INTRODUCTION
Toxoplasma gondii, the aetiological agent of toxoplasmosis, is an obligate intracellular protozoan parasite that infects most warm-blooded animals, including humans (Dubey and Jones, Reference Dubey and Jones2008; Dubey et al. Reference Dubey, Lago, Gennari, Su and Jones2012). Toxoplasmosis imposes adverse economic impact due to the induction of severe abortion and neonatal loss of domestic animals (Dubey and Jones, Reference Dubey and Jones2008; Dubey et al. Reference Dubey, Lago, Gennari, Su and Jones2012). During pregnancy, infection may give rise to serious foetal congenital mental retardation, blindness and hydrocephaly (McLeod et al. Reference McLeod, Beem and Estes1985; Fatoohi et al. Reference Fatoohi, Cozon, Greenland, Ferrandiz, Bienvenu, Picot and Peyron2002). Toxoplasmosis is also a major opportunistic infection in immunocompromised individuals, often resulting in lethal toxoplasmic encephalitis (Contini, Reference Contini2008). Although vaccines against T. gondii have been investigated for a long time, only one attenuated vaccine has been licensed for use in sheep (Innes et al. Reference Innes, Bartley, Maley, Katzer and Buxton2009; Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009). Therefore, the lack of effective vaccines has become a major burden in controlling toxoplasmosis.
Significant information obtained recently indicates that future investigations on Toxoplasma vaccine development have to include potent adjuvants capable of enhancing protective immunity against T. gondii in animals (Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009). Adjuvants can enhance the vaccine immunogenicity for induction of the ‘appropriate’ immune response (Singh and O'Hagan, Reference Singh and O'Hagan2003; Garlapati et al. Reference Garlapati, Facci, Polewicz, Strom, Babiuk, Mutwiri, Hancock, Elliott and Gerdts2009; Heegaard et al. Reference Heegaard, Dedieu, Johnson, Le Potier, Mockey, Mutinelli, Vahlenkamp, Vascellari and Sorensen2011), particularly cell-mediated immunity that is urgently required for eliminating tachyzoite-infected cells as well as inhibiting tachyzoite dissemination and cyst formation (Innes et al. Reference Innes, Bartley, Maley, Katzer and Buxton2009; Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009, Reference Jongert, Lemiere, Van Ginderachter, De Craeye, Huygen and D'Souza2010). In addition, IFN-γ, a key cytokine that can favourably strengthen Th1 cell-mediated immunity, has been considered as a decisive mediator of resistance to T. gondii (Jongert et al. Reference Jongert, Lemiere, Van Ginderachter, De Craeye, Huygen and D'Souza2010). Thus, efficacious adjuvants that are promised to induce IFN-γ-associated Th1 cell-mediated immunity seem more likely to be approved for use in Toxoplasma vaccine development.
In recent years, microparticles (MPs) made from biodegradable poly(lactide-co-glycolide) (PLG) polymer have been developed as potent adjuvants or delivery systems to encapsulate antigens for preparation of controlled-release MP vaccines (Sinha and Trehan, Reference Sinha and Trehan2003; Heegaard et al. Reference Heegaard, Dedieu, Johnson, Le Potier, Mockey, Mutinelli, Vahlenkamp, Vascellari and Sorensen2011; Jain et al. Reference Jain, O'Hagan and Singh2011). The PLG encapsulation not only allows the sustained release of antigens for a long period (Lim et al. Reference Lim, Poh and Wang2009; Jain et al. Reference Jain, O'Hagan and Singh2011) but also promotes the antigen uptake via antigen-presenting cells (APCs) (Newman et al. Reference Newman, Elamanchili, Kwon and Samuel2002), thereby favourably eliciting strong cell-mediated immunity accompanied by production of Th1 cytokines, such as IFN-γ (Sinha and Trehan, Reference Sinha and Trehan2003; Heegaard et al. Reference Heegaard, Dedieu, Johnson, Le Potier, Mockey, Mutinelli, Vahlenkamp, Vascellari and Sorensen2011). Therefore, the MP vaccines made from the PLG polymer can achieve the need for induction of functional IFN-γ-associated Th1 cell-mediated immunity against T. gondii.
Recent development efforts of subunit vaccines against T. gondii have been focused mainly on the tachyzoite surface antigens (SAGs) (Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009; Machado et al. Reference Machado, Caetano, Barbosa, Salgado, Rabelo, Garcia, Bruna-Romero, Escriou and Gazzinelli2010; Meng et al. Reference Meng, He, Zhao, Bai, Zhou, Cong, Lu, Zhao and Zhu2012; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb) such as SAG2, due to their attachment roles in the process of host-cell invasion (Grimwood and Smith, Reference Grimwood and Smith1996). The immune responses elicited by vaccination with SAG2 have previously been demonstrated to protect mice against tachyzoite infection (Yang et al. Reference Yang, Chang and Chao2003; Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009; Meng et al. Reference Meng, He, Zhao, Bai, Zhou, Cong, Lu, Zhao and Zhu2012; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb) and cyst formation (Mishima et al. Reference Mishima, Xuan, Shioda, Omata, Fujisaki, Nagasawa and Mikami2001; Machado et al. Reference Machado, Caetano, Barbosa, Salgado, Rabelo, Garcia, Bruna-Romero, Escriou and Gazzinelli2010). In addition, the SAG2 sequence was cloned in our previous work to produce a recombinant SAG2 (rSAG2) protein (Yang et al. Reference Yang, Chang and Chao2004). However, the further animal study in mice demonstrated that the rSAG2 protein emulsified with an oil adjuvant, Vet L-10, induced only partial protection (53%) against a lethal subcutaneous challenge of T. gondii tachyzoites (Yang et al. Reference Yang, Chang and Chao2004). As long as alternative effective adjuvants such as the PLG polymer are used to make the rSAG2 protein more immunogenic, more protective immunity against T. gondii may be achieved in animals.
Although use of PLG as an adjuvant to extend SAG1- and SAG1/2-induced immunity has been described in our previous studies (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb), both improved immunity and advanced protection against T. gondii induced by the more potential antigen, SAG2, encapsulated in PLG MPs are certainly worthy of being studied. In the present study, the Escherichia coli-based recombinant SAG2 (rSAG2) protein was prepared to be encapsulated with the PLG polymer for production of PLG-encapsulated rSAG2 (PLG-rSAG2) MPs. The ability of PLG-rSAG2 MPs to induce SAG2-specific protective immunity was then evaluated in BALB/c mice, and compared with rSAG2 emulsified with a Vet L-10 oil adjuvant (rSAG2 (Vet L-10)).
MATERIALS AND METHODS
Parasites
The RH strain of T. gondii tachyzoites used in the present study were kindly given by Dr David Chao (Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan) and maintained in Vero cells or BALB/c mice (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a). Only two or three passages of tachyzoites in the ascites of BALB/c mice were carried out to recover their virulence before the tachyzoite challenge in mice. The tachyzoites harvested from the mouse peritoneal fluid were used for subcutaneous tachyzoite challenge in immunized mice. On the other hand, for all experiments in our laboratory except the challenge study, the tachyzoites were maintained in tissue culture by passage in Vero cells according to the guidelines of the ‘OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2013, Chapter 2.9.10. Toxoplasmosis’.
Animals
BALB/c mice (6–8 weeks of age) purchased from the National Laboratory Animal Center, National Science Council, Taiwan, were used to maintain and passage T. gondii tachyzoites as well as to perform the immunization experiments. All mice were housed in high containment facilities and managed in compliance with the Animal Welfare Act. All administrations were reviewed and approved by the Institutional Animal Care and Use Committee, National Pingtung University of Science and Technology.
