the remainder of the family, there is often a dramatic reflection in the parasite fauna. Thus, apogonids, blenniids and labrids are rarely piscivores but in those few cases where they are, bucephalids have taken independent advantage of the dietary specialisation. Thus, cleaner wrasse (Labroides), fang blennies (Plagiotrema) and the large piscivorous apogonid Cheilodipterus are all infected by bucephalids (Jones et al. Reference Jones, Grutter and Cribb2003; Bott and Cribb, Reference Bott and Cribb2005; Roberts-Thomson and Bott, Reference Roberts-Thomson and Bott2007) whereas their non-piscivore relatives lack such infections.
We do not know whether individual fish species are infected with particular trematode species because of the particular alga, fish or invertebrate that they ingest. Specificity based on such precise encounter parameters is possible, but we suspect that it is not generally significant. Despite the amount of feeding specialisation seen in marine fishes, it is evident that many fishes share far more dietary items than parasites. We can make the simple but telling observation that a line baited with a prawn or a pilchard in these waters will catch a wide range of fishes which, nevertheless, will share few parasite species. In addition, we conclude that the discrepant host distribution of bucephalids and cryptogonimids whose hosts certainly have overlapping diets indicates that physiological compatibility is an important part of the pattern of host specificity. Perhaps certain hemiuroids are exceptions to this rule and, almost uniquely, will develop in almost any fish that ingests their metacercariae. The basis of physiological incompatibility has not been explored for these parasites and we can only speculate that the kinds of processes demonstrated in other systems (Randhawa and Burt, Reference Randhawa and Burt2008) may apply here too.
A further indication that compatibility plays a vital role in the expression of host specificity can be drawn from the two trematode families that are not transmitted trophically. Aporocotylids (fish blood flukes) are transmitted to fishes by the direct penetration of the cercaria and thus largely independent of host feeding behaviour except in terms of where the fish occurs. There are 24 species of aporocotylids in the present data-set. Of these, 15 are known from only one species and the widest reported host range is that of Pearsonellum corventum Overstreet and Køie, 1989, which is reported in 7 serranid species; no species has been reported from more than one family of fishes (Nolan and Cribb, Reference Nolan and Cribb2004). Quite evidently, physiological compatibility is a major restricting force in this system. Perhaps this is no surprise given the intimate association between a blood fluke and its host. The second family transmitted independent of host diet is the Transversotrematidae. These parasites infect their hosts by direct attachment of the cercaria to the outside of the fish; the adult worms live beneath the trailing edges of the scales. Hunter and Cribb (Reference Hunter and Cribb2010) have shown that transversotrematids are far more common than was previously recognised, but still the majority of scaled fishes do not harbour transversotrematids and the host specificity of individual species is generally stenoxenic in this system (Hunter et al. Reference Hunter, Ingram, Adlard, Bray and Cribb2010). Thus, again we can conclude that physiological compatibility is a major determinant of host specificity for this family and, by extension, probably for most others as well.
In summary, we conclude that the physical and dietary attributes of a fish (usually contingent on the family to which it belongs) will predetermine the range of families of trematodes to which it is susceptible. The individual trematode species found in the fish will then be determined and perhaps explained by a combination of factors that range from the historical, the dietary, the physiological and the local distribution of the particular individual and intermediate hosts. The complexity of these factors means that many infections are neither reliably predictable a priori or convincingly explicable a posteriori. Examples of unexplained distributions include:
• Hurleytrematoides Yamaguti, 1953 (Monorchiidae) has a single species known in tetraodontid fishes (McNamara and Cribb, Reference McNamara and Cribb2009) whereas all 10 other species occur in chaetodontids (McNamara, personal communication).
• Just two of 15 examined species of Lutjanus, L. bohar and L. argentimaculatus, have revealed aporocotylids (Nolan and Cribb, Reference Nolan and Cribb2006a). Can the explanation be as simple as that these are the two largest species present?
• There are multiple species of the lepocreadiid genus Multitestis Manter 1931 in species of the ephippid genus Platax, but just one in one genus of Chaetodontidae (Bray et al. Reference Bray, Cribb and Justine2010).
• Preptetos impar Bray and Cribb, Reference Bray and Cribb1996 occurs in two species of primarily piscivorous lutjanids whereas other Preptetos species occur predominantly in herbivores such as acanthurids and siganids (Bray and Cribb, Reference Bray and Cribb1996).
