INTRODUCTION
Synchronization of life cycles with the availability of resources is basic for many organisms among which parasites are not an exception (Poulin, Reference Poulin1998). Parasitic species, particularly those that feed on ephemeral resources, must ensure that their host-feeding stages are synchronized with the times when those hosts provide the appropriate food resource. Therefore, many parasites have evolved dispersal and developmental mechanisms, like diapause, that allow individuals to survive when conditions are unfavourable and ensure synchronization of active stages with favourable conditions (Danks, Reference Danks1987; Masaki, Reference Masaki2002). Diapause regulation mechanisms must achieve that induction, development and diapause completion are realized in most suited periods for every individual, responding to reliable external stimuli and cues that modulate direct or indirectly different physiological events during the phases of diapause (Kostal, Reference Kostal2006), regulating its duration and intensity (Hodek, Reference Hodek2002; Masaki, Reference Masaki2002). Although these environmental factors are diverse, numerous studies distinguish temperature and photoperiod (single or combined) as the most important controlling signals (see references in Tauber et al. Reference Tauber, Tauber and Masaki1986; Danks, Reference Danks1987).
Temperature has long been acknowledged as an influential variable for insects and development thermal responses in diapause are well understood in parasitic insects (Feder et al. Reference Feder, Stolz, Lewis, Perry, Roethele and Rogers1997; Wharton, Reference Wharton1999; Randolph, Reference Randolph2004). Ambient temperature is a reliable signal, especially in long winter diapauses (several months), due to the annual periodicity at regional level; or in places where photoperiodic changes are small (tropical areas) or not appreciable and daily temperature fluctuations are buffered (e.g. species in which some phases of the diapause take place in holes, caves or into the soil) (Danks, Reference Danks1987, Reference Danks2006). Several authors have pointed out that, in ectoparasites of endothermic hosts, temperature of the host or its surroundings (burrows or nests where they reside or breed) can control and modify parasite life-cycle duration and intensity (Marshall, Reference Marshall1981, Danks, Reference Danks1992). This could be the case of hosts of polyphagous parasites, which can show distinct thermal characteristics determined by physiological mechanisms and/or behavioural features (e.g. breeding phenology, roosting behaviour, type of nest with varying insulation characteristics …). Moreover, for those parasites that do not actively choose their host, changes in the habitual host (host shifting) are likely to represent a significant challenge for the parasite's ability to exploit the new host. Variation in key features of the host, like temperature, is likely to influence diapausing or quiescent parasites that should synchronize their infection phase with the new resource. Nest parasites can be particularly the case. Given that some nests can be re-used by different bird species, successive generations of a parasite may be exposed to different host species, which may have similar or very different breeding biology and vary in important features (e.g. body temperature). This raises the question of whether the parasite is able to modify its life cycle in relation to the host species (i.e. degree of host specificity, Roulin, Reference Roulin1998; Valera et al. Reference Valera, Casas-Crivillé and Hoi2003). The effect of host temperature and host shifting on synchronization between trophic levels is a largely unexplored topic (but see Marshall, Reference Marshall1981; Danks Reference Danks1992) and, to our knowledge, there is no study focusing specifically on how host temperature variability and host-shifting could influence the phenology of diapausing parasites.
The system formed by the ectoparasite fly Carnus hemapterus Nitzsch and its avian host species provides us an excellent opportunity to address these issues. Carnus hemapterus parasitizes nestlings of bird species with very different breeding phenologies, with some preference for birds nesting in cavities (Grimaldi, Reference Grimaldi1997). This fly overwinters as a pupae in the nest and the emergence of the infecting phase is partly synchronized with the occurrence of their hosts (i.e. hatching of nestlings) (Liker et al. Reference Liker, Markus, Vozár, Zemankovics and Rózsa2001; Valera et al. Reference Valera, Casas-Crivillé and Hoi2003). Involuntary host shifting can occur if the nest is re-used by a host different from the one that used it in previous breeding seasons. In this paper we study the mechanisms involved in the synchronization of host-parasite cycles by analysing the influence of host temperature on termination of diapause in an ectoparasitic fly and by examining the response of diapausing parasites to changes in its intimate environment as a result of involuntary host shifting. To achieve these goals, we address the following questions. (i) What are the environmental signals used by C. hemapterus to terminate metabolic arrest and thus ensure the availability of resources? (ii) How do host temperature characteristics influence parasite diapause? (iii) How does the parasite respond to host shifting and to what extent can Carnus adapt its life cycle to different host species? We hypothesize that differences in breeding phenology among alternative host species will have an influence on Carnus diapause traits and that the parasite will respond to changes in host temperature. To answer these questions we experimentally simulate natural cases of host shifting by exposing overwintering pupae of carnid flies parasitizing European Bee-eaters (Merops apiaster) to the incubation periods of 2 common Carnus hosts (the Little Owl Athene noctua, and the Hoopoe Upupa epops) that frequently re-occupy Bee-eater nests.