Monoclonal antibody (mAb)
Hybridoma cells producing mouse mAb TG-2 (isotype G, subclass 1, κ light chain), which is specific to the tachyzoite SAG2 protein, were prepared by fusion, selection and cloning as described previously (Yang et al. Reference Yang, Chang and Chao2003; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b).
Antigens
The tachyzoites were harvested, purified and sonicated to prepare the tachyzoite sonicated antigen (TsoAg) as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). The rSAG2 protein used in the present study was produced as the following procedure. The full-length SAG2 sequence was amplified with SAG2 specific primers (the forward primer: 5′-CGCCCCGGGATGTCATTTTCGAAAACCAC-3′ and the reverse primer: 5′-ATACCCGGGTCACACAAACGTAATCAGCA-3′ contained the underlined SmaI sequences) by PCR (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a) and then cloned into the plasmid pGEX-6P-1 (GE Healthcare) to be expressed as a glutathione s-transferase (GST) fusion protein, GST-SAG2, in BL21 (DE3) E. coli (Yeastern Biotech). Protein expression and purification were then carried out as described previously (Yang et al. Reference Yang, Chang and Chao2004). The GST-SAG2 protein was expressed by induction with 1 mm IPTG (isopropyl-β-D-thiogalactopyranoside) for 4 h at 37 °C. Induced bacteria were obtained by centrifugation at 3000 g for 10 min and resuspended in PBS (140 mm NaCl, 2·7 mm KCl, 10 mm Na2HPO4, 1·8 mm KH2PO4, pH 7·3) supplemented with 1% Triton X-100, 100 μg mL−1 lysozyme, 1 mm PMSF (phenylmethylsulfonyl fluoride) and 1 mm dithiothreitol (DTT). After incubation at 4 °C for 10 min, cells were lysed by repeated freeze/thawing followed by ultrasonication. After centrifugation, supernatants were collected, diluted with an equal volume of PBS containing 1% Triton X-100, filtered through 0·22 μm disk filters (Millipore) and then applied into the GSTrap FF columns (GE Healthcare). The charged columns were washed with 5 mL of PBS and then digested with PreScission protease (160 units) (GE Healthcare) to remove GST protein tag at 4 °C for 16 h. Finally, recombinant SAG2 (rSAG2) was eluted from columns by PreScission protease buffer (50 mm Tris-HCl, 100 mm NaCl, 1 mm EDTA, 1 mm DTT, pH 8·0). The lipopolysaccharide (LPS) in the rSAG2 samples was removed by the Detoxi-Gel Endotoxin Removing column (Thermo Scientific). The LPS level was confirmed by the Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific) to be below 0·1 EU mL−1. The resulting rSAG2 (22 kDa) protein was prepared and its antigenicity was analysed by Western blot analysis using the mouse mAb TG-2, which is specific to SAG2 of T. gondii tachyzoites.
Preparation of PLG-encapsulated rSAG2 MPs
In this study, PLG MPs containing the rSAG2 protein were fabricated by the water/oil/water double emulsion solvent evaporation technique as described previously (Jeffery et al. Reference Jeffery, Davis and O'Hagan1993; Ghaderi and Carlfors, Reference Ghaderi and Carlfors1997; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb), with minor modifications. Briefly, 20 mL of a 10% solution of 50:50 PLG (Sigma) in dichloromethane (Sigma) was mixed with 2 mL of a rSAG2 solution (5 mg mL−1) by using a PRO200 homogenizer (PRO Scientific) equipped with 10 mm×150 mm generator at 10 000 rpm for 10 min to produce a water/oil emulsion. The resulting emulsion was further homogenized with 20 mL of a 2% polyvinyl alcohol (Sigma) solution at 10 000 rpm for 10 min to generate a stable water/oil/water emulsion. The water/oil/water emulsion was then stirred for 18 h at room temperature (RT) and pressurized to promote solvent evaporation and PLG-rSAG2 MP formation in a laboratory fume hood. The MPs were collected by centrifugation at 4000 g for 30 min, washed three times with distilled water to remove non-entrapped rSAG2 and then lyophilized by a FD-5030 freeze dryer (Panchum) for storage at −20 °C. The particle morphology was inspected using a S3000N scanning electron microscope (Hitachi) and the particle size was determined by N5 submicron particle size analyser (Beckman Coulter) as before (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb).
Protein entrapment
The entrapment of rSAG2 protein in PLG MPs was then measured with the following procedure. A total of 5 mg of PLG-rSAG2 MPs was first dissolved in 500 μL of 0·1 m NaOH with 2·5% SDS to extract the encapsulated rSAG2 as described previously (Jeffery et al. Reference Jeffery, Davis and O'Hagan1993; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). After centrifugation at 4000 g for 10 min, the content of rSAG2 in the supernatant was assessed with the BCA protein assay (Pierce) and compared with BSA standards and adjusted against empty PLG MPs. Based on this result, the ratio (w/w) of rSAG2 entrapped per dry weight of MPs was determined and the entrapment efficiency (%) was expressed as a ratio of the actual rSAG2 entrapment to the theoretical rSAG2 entrapment (Jeffery et al. Reference Jeffery, Davis and O'Hagan1993; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). All measurements were performed in triplicate on samples from different batches.
In vitro release study
A total of 5 mg of PLG-rSAG2 MPs was suspended in 1 mL of PBS (pH 7·4) with 0·02% sodium azide and shaken at 37 °C in 1·5 mL microfuge tubes. Every 3 days, 1 mL of supernatant was sampled and the amount of rSAG2 in the supernatant was measured using the BCA protein assay (Pierce), compared with BSA standards and adjusted against empty PLG MPs as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). In addition, Western blot analysis using the mouse mAb TG-2, which is specific to SAG2 of T. gondii tachyzoites, was performed to determine if released rSAG2 samples on days 3, 9, 15, 21, 27 and 33 could be recognized by mAb TG-2 (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). The Amicon Ultra-0.5 Centrifugal Filter Device (10 kDa limit) was used to concentrate these release samples. The same volume (10 μL) of each concentrated sample was then separated by 12% SDS-PAGE and analysed with mouse mAb TG-2.
Immunization
Five groups of 35 BALB/c mice each were intraperitoneally injected twice at a 14-day interval with PBS, blank PLG, 10 μg of soluble rSAG2 alone, 10 μg of rSAG2 emulsified with Vet L-10 (Invitrogen) oil adjuvant (rSAG2 (Vet L-10)) as described previously (Yang et al. Reference Yang, Chang and Chao2004) or PLG-rSAG2 MPs containing 10 μg of rSAG2. During the immunization schedule, the specific anti-Toxoplasma immune responses were analysed by the following immunoassays.
Serum assay
Two weeks after boosting (week 4), Western blot analysis was performed to study the antigenic specificity of the immunized mouse sera. TsoAg (15 μg well−1) was separated by 12% SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). After blocking, strips of the membrane were cut and probed with sera from mice immunized with PLG-rSAG2 MPs, rSAG2 (Vet L-10), soluble rSAG2 alone, PLG or PBS for 1 h at 37 °C. The mouse mAb TG-2 was also conducted. The subsequent colour development was processed as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb).
Lymphocyte proliferation assay
Following immunization, three mice per group were euthanized every 2 weeks to isolate spleen lymphocytes under sterile conditions. Then, 1×105 cells in 200 μL of RPMI-1640 culture medium (CM) were cultured in each well of 96-well culture plates and stimulated with 5 μg mL−1 of rSAG2 as the procedure described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). In addition, CM-treated cultures without rSAG2 stimulation were conducted to use as controls. The rSAG2-induced lymphocyte proliferation was then monitored by using the BrdU (5-bromo-2′-deoxyuridine) Colorimetric Cell Proliferation ELISA Kit (Roche) according to the manufacturer's instructions. Finally, the stimulation index (SI = OD450 values from rSAG2-treated cultures/OD450 values from CM-treated control cultures) of each group was calculated as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb).