• Alone of over 60 species of Pomacentridae that we have examined from the GBR, Acanthochromis polyacanthus is regularly infected by a bivesiculid, Bivesicula unexpecta Cribb, Bray and Barker, Reference Barker, Cribb, Bray and Adlard1994. No aspect of the biology of the host fish explains this anomalous distribution.
The uncertainty about precisely what species of trematodes a GBR fish will habour on the basis of its family (or even its specific) characteristics returns us to the starting point of our initial iterative loop – the collections and identification of host/parasite records. Because there is so much unpredictability in the system, we conclude that every species must be examined if we are to develop a full understanding of the system. In addition to the obvious need for care in identifications, we draw attention to the need for extensive sample sizes. Some of the “inconsequentially rare” host/parasite combinations discussed above were reported by us when the sample sizes were too low to allow understanding that they probably were inconsequential. Naturally there are limits to the numbers of animals that can be examined (both practical and ethical) but we point to the recent study by Downie et al. (Reference Downie, Bray, Jones and Cribb2011) who analysed records from over 2,400 fishes to convincingly establish the nature of the host specificity and distribution of two species of Symmetrovesicula in chaetodontid fishes.
What will change?
Previous estimates have suggested that the trematode fauna of fishes of the GBR might exceed 2,000 species (Cribb et al. Reference Cribb, Bray, Barker, Adlard and Anderson1994b). Given that here we are analysing only 290 species it is clear that current records are far from comprehensive. In our view there are several ways in which the overall understanding of this fauna will evolve. First, clearly many more species will be described and many more host/parasite combinations will be reported. We expect that the overall pattern, i.e. that most species are oioxenous or stenoxenous, will be largely unaffected (Fig. 2). As a greater number of fish species is examined, numbers of species will probably transfer from the category of oio- to stenoxenous; however, this will only occur in such cases where there are other closely related fish species with which trematodes might be shared. On the other hand we already see that that some apparently euryxenous species are proving to be complexes of oio/stenoxenous species.
The rapidly increasing capabilities of DNA sequencing machines and the consequent reduction in costs will revolutionise our understanding of the relationships not only between parasites, but between the parasites and their hosts. Until recently it has only been financially possible to study important human pathogens, such as Schistosoma Weinland, 1858, in any detail, but soon it will be possible to afford to study a wide variety of worms in similar detail. The lack of genetic uniformity revealed in Schistosoma mansoni Sambon, 1907 in Ugandan lakes, reflected in their epidemiological heterogeneity (Stothard et al. Reference Stothard, Webster, Weber, Nyakaana, Webster, Kazibwe, Kabatereine and Rollinson2009), makes one ponder the likely intraspecific complexity of reef species, inhabiting a complex three-dimensional environment.
What we don't know
A major theme of this paper has been the recognition that euryxenicity is rather rare on the GBR (and presumably in general) in coral reef fish trematodes. Several new questions arise from this observation. First, just what is the basis of the specificity of the euryxenic species given that, as we have seen, all still have distinct specificities? Second, is the broad pattern of dominance of oio/stenoxenicity reflected in other ecosystems? Does this reflect a fundamental difference in the nature of host specificity in higher latitudes? Finally we suspect that there is something interesting to be explored at the interface between oio- and stenoxenicity. Numerous species in this system infect only a single species despite the availability of seemingly comparable multiple congeners whereas there are also large numbers of species that do exploit such ranges. A description and an explanation for these contrasts are so far lacking. While many questions regarding host-parasite interactions in tropical marine trematodes remain for the moment unanswered, we will continue to use Systematics to strengthen the cornerstone that supports our edifice of understanding this system.
ACKNOWLEDGEMENTS
The collection and descriptions of the trematodes discussed here has been underpinned by research grants to THC from the Australian Research Council (grant numbers A00104962, DP0559217) and the Australian Biological Resources Study (ABRS). This study was also funded by, and is a contribution from, the Australian node of the CReefs global research initiative (grant number: 209/29), a partnership between the ABRS, BHP Billiton, the Great Barrier Reef Foundation, the Census of Marine Life and the Australian Institute of Marine Science (AIMS). The CReefs Australia Project is generously sponsored by BHP Billiton in partnership with The Great Barrier Reef Foundation, the Australian Institute of Marine Science, the Australian Biological Resources Study and the Alfred P. Sloan Foundation. CReefs is a field program of the Census of Marine Life. We thank the many colleagues and students who helped collect and identify the fish and the parasites over many years. We also gratefully thank the staff of the Heron and Lizard Island research stations for their support and hospitality during our many long stays.