MATERIALS AND METHODS
Study area and species
The study area is located at La Palma del Condado (Huelva, South-west Spain 37° 35′N, 6° 45′W) where Bee-eaters breed in several colonies. The colony from which samples were collected is situated in an old sand quarry and has been occupied by Bee-eaters for years. More than 40 pairs bred during the study years 2005 and 2006. Climate in this area is typically Mediterranean with Atlantic influence. Precipitations are abundant mainly in autumn/spring (mean annual rainfall during 2001–2006 period=588·2 mm) (Junta de Andalucía meteorological data).
Carnus hemapterus is a 2 mm long blood-sucking fly that parasitizes nestlings of a variety of bird species (Grimaldi, Reference Grimaldi1997). Its life cycle comprises an adult stage, 3 larval phases encompassing around 21 days at 22°C and 95% relative humidity and a pupal stage (Guiguen et al. Reference Guiguen, Launay and Beaucournu1983). The puparia are black, short barrel shaped and very cryptic, simulating nest remains of chitinous parts of arthropods consumed by the hosts. After several months of winter diapause (Guiguen et al. Reference Guiguen, Launay and Beaucournu1983) adult flies emerge in the following spring approximately when their nestling hosts hatch (Valera et al. Reference Valera, Casas-Crivillé and Hoi2003). Adult flies are initially winged, but typically lose their wings once they locate a suitable host (Roulin, Reference Roulin1998). Since neither the adults nor the larvae have been found on adult birds, flies are assumed to colonize new host nests actively during the winged phase of their life cycle (Grimaldi, Reference Grimaldi1997; Roulin, Reference Roulin1998). Nonetheless, Carnus can persist by itself in the nest for several years since prolonged diapause has been recorded for this species (Valera et al. Reference Valera, Casas-Crivillé and Calero-Torralbo2006a). Adult flies are short lived during dispersion (around 2 days; MACT, unpublished observations).
The European Bee-eater Merops apiaster is a single-brooded, migrant bird that nests in cavities at the end of long burrows. It usually forms breeding colonies that can be used for many years, becoming traditional breeding areas. Eggs are laid directly on the sandy soil of the incubation chamber. Incubation lasts around 20 days starting before the clutch is complete (Cramp, Reference Cramp1985). The female usually sleeps in the nest (Cramp, Reference Cramp1985). Ar and Piontkewitz (Reference Ar and Piontkewitz1992) estimated that the mean temperature in a Bee-eater incubation chamber during the day was of 27·8±1·6°C.
Bee-eater nests are commonly used by a variety of birds for breeding among which Hoopoes Upupa epops and Little Owls Athene noctua have been reported (Casas-Crivillé and Valera, Reference Casas-Crivillé and Valera2005). The latter species are resident, cavity nesting birds commonly parasitized by C. hemapterus (Valera et al. Reference Valera, Casas-Crivillé and Calero-Torralbo2006a). They frequently occur in sympatry with the Bee-eater in Spain. In southern Spain, Hoopoes breed from February to June, with about 20% of pairs laying a second clutch (Martín-Vivaldi et al. Reference Martín-Vivaldi, Palomino, Soler and Soler1999). Incubation lasts around 17 days (Martín-Vivaldi et al. Reference Martín-Vivaldi, Palomino, Soler and Soler1999). Little Owls lay a single clutch although replacement clutches occur. Incubation lasts around 27–28 days and the breeding period is usually from April to June (Cramp, Reference Cramp1985). Thus, in our study area Hoopoes start breeding first, followed by Little Owls and Bee-eaters being the latest breeders.