IFN-γ assay
Every 2 weeks, spleen lymphocytes derived from three mice of each group were collected and stimulated with 5 μg mL−1 of rSAG2 for 96 h as the procedure for the proliferation assay. Cell-free supernatants were harvested to analyse their IFN-γ concentrations by the sandwich ELISA using the OptEIA Mouse IFN-γ Set (BD Biosciences) as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). The IFN-γ concentrations were determined by comparison to a standard curve created with known amounts of standard recombinant mouse IFN-γ.
Tachyzoite challenge
Eight weeks after boosting (the 10th week), five groups of 15 mice each were challenged with a subcutaneous injection of 1×104 live tachyzoites of T. gondii (RH strain). The mice were monitored daily for definite distressful signs of acute toxoplasmosis for additional 28 days. Signs of acute toxoplasmosis included ruffled fur, trembling, huddling and loss of activity. The end point occurred when mice showed severest signs of distress for 3 successive days. At the end point, mice with severest signs should be killed by a humane method. The survival rate (number of surviving mice after challenge/number of tested mice in each group) in each group was calculated as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb).
Statistical analysis
The data in the present study were statistically analysed as described previously (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). Particle size and entrapment efficiency of PLG-rSAG2 MPs from different batches were statistically compared using one-way ANOVA. Proliferation SI values and IFN-γ production of different immunization groups were statistically compared by using the nested design and the means at each time point in each group were tested by least significant difference (LSD) multiple comparison. The survival rates of different groups were analysed by the chi-square test. A P value of less than 0·05 was considered to be significant.
RESULTS
Antigenicity of E. coli-based rSAG2
The purified rSAG2 protein prepared for the subsequent PLG encapsulation was analysed by Western blot analysis using the mouse mAb TG-2, which is specific to SAG2 of T. gondii tachyzoites. The result demonstrated that the rSAG2 protein (22 kDa) showed the native SAG2 antigenicity recognized by the mouse mAb TG-2 (data not shown).
Characteristics of PLG-rSAG2 MPs
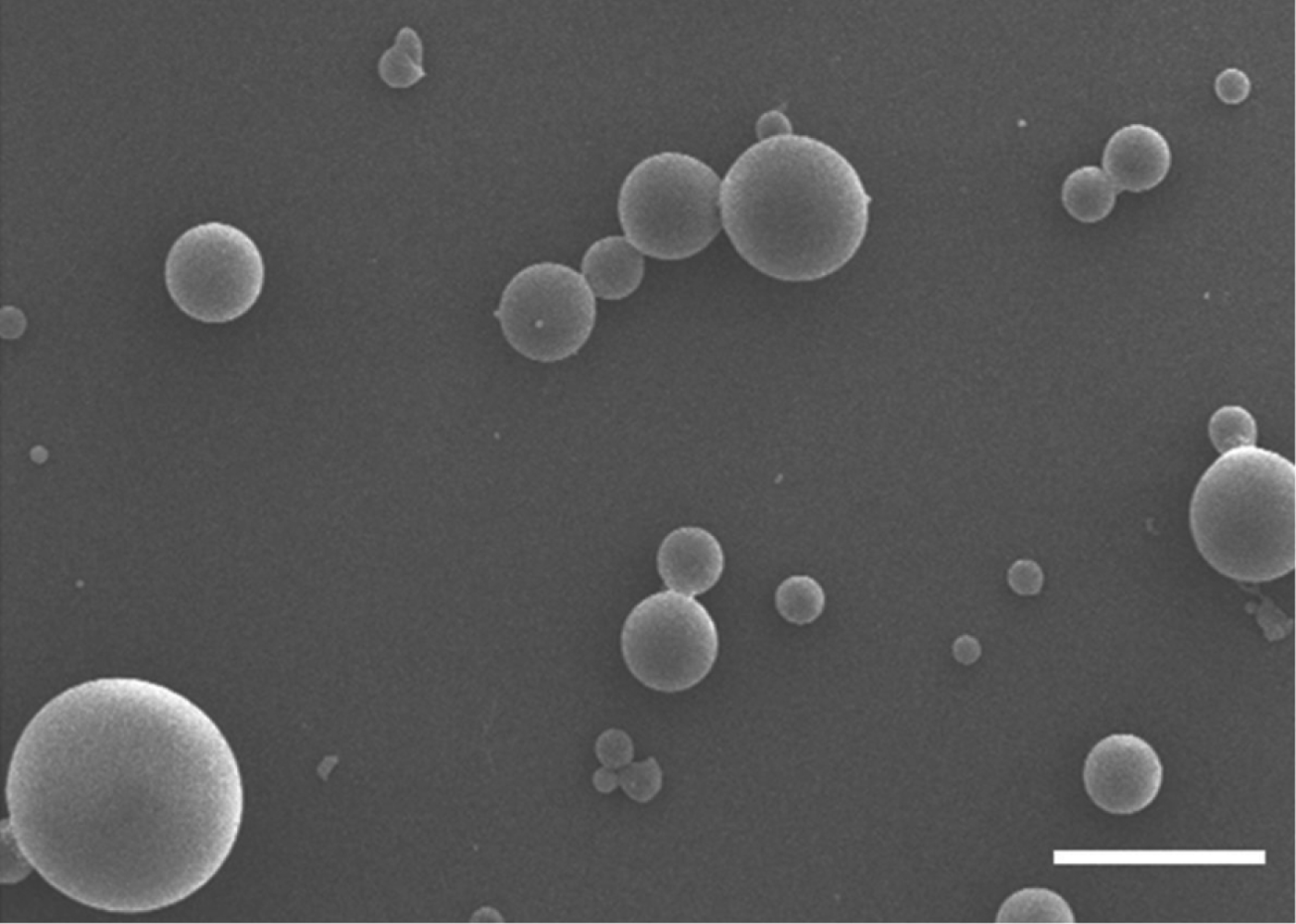
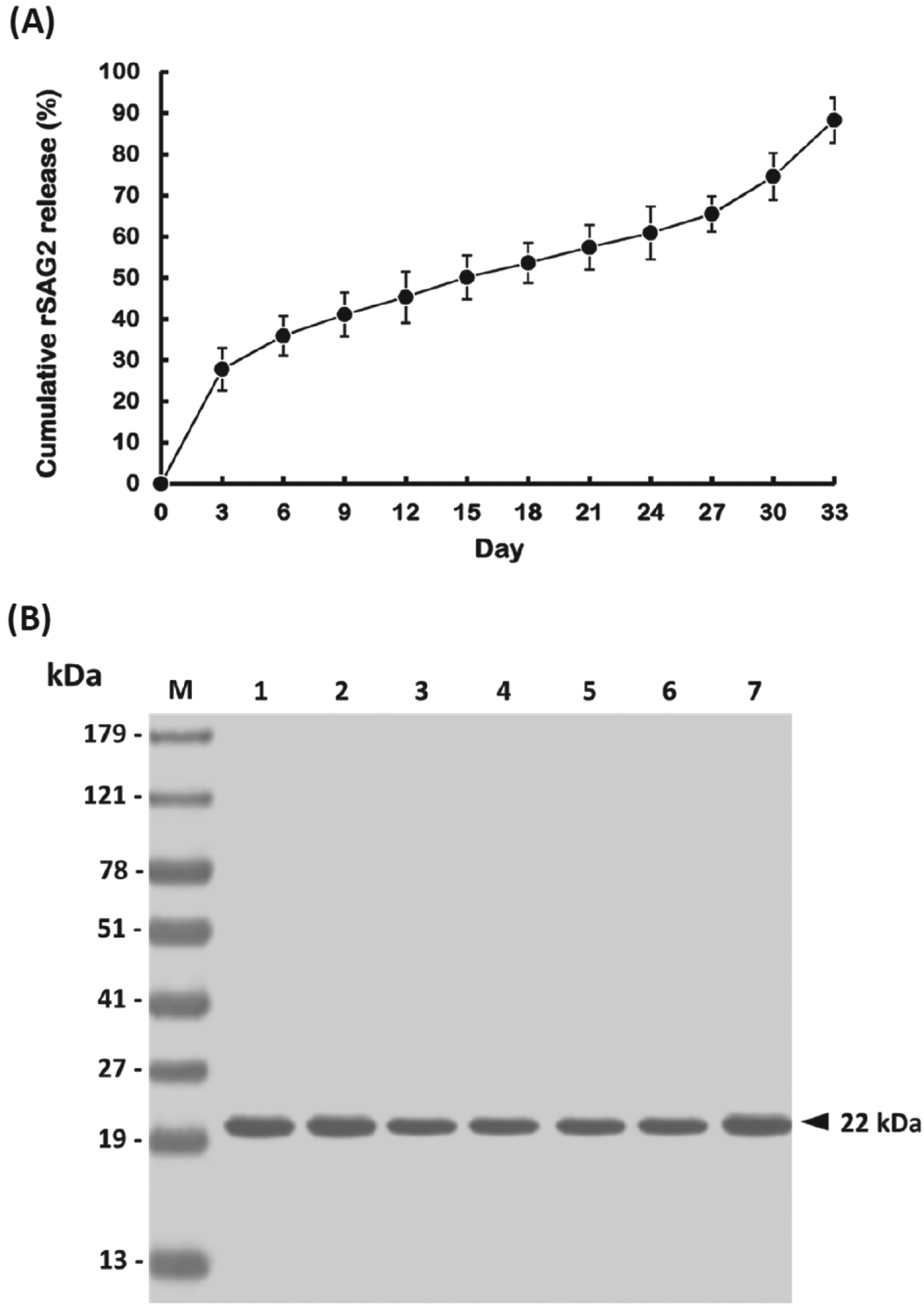