Material collection
During 21–22 February 2005 and 16 February 2006 nests with evident cues of having been used the previous breeding season (abundance of arthropod remains and bird pellets in the nest), and therefore more likely to contain Carnus pupae, were sampled. Twenty-five samples were collected each year from the breeding chamber by using a spoon attached to a stick. The amount of nest material collected varied among nests (range: 417–1623 g). Nonetheless, the number of emerged flies is not related to the amount of nest material collected (Spearman rank correlations for each treatment and year, P>0·1 for all cases; see also Valera et al. Reference Valera, Casas-Crivillé and Calero-Torralbo2006b). After collection, samples were kept in transparent plastic bags, carried to the Estación Experimental de Zonas Áridas (Almería, South-east Spain, 36° 50′N 02° 28′W) and stored in a dark room with open windows to resemble natural conditions (i.e. ambient temperature moderated by partial enclosure and semi-darkness).
Experimental design
Our experimental design consists of exposing carnid pupae to 3 different situations: occurrence of the usual host (the Bee-eater), occurrence of a different host (Little Owl in 2005, Hoopoe in 2006) and control (resembling pupae in unoccupied nests). In 2005 our experiment included a Little Owl, a Bee-eater and a control treatment whereas in 2006 it comprised a Hoopoe, a Bee-eater and a control treatment (Fig. 1).

Fig. 1. Experimental design diagram for simulations of host occurrence and host shifting in 2005 and 2006.
Samples of Bee-eater nests were thoroughly mingled, randomly split into 3 subsamples of the same mass and arbitrarily subjected to the following 3 treatments. (i) Incubation by the original host: we artificially reproduced the Bee-eater incubation period, keeping the subsamples during 21 days at 27·5°C (JP Selecta, model Incubat 150, ref. 2000994). Following the Bee-eater breeding period in our latitude (Cramp, Reference Cramp1985; personal observations), the treatment began from 11 May and lasted until 31 May (both in 2005 and 2006). From then onwards the subsamples remained in the store room. (ii) Host-shifting incubation: we artificially reproduced the Little Owl and the Hoopoe incubation periods. Given the narrow range of incubation temperature reported for very different bird species (Webb, Reference Webb1987) and that the thermal microenvironment of burrows is very constant (Ar and Piontkewitz, Reference Ar and Piontkewitz1992; Lill and Fell, Reference Lill and Fell2007) we assume that temperature in the breeding chamber in nests occupied by these two species is similar to the one in Bee-eater nests. Following the breeding dates and duration in our latitudes (Cramp, Reference Cramp1985; Martín-Vivaldi et al. Reference Martín-Vivaldi, Palomino, Soler and Soler1999), we kept the samples during 28 days at 27·5°C, from 19 April to 16 May 2005 (Little Owl treatment), and for the Hoopoe treatment during 18 days at 27·5°C, from 24 March to 10 April 2006. From the end of the treatment onwards subsamples remained in the store room. (iii) Control treatment: subsamples remained the whole time in the storeroom.
Samples were periodically monitored (every 2–3 days) for Carnus emergence from 14 March 2005 until 9 July 2005 and from 14 March 2006 until 23 June 2006 (in both cases 10 days after the last emerged fly was recorded). Flies emerging from each subsample and date were separately preserved in 99% ethanol and subsequently counted and identified with the aid of a binocular microscope. Emergence dates were grouped into weeks starting from the first week of April (when earliest emergence was recorded). During 2006, the temperature in the store room was checked every 3 h by means of a temperature data logger (Maxim/Dallas Integrated Products, Inc.). At the time of the Bee-eater treatment it averaged 25·5°C (range: 23·5–28·0°C).
Carnus hemapterus emergence was registered in 12 out of 25 nests sampled in 2005. In 8 nests emergence was recorded in all treatments, in 1 nest emergence was recorded only in the Little Owl and control treatment and in 3 nests emergence was recorded in just a single treatment. During 2006, 15 nests had emergence in all 3 treatments. In 1 nest emergence was recorded only in the Hoopoe and control treatments and in 2 nests emergence was recorded in just a single treatment.
A subsample of flies emerged during the study period was deposited in the Zoological Collection of the Estación Experimental de Zonas Áridas (Almería, Spain) (reference numbers 6488-6496).
Statistics
Except for the calculation of prevalence, analyses were restricted to those nests where emergence was recorded in all 3 treatments since the number of emerged flies in the remaining nests was very low. Prevalence (proportion of infected samples among all the samples examined) of parasites and mean intensity (number of individuals found in the infected samples) of flies was calculated and χ2 tests were used for comparing prevalences. One-way within-subjects (repeated measures) ANOVAs were used to test the effect of treatments on the number of flies emerged per sample, length of the emergence period and mean date of emergence.
For the analysis of the effect of treatments on the emergence pattern of flies our experimental design encompasses 2 within-subject factors: (i) emergence time and (ii) subsamples of the same nest exposed to different treatments. Thus, we used multi-way within-subjects repeated measure ANOVA tests (von Ende, Reference Von Ende, Scheiner and Gurevitch2001). The dependent variable was the cumulative percentage of emerged flies per week after checking for normality. Given that the earliest emergences occurred intermittently, only in some treatments and at a low number (see Results section) we focused on the main period of emergence and excluded from the analyses during the first week of emergence in 2005 and the first 3 weeks in 2006. However, results did not change when including such weeks. Weeks when most subsamples had reached 100% emergence were also discarded since they do not influence the overall emergence pattern. Thus, 7 weeks (from the 4th week of April until the 2nd week of June) and 5 weeks (from the 4th week of April until the last week of May) were included in the 2005 and 2006 analyses respectively.
We used Mauchly's test to check the assumption of sphericity and, when the latter was not met, we adjusted the degrees of freedom by using the Greenhouse-Geisser and Huynh and Feldt estimators (von Ende, Reference Von Ende, Scheiner and Gurevitch2001). Here we provide the conservative Greenhouse-Geisser corrected probability. We followed both the univariate and the multivariate approach when possible (i.e. sample size did not allow testing the effect of the interaction between time and treatment in 2005). Since both approaches gave the same results here we report the results obtained with the more powerful univariate approach (von Ende, Reference Von Ende, Scheiner and Gurevitch2001). Given that the sample size in 2005 was low in comparison to the number of dependent variables (i.e. 7 weeks and 3 treatments; von Ende, Reference Von Ende, Scheiner and Gurevitch2001) we first analysed our data pooled into 3 periods (2 first weeks, the main emergence period of 3 weeks, and the latest 2 weeks). Since the results do not change when compared with those obtained when considering 7 periods (i.e. 7 weeks) and since a more accurate view of the effect of treatments on Carnus emergence is gained in this way we prefer to show the latter results.
Because we hypothesize that both experimental treatments have a differential effect on Carnus emergence both when compared to each other and when compared with the control, we performed univariate tests of significance for planned comparisons when opportune.
RESULTS
Effect of the incubation treatments on prevalence and abundance of flies
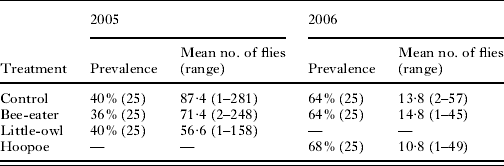
Prevalence of C. hemapterus was not affected by the treatments either in 2005 or in 2006 (Chi-square tests, χ2=0·11, P>0·10; χ2=0·11, P>0·10, respectively) (Table 1).
Table 1. Prevalence (sample size in parentheses) and abundance of Carnus hemapterus flies in subsamples of each treatment in 2005 and 2006
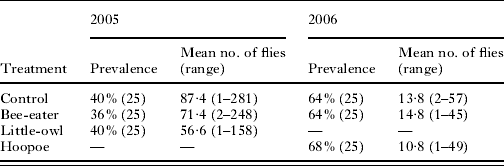
Experimental treatments performed in 2005 did influence the number of emerged flies (Repeated Measures ANOVA, F 2,14=4·70, P=0·027) since more flies emerged in the control subsamples than in the Little Owl and the Bee-eater subsamples (Univariate tests of significance for planned comparisons, P=0·045 and P=0·024, respectively) (Table 1). In contrast, treatments in 2006 did not influence the number of emerged flies per nest (Repeated Measures ANOVA, F 2,28=1·8, P=0·18) (Table 1).
Effect of the incubation treatments on the phenology of emergence of Carnus hemapterus
In 2005 emergence was first recorded during the 3rd week of April in some subsamples of all treatments (Fig. 2A) and became common in all treatments during the 4th week of April. The earliest emergence in 2006, as early as the 1st week of April, was recorded in subsamples under the Hoopoe treatment. At the end of April it became common in the Hoopoe treatment and it was not until the 2nd week of May when emergence occurred regularly in the Bee-eater and control treatments (Fig. 2B). Nonetheless, treatments did not influence the length of the emergence period (number of weeks when emergence occurred) in any year (Repeated measures ANOVA, 2005: F 2,14=0·05, P=0·94; 2006: F 2,28=0·57, P=0·57). Most nests reached 100% emergence by mid-June in 2005 and during the 1st week of June in 2006.