The characteristics of PLG-rSAG2 MPs were analysed after PLG encapsulation. The MPs were inspected by scanning electron microscopy and their morphology showed spherical particles with a smooth surface (Fig. 1). In addition, the mean diameter of different batches of PLG-rSAG2 MPs ranged from 2·14 to 3·63 μm (Table 1). These MPs displayed their entrapment efficiency for the rSAG2 protein ranging from 74 to 80%, without significant differences (P>0·05, ANOVA) among different batches (Table 1). In the release study, the cumulative release of rSAG2 protein from PLG MPs gradually increased over the course of a 33-day period with three distinct phases (Fig. 2A). An initial burst released approximately 27·8% of the total protein load in the first 3 days. Afterwards, there was a very slow release (37·7% of the total protein load) for 24 days followed by a rapid release (22·8% of the total protein load) during the last 6 days. Altogether, 88·3% of the total protein load was released from the MPs during the 33-day study (Fig. 2A). Moreover, mouse mAb TG-2, which specifically reacted with the rSAG2 protein (Fig. 2B, lane 1), recognized the released rSAG2 proteins (22 kDa) collected on days 3, 9, 15, 21, 27 and 33 (Fig. 2B, lanes 2–7).

Fig. 1. Scanning electron micrograph of PLG-rSAG2 MPs. PLG-rSAG2 MPs showed spherical particles with a smooth surface (bar represents 2 μm).

Fig. 2. In vitro release of rSAG2 protein from PLG MPs. (A) PLG-rSAG2 MPs were suspended in PBS as described in Materials and Methods. The amount of rSAG2 in the supernatant sampled every 3 days was measured using the BCA protein assay. The release studies were carried out in triplicate, with each point representing the mean±s.d. (B) Western blot analysis of the antigenicity of released rSAG2 protein from PLG MPs. The soluble rSAG2 (lane 1) and released rSAG2 samples on days 3 (lane 2), 9 (lane 3), 15 (lane 4), 21 (lane 5), 27 (lane 6) and 33 (lane 7) were electrophoretically separated on a 12% SDS-PAGE, transferred to a PVDF membrane, and then analysed with mouse mAb TG-2. Standard protein markers (lane M) are shown at the left.
Table 1. Particle size and entrapment efficiency of PLG-rSAG2 MPs

a The particle size in diameter was measured and expressed as the mean±s.d.
b The entrapment efficiency was expressed as a ratio of the actual rSAG2 entrapment to the theoretical rSAG2 entrapment as described in the Materials and Methods.
c,d A significant difference (P<0·05) exists between SAG2 particle vaccine (SAG2-PV) batches with different superscript letters.
SAG2-specific serum response of immunized mice
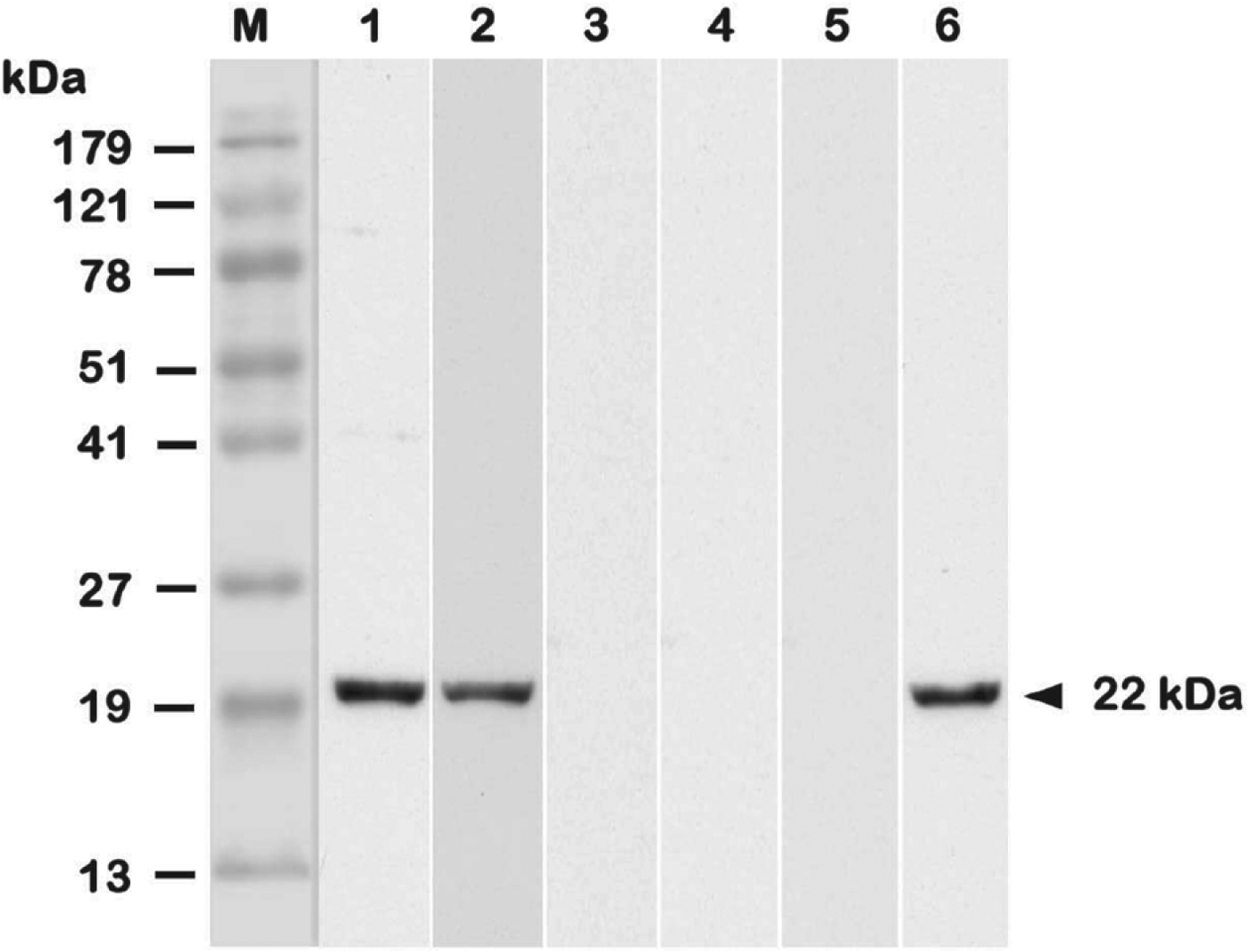
Two weeks after boosting (week 4), mouse sera were collected to study their antigenic specificity by Western blot analysis (Fig. 3). Results showed that mice immunized with PLG-rSAG2 MPs or oil formulation rSAG2 (Vet L-10) produced serum IgG antibodies in response to the native SAG2 protein in TsoAg (Fig. 3, lanes 1 and 2), which was also recognized by the mAb TG-2 (Fig. 3, lane 6). However, sera from mice immunized with soluble rSAG2 alone, PLG or PBS did not recognize any proteins in TsoAg (Fig. 3, lanes 3–5). Therefore, SAG2-specific serum response could be elicited in mice by immunization with rSAG2 formulated with adjuvants, but not in its soluble form.

Fig. 3. The antigenic specificity of immunized mouse sera. Two weeks after boosting (week 4), immunized mouse sera were collected and their antigenic specificity analysed. TsoAg was probed with sera from mice immunized with PLG-rSAG2 MPs (lane 1), rSAG2 (Vet L-10) (lane 2), soluble rSAG2 alone (lane 3), PLG (lane 4) or PBS (lane 5). The mouse mAb TG-2 (lane 6) was conducted as a positive control for the native SAG2 in TsoAg. Standard protein markers (lane M) are shown at the left.
Prolonged SAG2-specific lymphocyte proliferation induced by PLG-rSAG2 MPs
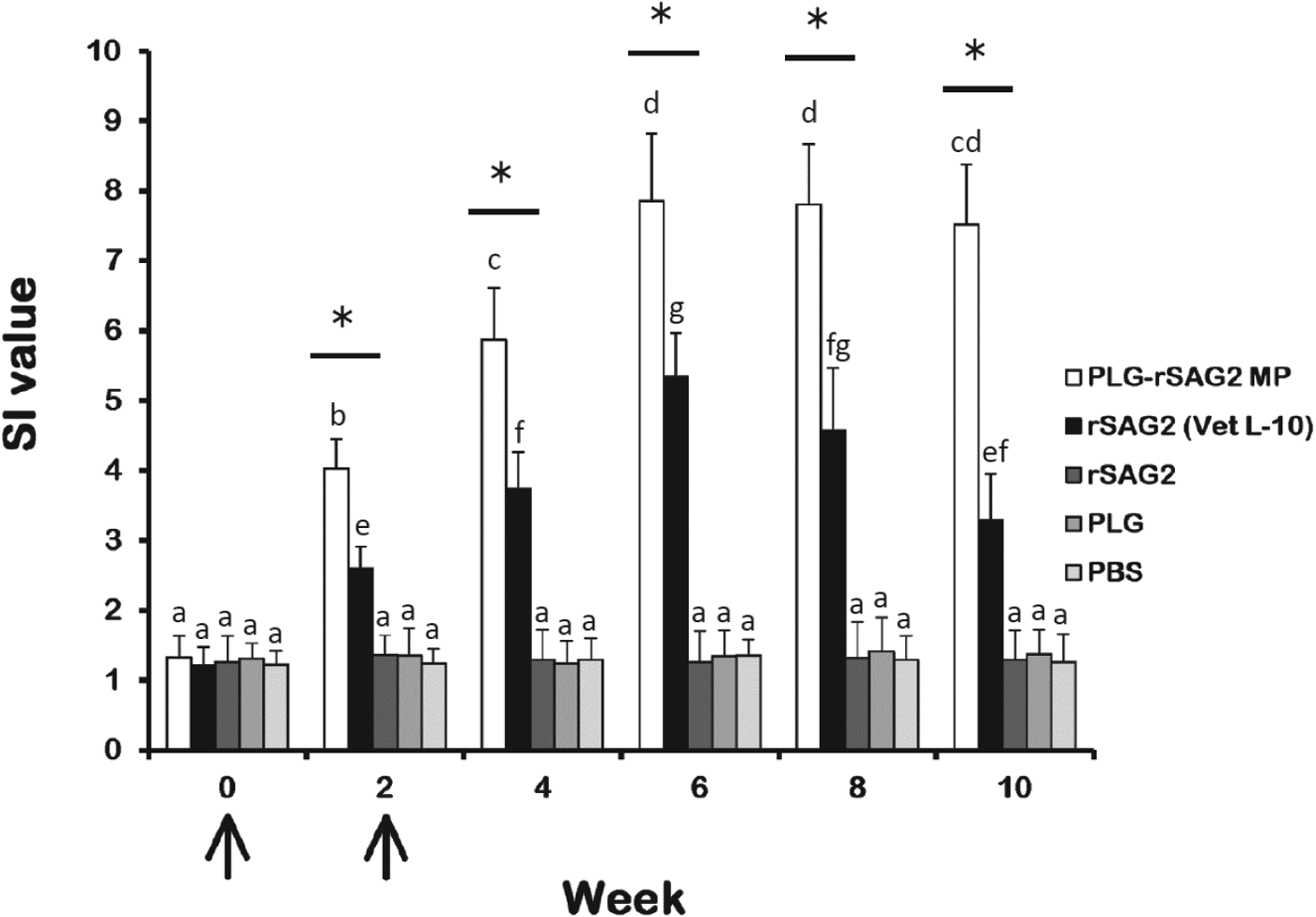
Spleen lymphocytes stimulated with the purified rSAG2 protein were prepared from mice of different groups every 2 weeks and their SAG2-specific proliferation responses were studied and expressed as SI values (Fig. 4). Before immunization (week 0), rSAG2-stimulated lymphocytes from different groups of mice were found to show similar low proliferative SI values. Two weeks after immunization, PLG-rSAG2 MPs elicited significantly higher SI values (P<0·05, nested design) than rSAG2 (Vet L-10) (Fig. 4). The SI values in the PLG-rSAG2 MP group increased from week 0 to week 6 (P<0·05, LSD multiple comparison). However, in the same group, there were no significant differences in the SI values from week 6 to week 10 (P>0·05, LSD multiple comparison). Therefore, the SI values induced by PLG-rSAG2 MPs reached a maximal level in the 6th week and the enhanced SI values could be maintained until the 10th week (Fig. 4). Although high SI values were also enhanced by rSAG2 (Vet L-10) from week 0 to week 6 (P<0·05, LSD multiple comparison), they gradually decreased starting from week 6 to week 10 (P<0·05, LSD multiple comparison) (Fig. 4). Administration with soluble rSAG2 alone, PLG or PBS induced a low proliferation response in mice (Fig. 4). Thus, the PLG encapsulation was better than the oil emulsification in eliciting and maintaining strong SAG2-specific lymphocyte proliferation in mice, indicating the importance of use of the PLG adjuvant.