Fig. 2. Mean cumulative emergence (±s.e.), of Carnus hemapterus under different treatments performed during 2005 (A), and 2006 (B). Horizontal dotted lines represent the duration of the habitual host (Bee-eater) incubation treatment in both 2005 and 2006; horizontal continuous lines represent the duration of the host-shifting incubation treatment (Little Owl in 2005 and Hoopoe in 2006, the latter starting 24 March).
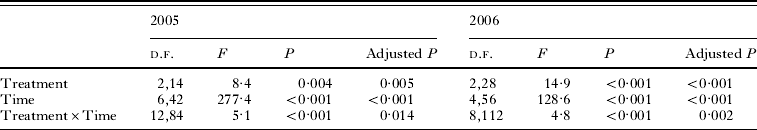
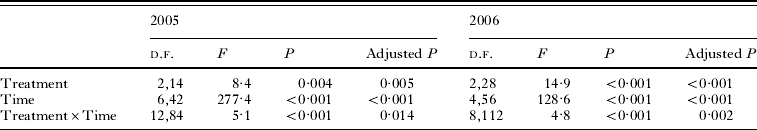
Treatments influenced the mean date of emergence both in 2005 and in 2006 (Repeated measures ANOVA, 2005: F 2,14=5·9, P=0·013, adjusted P=0·027; 2006: F 2,28=12·1, P<0·001, adjusted P<0·001, respectively) so that the mean emergence of flies from subsamples under the Little Owl treatment (in 2005) and the Hoopoe treatment (in 2006) occurred earlier in comparison to the one of flies under the control (Univariate tests of significance for planned comparisons, P=0·004 and P=0·005, respectively) and Bee-eater treatment (P=0·063 and P<0·001, respectively) (Fig. 3A and B). The Hoopoe treatment had a stronger effect than that of Little Owl since Hoopoe-incubated flies emerged on average 1·07 weeks earlier in comparison to control flies (Fig. 3B) whereas Little Owl-incubated flies emerged just 0·64 weeks earlier than control flies (Fig. 3A).

Fig. 3. Mean emergence date (±s.e.), ranging from 26 March to 11 July (2 May=8th – 14th) of Carnus hemapterus under different treatments during 2005 (A) and 2006 (B).
Analysing the weekly emergence of flies in each treatment we found a significant treatment×time interaction in both years (Table 2), indicating that treatments influenced the emergence pattern of adult flies. Specifically, in 2005 pupae started emerging at the same time regardless of the treatment, but from mid-May onwards pupae under the Little Owl incubation treatment emerged at a faster rate (Fig. 2A). In 2006, some Hoopoe-incubated pupae (2 out of 176 emerged in this treatment, 1·13%) appeared 2 weeks earlier than pupae under the other treatments. Nonetheless, the differential effect of the treatments is evident only from the end of April, when the Hoopoe treatment produced a faster emergence than the Bee-eater and the control treatments (Fig. 2B). The Bee-eater treatment had no significant effect on Carnus emergence when compared with the control treatment (Univariate test of significance for planned comparisons, 2005: P=0·21, 2006: P=0·41).
Table 2. Results of the multi-way within-subjects repeated measure ANOVA for the experiments in 2005 and 2006
(The dependent variable is the cumulative percentage of emerged flies per subsample, and treatment (Little Owl, Bee-eater and control for 2005, and Hoopoe, Bee-eater and control for 2006,) and time (7 weeks from 25 April until 11 June for 2005, and 5 weeks from 24 April until 28 May for 2006) are the predictors. Adjusted P values refer to the Greenhouse-Geisser corrected probability (see Materials and Methods section).)
DISCUSSION
Our experimental study shows the ability of a diapausing ectoparasitic fly to respond to thermal changes in its intimate environment caused by the type of host. Pupae exposure to the incubation of the usual or a different host did not influence the prevalence, the length of the emergence period nor the number of emerged flies (with the exception of control subsamples in 2005). In contrast, experimental simulations of host shifting resulted in significant changes in the phenology of emergence of the parasite, modifying both the mean date and the rate of emergence. As a result, flies under the new host emerged earlier and faster in comparison with flies under the habitual host and the control treatments. The effect was more evident for flies under the Hoopoe treatment, the host with the most dissimilar phenology to the usual one, the Bee-eater, since emergence of the parasite started some weeks earlier than in subsamples under the other treatments (even though only few flies emerged at that time). The treatment reproducing the occurrence of the usual host had no discernible effect when compared with the results obtained with the control treatment (absence of host). This is not surprising since differences in temperature between both treatments (27·5°C versus an average temperature of 25·5°C in the store room at the time of the Bee-eater treatment in 2006) was small and, thus, they had a negligible effect on the general pattern of emergence of flies under both treatments. For comparison, differences in temperature between the Hoopoe treatment (27·5°C) and the control at the time when the former was applied were 3-fold higher (mean temperature in the store room during the Hoopoe treatment=21·6°C). The question remains whether differences in temperature between the control and an experimental treatment resembling the same host in a different (colder) climatic area (where ambient and nest temperature differences would be larger), or another usual host breeding earlier in the season (e.g. Hoopoe) (and thus causing larger differences between both treatments) would have resulted in different emergence patterns.
Thermal response of diapausing insects is well-known since long and decreased diapause duration in many different arthropods as a consequence of gradual increase of environmental temperature has been experimentally shown (e.g. Broufas and Koveos, Reference Broufas and Koveos2000; Kemp and Bosch, Reference Kemp and Bosch2005; Teixeira and Polavarapu, Reference Teixeira and Polavarapu2005). Surprisingly, the effect of host temperature on parasites has been seldom investigated (but see Wetzel and Weigl, Reference Wetzel and Weigl1994; Tripet and Richner, Reference Tripet and Richner1999) and, to our knowledge, there is no work paying specific attention to the effect of host temperature on the phenology of emergence of diapausing parasites and its ecological consequences in terms of host-parasite synchronization. The use of host temperature as a cue to break dormancy has been described for ectoparasites of endothermic hosts like some flea or bug species (Marshall, Reference Marshall1981; Danks, Reference Danks1992). However, our results show that the rate of Carnus emergence increased just before the end of the Hoopoe and Little Owl treatments, suggesting that the underlying mechanism is not the above-mentioned break dormancy, that is an aseasonal quiescence where duration of dormancy is highly variable and generally depends on the absence/presence of the host (Marshall, Reference Marshall1981; Tauber et al. Reference Tauber, Tauber and Masaki1986). In contrast, C. hemapterus dormancy is a long-cycle seasonal diapause (Guiguen et al. Reference Guiguen, Launay and Beaucournu1983; Valera et al. Reference Valera, Casas-Crivillé and Calero-Torralbo2006b) and, thus, differences observed among treatments are probably the result of faster heat accumulation (Tauber et al. Reference Tauber, Tauber and Masaki1986; Kostal, Reference Kostal2006).
What are the consequences of Carnus response to host temperature and host shifting? Synchronization of host-parasite cycles is expected (Poulin, Reference Poulin1998) and, in fact, it has been reported elsewhere (see, for instance, Andres and Cordero, Reference Andres and Cordero1998; Rolff, Reference Rolff2000, Valera et al. Reference Valera, Casas-Crivillé and Hoi2003). However, appearance of the most adequate resource can vary according to the characteristics of the particular host exploited by the parasite (e.g. breeding phenology, see, for instance, Valera et al. Reference Valera, Casas-Crivillé and Hoi2003) and/or the geographical location. Spatial and/or temporal adaptations to different hosts' phenologies can help to diminish such mismatches (Carroll and Boyd, Reference Carroll and Boyd1992; Filchak et al. Reference Filchak, Roethele and Feder2000; Nyman, Reference Nyman2002). Intraspecific clinal variation in the thermal regulation of the rate of diapause development has been demonstrated in a variety of organisms (Tauber et al. Reference Tauber, Tauber and Masaki1986; Hoffmann et al. Reference Hoffmann, Sorensen and Loeschcke2003) and, thus, selection for various C. hemapterus thermal phenotypes (Nijhout, Reference Nijhout1999; Weinig and Schmitt, Reference Weinig and Schmitt2004) with rates of development at different optima of temperature adapted to the breeding phenology of different hosts could be possible.