Fig. 4. The proliferation responses of mouse spleen lymphocytes. Groups of mice were intraperitoneally immunized twice (↑) with PLG-rSAG2 MPs (□), rSAG2 (Vet L-10) (■), soluble rSAG2 alone (![]() ), PLG (
), PLG (![]() ) or PBS (
) or PBS (![]() ). After immunization, rSAG2-stimulated spleen lymphocytes were prepared from three mice per group every 2 weeks and their subsequent proliferation responses were analysed and expressed as stimulation index (SI) values. Results were presented as the mean of SI values±s.d. All groups were analysed by the nested design and an asterisk (*) indicated P<0·05 when comparing the PLG-rSAG2 group to the rSAG2 (Vet L-10) group. Different letters meant a significant difference at each time point in a group (P<0·05, LSD multiple comparison).
). After immunization, rSAG2-stimulated spleen lymphocytes were prepared from three mice per group every 2 weeks and their subsequent proliferation responses were analysed and expressed as stimulation index (SI) values. Results were presented as the mean of SI values±s.d. All groups were analysed by the nested design and an asterisk (*) indicated P<0·05 when comparing the PLG-rSAG2 group to the rSAG2 (Vet L-10) group. Different letters meant a significant difference at each time point in a group (P<0·05, LSD multiple comparison).
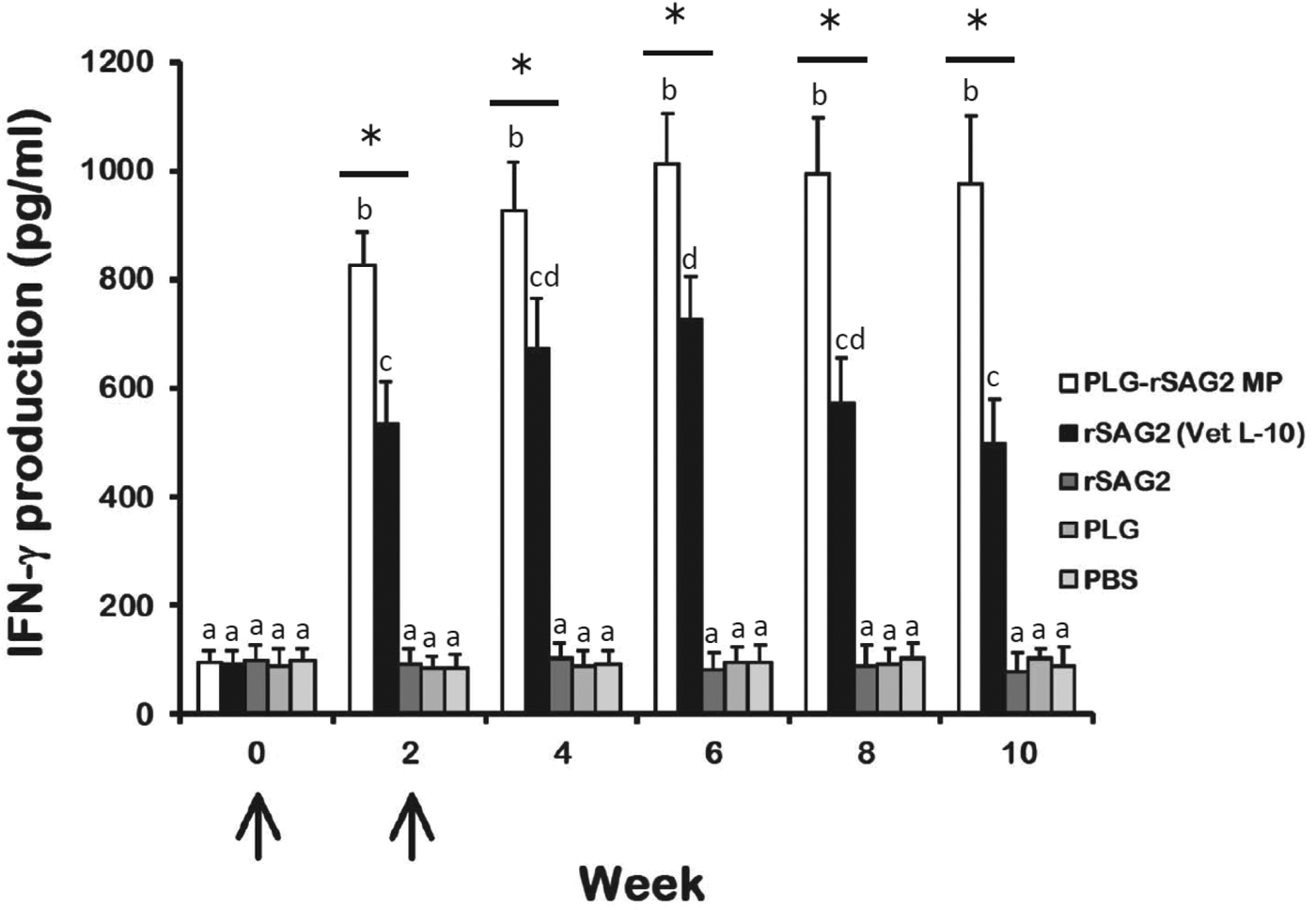
Sustained SAG2-specific IFN-γ production elicited by PLG-rSAG2 MPs
To evaluate whether immunization with PLG-rSAG2 MPs could induce Th1 cell-mediated immunity, the ability of spleen lymphocytes to produce IFN-γ, a Th1 cytokine that plays an important role in cell-mediated immunity, was determined every 2 weeks by sandwich ELISA (Fig. 5). Upon rSAG2 stimulation, lymphocytes from mice immunized with either PLG-rSAG2 MPs or rSAG2 (Vet L-10) produced large amounts of IFN-γ following intraperitoneal immunization (Fig. 5). More importantly, 2 weeks after immunization, PLG-rSAG2 MPs elicited significantly higher levels of IFN-γ (P<0·05, nested design) than rSAG2 (Vet L-10). In the PLG-rSAG2 MP group, the IFN-γ levels raised from week 0 to week 6 (P<0·05, LSD multiple comparison). However, in the same group, there were no significant differences in the IFN-γ levels from week 6 to week 10 (P>0·05, LSD multiple comparison). Therefore, the enhanced IFN-γ levels induced by PLG-rSAG2 MPs in the first 6 weeks were maintained until the 10th week (Fig. 5). However, immunization with rSAG2 (Vet L-10) resulted in an initial IFN-γ induction in the first 6 weeks (P<0·05, LSD multiple comparison) followed by an IFN-γ reduction from week 6 to week 10 (P<0·05, LSD multiple comparison) (Fig. 5). Lymphocytes isolated from mice immunized with soluble rSAG2 alone, PLG or PBS produced low amounts of IFN-γ (Fig. 5). Therefore, immunization with PLG-rSAG2 MPs in mice could effectively elicit and maintain high levels of SAG2-specific IFN-γ.

Fig. 5. IFN-γ production in lymphocyte cultures of immunized mice. Groups of mice were intraperitoneally immunized twice (↑) with PLG-rSAG2 MPs (□), rSAG2 (Vet L-10) (■), soluble rSAG2 alone (![]() ), PLG (
), PLG (![]() ) or PBS (
) or PBS (![]() ). The production of IFN-γ in the culture supernatants of rSAG2-stimulated lymphocytes, collected every two weeks, was calculated and expressed as mean±s.d. All groups were analysed by the nested design and an asterisk (*) indicated P<0·05 when comparing the PLG-rSAG2 group to the rSAG2 (Vet L-10) group. Different letters meant significant difference at each time point in a group (P<0·05, LSD multiple comparison).
). The production of IFN-γ in the culture supernatants of rSAG2-stimulated lymphocytes, collected every two weeks, was calculated and expressed as mean±s.d. All groups were analysed by the nested design and an asterisk (*) indicated P<0·05 when comparing the PLG-rSAG2 group to the rSAG2 (Vet L-10) group. Different letters meant significant difference at each time point in a group (P<0·05, LSD multiple comparison).
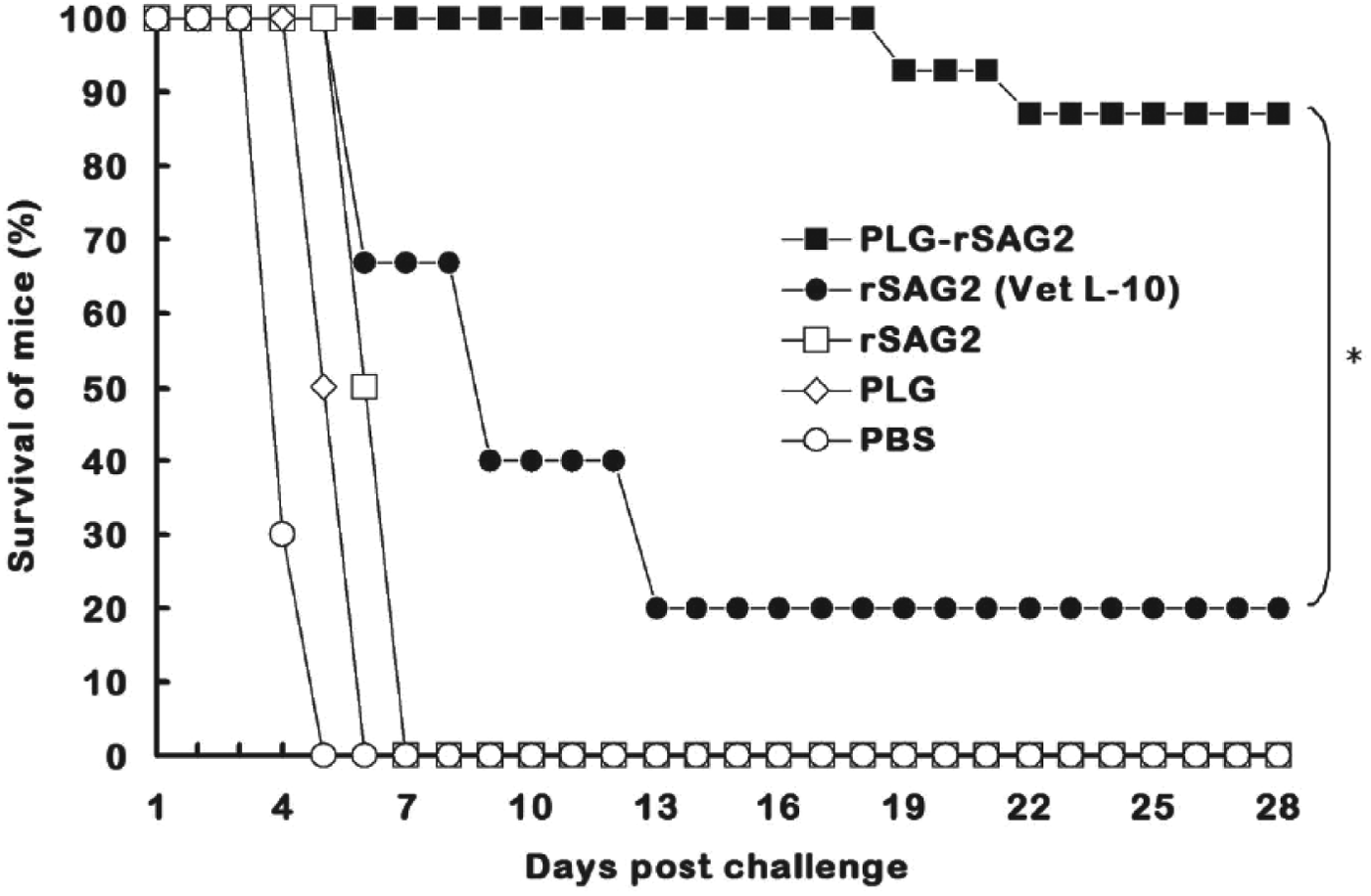
Strong protection against T. gondii tachyzoites induced by PLG-rSAG2 MPs
After T. gondii tachyzoite challenge, all mice administrated with soluble rSAG2 alone, PLG or PBS died within 1 week and displayed no protection (Fig. 6). Three out of 15 mice immunized with rSAG2 (Vet L-10) survived during the challenge study and showed a low protection rate of 20% (Fig. 6). However, in the group of mice immunized with PLG-rSAG2 MPs, only 2 mice died on the 19th day and the 22nd day after challenge. In other words, intraperitoneal immunization with PLG-rSAG2 MPs in mice resulted in the highest survival rate (87%), which was significantly higher (P<0·05, chi-square test) than that of the rSAG2 (Vet L-10) group (Fig. 6). Therefore, the immunity induced by PLG-rSAG2 MPs conferred a substantial resistance to the experimental challenge of T. gondii tachyzoites.

Fig. 6. Survival of immunized mice after a lethal tachyzoite challenge. Groups of mice were intraperitoneally immunized twice with PLG-rSAG2 MPs (■), rSAG2 (Vet L-10) (●), soluble rSAG2 alone (□), PLG (◊) or PBS (○). Eight weeks after boosting (the 10th week), five groups of 15 mice each were subcutaneously infected with 1×104 live tachyzoites of T. gondii (RH strain). Animals were observed daily for an additional month (28 days) and the final survival rates were counted. All groups were analysed by the chi-square test and an asterisk (*) indicated P<0·05 when comparing the PLG-rSAG2 group with the rSAG2 (Vet L-10) group.
DISCUSSION
Most modern vaccines based on subunits of pathogens, such as purified proteins, are often poorly immunogenic and therefore require potent adjuvants to enhance specific immune responses and protection against the given pathogens in vaccinated animals (Singh and O'Hagan, Reference Singh and O'Hagan2003; Garlapati et al. Reference Garlapati, Facci, Polewicz, Strom, Babiuk, Mutwiri, Hancock, Elliott and Gerdts2009; Heegaard et al. Reference Heegaard, Dedieu, Johnson, Le Potier, Mockey, Mutinelli, Vahlenkamp, Vascellari and Sorensen2011). In order to prepare efficacious subunit vaccines against toxoplasmosis, more efforts have been put into the adjuvant design and application for induction of anti-Toxoplasma protective immune responses, particularly sustained T cell-dependent immunity (Innes et al. Reference Innes, Bartley, Maley, Katzer and Buxton2009; Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009, Reference Jongert, Lemiere, Van Ginderachter, De Craeye, Huygen and D'Souza2010). However, the biodegradable PLG polymer is so far seldom used as an adjuvant for Toxoplasma vaccine development. To the best of our knowledge, only three investigations, including our two recent studies, have shown PLG MP-induced immunity and protection against toxoplasmosis in animals (Stanley et al. Reference Stanley, Buxton, Innes and Huntley2004; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). However, Stanley and co-authors have found that a PLG MP vaccine containing a tachyzoite extract plus a mucosal adjuvant, cholera toxin, failed to provide protection in sheep (Stanley et al. Reference Stanley, Buxton, Innes and Huntley2004). In our two previous studies, although the adjuvant effects of the PLG encapsulation have been exercised to maintain anti-SAG1 or anti-SAG1/2 immunity (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb), more effort is needed to improve the potency of a Toxoplasma MP vaccine in animals by encapsulating other Toxoplasma antigens in PLG MPs. In the present study, we have demonstrated that the rSAG2 protein encapsulated in PLG MPs can induce sustained lymphocyte proliferation and IFN-γ production. PLG-rSAG2 MPs also improved anti-Toxoplasma protection (87%), which is higher, though not statistically significant, than either 80% of PLG-rSAG1 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b) or 83% of PLG-rSAG1/2 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a).