Most insects in temperate climates use either photoperiod or temperature, or a combination of both, as cues for their timing decisions (Tauber and Tauber, Reference Tauber and Tauber1981; Smith and McIver, Reference Smith and McIver1984; Leather et al. 1993). Our data suggest that, by using the same signal (temperature) but from different sources (abiotic-ambient temperature and biotic-host temperature), Carnus is able to respond both to seasonal, predictable unsuitable periods (e.g. autumn-winter) as well as, at least partially, to unpredictable short-cycle modifications (host shifting involving differences in breeding phenologies), adjusting its emergence to the appearance of the nestlings of the new hosts and increasing its chances of survival and future reproduction.
It could be argued that the observed differences in emergence here revealed could be due to genetic variation within the fly population for emergence date. However, mixing of samples during collection and randomization of assignment to each treatment excludes this explanation. Rather, we propose that C. hemapterus displays some degree of phenotypic plasticity to adjust its emergence to different hosts. When appropriate food resource appearance is irregularly distributed along the season, polymorphism in diapause duration between individuals feeding on particular hosts can take place (Feder et al. Reference Feder, Hunt and Bush1993; Tikkanen and Lyytikainen-Saarenmaa, Reference Tikkanen and Lyytikainen – Saarenmaa2002). Nonetheless, when a set of individuals are specialized to parasitize a particular resource, they can lose the ability to exploit others (Giorgi et al. Reference Giorgi, Arletazz, Guillaume, Nussle, Ossola, Vogel and Christe2004), jeopardizing their future reproductions when the preferred host is not present. Therefore, selection for ability to respond to eventual alternative host appearance by means of phenotypic plasticity in diapause traits could be advantageous. Moreover, it is known that the development system of insects is pre-adapted in many ways for the rapid evolution of phenotypic plasticity (Nijhout, Reference Nijhout1999). For generalist species that occupy a wide range of habitats, like C. hemapterus, a high degree of plasticity is advantageous and could consequently be a result of natural selection (Blanckenhorn, Reference Blanckenhorn1998).
We cannot discriminate whether this species shows a ‘general-purpose genotype’ (Baker, Reference Baker, Baker and Stebbins1965), capable of responding to any host, or whether the plastic response is limited either because there exists some physiological limit, or because there is a loss of specific thermal phenotypic responses in local populations (Kemp and Bosch, Reference Kemp and Bosch2005). Our results do suggest that there may exist some kind of limited thermal phenotypic plasticity (West-Eberhard, Reference West-Eberhard2003) because, in the most strenuous case (the Hoopoe treatment), only a small fraction of flies (9·2% of the total number of flies emerged in that treatment) appeared during April, when most first Hoopoe clutches are hatching in our latitudes (Martín-Vivaldi et al. Reference Martín-Vivaldi, Palomino, Soler and Soler1999). Thermal phenotypic plasticity may, on the other hand, be costly since responses to changes in temperature, when combined with other factors affecting insect survival (e.g. heavy competition for resources during the larval stage due to high population density), could jeopardize survival. This could be the case in 2005, when the temperature treatments, together with the high density of larvae, could result in the lower number of emerged flies in the experimental treatments in comparison to the control.
Previous work on the interactions between host and parasites has focused primarily on the importance of host immunology, morphology or behaviour. Our study rather explores intimate mechanisms regulating the synchronization of host-nest-dwelling parasite cycles and promotes the idea that host temperature is an ecological factor that must be considered when host-parasite relationships are studied. Differences in temperature traits among hosts could be an important selective pressure driving ecological specialization processes via diapause adaptations to particular temperature attributes in parasites. Testing whether host temperature could be a mechanism of initial divergence of populations in Carnus and other nest-dwelling haematophagous parasites would require additional research on within-population variance and range of phenotypic plasticity in the emergence patterns of such parasites.
We thank Radovan Václav for useful comments, Jose Manuel Sayago for his fieldwork assistance and Juana Ortiz and Gerardo Espeso for logistic support. Junta de Andalucía provided meteorological data and permits to sample Bee-eater nests. M.A.C.T. was funded by a pre-doctoral FPU grant from the Spanish Ministry of Education and Science (AP20043713). F. V. was funded by the Programa de Ayudas para la Incorporación de Personal Investigador al CSIC (Proyectos Intramurales Especiales 2006).