In the present study, PLG-rSAG2 MPs suspended in PBS were able to show a triphasic rSAG2 release manner consisted of an initial burst release, a very slow release and a final rapid release (Fig. 2A and B). Similarly, both PLG-rSAG1 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b) and PLG-rSAG1/2 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a), other two PLG MP vaccines prepared in our previous studies, also sustained their critical release of entrapped recombinant proteins in a triphasic controlled-release manner, which has also been addressed by others (Sturesson and Carlfors, Reference Sturesson and Carlfors2000; Kavanagh et al. Reference Kavanagh, Earley, Murray, Foster and Adair2003; Uchida et al. Reference Uchida, Natsume, Kishino, Seki, Ogihara, Juni, Kimura and Morimoto2006). This reproducible triphasic release manner in PBS indicates the significance of our encapsulation conditions for long, stable in vitro release of recombinant proteins from PLG MPs. In the present study, the release assay was done in vitro in PBS and therefore may not completely reflect in vivo release in mice. However, both sustained lymphocyte proliferation (Fig. 4) and prolonged IFN-γ production (Fig. 5) detected in mice immunized with PLG-rSAG2 MPs, rather than rSAG2(Vet L-10), provided notable evidence that the sustained protein release from PLG MPs can repeatedly stimulate the immune effector cells to maintain specific immunity for a long period (Lim et al. Reference Lim, Poh and Wang2009; Jain et al. Reference Jain, O'Hagan and Singh2011).
According to previous studies, an indicative hallmark of an efficacious vaccine against T. gondii is the ability to induce strong Th1 cell-mediated immunity in vaccinated animals (Innes et al. Reference Innes, Bartley, Maley, Katzer and Buxton2009; Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009, Reference Jongert, Lemiere, Van Ginderachter, De Craeye, Huygen and D'Souza2010). In the present study, after peritoneal immunization in mice, we focused much attention on lymphocyte proliferation and IFN-γ production, two activities that positively correlate with Th1 cell-mediated immunity against T. gondii (Yang et al. Reference Yang, Chang and Chao2003, Reference Yang, Chang and Chao2004; Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a, Reference Chuang, Ko, Chen, Du and Yangb). Results revealed that vaccination with PLG-rSAG2 MPs resulted in induction of both sustained lymphocyte proliferation and prolonged IFN-γ production. These data obviously indicate that PLG-rSAG2 MPs really elicit prolonged Th1 cell-mediated immunity, the expected response that we aimed to induce in mice in the present study. We also found that the two activities displayed similar 10-week profiles. Based on previous studies, IFN-γ has been demonstrated to be a critical mediator that has to be secreted for as long as possible in order to maintain anti-Toxoplasma immunity (Subauste and Remington, Reference Subauste and Remington1993; Casciotti et al. Reference Casciotti, Ely, Williams and Khan2002). Thus, the sustained IFN-γ production detected in mice immunized with PLG-rSAG2 MPs (Fig. 5) could support the prolonged SAG2-specific proliferation response (Fig. 4). However, 6 weeks after immunization, the oil formulation rSAG2 (Vet L-10) could not induce a sufficient amount of IFN-γ (Fig. 5) to keep an immunological memory response (Fig. 4).
Based on previous studies, PLG MPs appear to favourably facilitate a size-dependent interaction with APCs, such as macrophages and dendritic cells (Men et al. Reference Men, Audran, Thomasin, Eberl, Demotz, Merkle, Gander and Corradin1999; Newman et al. Reference Newman, Elamanchili, Kwon and Samuel2002). Generally, the particles, like PLG-rSAG2 MPs (2·14–3·63 μm as indicated by Table 1), smaller than 10 μm in diameter are directly taken by APCs (Newman et al. Reference Newman, Elamanchili, Kwon and Samuel2002). In our previous studies, the direct size-dependent interaction has also been displayed by PLG-rSAG1 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b) and PLG-rSAG1/2 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a). In further comparison, the mean diameter of PLG-rSAG2 MPs prepared in the present study is smaller than that (4·25–6·58 μm) of PLG-rSAG1 MPs (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b).
More importantly, the immunity induced by PLG-rSAG2 MPs protected 87% of mice against a lethal subcutaneous challenge of 1×104T. gondii tachyzoites (RH strain), an amount 10 times higher than what we had used in the previous study (Yang et al. Reference Yang, Chang and Chao2004), and enabled mice to survive for a long period of 28 days after challenge (Fig. 6). Such a high survival rate caused by PLG-rSAG2 MPs has increased by 67% compared with the oil formulation, rSAG2 (Vet L-10). However, different tachyzoite doses used for the challenge experiments in mice immunized with rSAG2 (Vet L-10) led to different survival rates in our present (20%) and previous (53%) studies. In further comparison with our previous studies, PLG-rSAG2 MPs elicited a higher protective rate (87%) in mice than either PLG-rSAG1 MPs (80%) (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013b) or PLG-rSAG1/2 MPs (83%) (Chuang et al. Reference Chuang, Ko, Chen, Du and Yang2013a). Numerous investigations have shown that the T. gondii genotypes (Types 1, 2 and 3) (Jongert et al. Reference Jongert, Roberts, Gargano, Forster-Waldl and Petersen2009; Rajendran et al. Reference Rajendran, Su and Dubey2012) and host susceptibility (Li et al. Reference Li, Zhao, Zhu, Ren, Nie, Gao, Gao, Yang, Zhou, Shen, Wang, Lu, Chen, Hide, Ayala and Lun2012) are critical factors on the disease progression and severity of toxoplasmosis. Therefore, the protective capacity of a Toxoplasma vaccine should be evaluated with different parasite strains and different host models. Since the natural Toxoplasma infection is initiated by ingesting oocysts released in cat faeces or consuming meat from infected animals containing long-lived tissue cysts (Dubey and Jones, Reference Dubey and Jones2008; Dubey et al. Reference Dubey, Lago, Gennari, Su and Jones2012), further studies are needed to confirm whether administration with PLG-rSAG2 MPs protects different animal models against an oral challenge of T. gondii cysts (Types 2 and 3).
CONCLUSIONS
The controlled-release PLG-rSAG2 MPs can stably release rSAG2 protein in PBS for an extended period. Peritoneal immunization with PLG-rSAG2 MPs in mice not only maintains strong SAG2-specific Th1 cell-mediated immunity (proliferation response and IFN-γ production) for 10 weeks but also induces improved protection against T. gondii tachyzoite infection. These findings therefore indicate that the adjuvant effects of the PLG encapsulation can strongly enhance rSAG2 immunogenicity. In addition, the ability of the Toxoplasma SAG2 MP vaccine to control the stable rate of antigen release would be advantageous for its application in the development of durable vaccines for future use in humans and animals.
ACKNOWLEDGEMENTS
We thank the Animal Vaccine and Adjuvant Research Center, National Pingtung University of Science and Technology (NPUST), for providing the facility for studies on in vivo immune responses of mice. We also thank the Precision Instruments Center of NPUST for the use of the S3000N scanning electron microscope.
FINANCIAL SUPPORT
This work was supported by the National Science Council (grant No. 101-2313-B-020-029).