Introduction
Anthelmintic drugs are used to control nematode infections but the high frequency of treatment has promoted the selection of resistant populations impacting animal health and welfare (Molento, Reference Molento2009). Although several anthelmintic compounds are currently available, the amino acetonitrile derivatives (AAD) class is an alternative short-acting nematicide option. The AAD monepantel (MNT, C20H13F6N3O2S, MW 473.4 g mol−1) was developed to act against nematode parasites of small ruminants with almost no residual effect and a large safety margin of its sulphone derivative (Kaminsky et al., Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Mäser2008). The compound is also effective against fourth stage inhibited larvae of the most important gastrointestinal nematodes using the therapeutic dose of 2.5 mg kg−1 live weight (LW). MNT has a novel mode of action against livestock nematodes acting by hypercontraction at a nicotinic acetylcholine receptor subunit (Hco-MPTL-1) in nematode body muscle membrane receptors, affecting worm paralysis by blocking nerve signals (Kaminsky et al., Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Mäser2008; Martins et al., Reference Martins, Bergamasco, Felippelli, Tebaldi, Moraes, Testi, Lan, Lapera and Hoppe2017).
To determine the mechanism of action and resistance of MNT, the first studies were conducted in Caenorhabditis elegans determining the acr-23 gene as the main target (Kaminsky et al., Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Mäser2008). However, it was not possible to determine whether the acr-23 gene was solely responsible for the AAD resistance (Rufener et al., Reference Rufener, Mäser, Roditi and Kaminsky2009). Scott et al. (Reference Scott, Pomroy, Kenyon, Smith, Adlington and Moss2013) reported for the first time the resistance of Teladorsagia circumcincta and Trichostrongylus colubriformis to MNT in sheep in New Zealand. The first report of Haemonchus spp. resistance to MNT was from Australia (Sales and Love, Reference Sales and Love2016). Other reports followed from Uruguay (Mederos et al., Reference Mederos, Ramos and Banchero2014), the Netherlands (van den Brom et al., Reference van Den Brom, Moll, Kappert and Vallema2015) and Sweden (Höglund et al., Reference Höglund, Enwejib and Gustafsson2020). In Brazil, cases of MNT resistance of Haemonchus contortus have been described in São Paulo (Albuquerque et al., Reference Albuquerque, Basseto, Almeida and Amarante2017; Martins et al., Reference Martins, Bergamasco, Felippelli, Tebaldi, Moraes, Testi, Lan, Lapera and Hoppe2017) and Rio Grande do Sul (Ramos et al., Reference Ramos, Portella, Rodrigues, Reginato, Cezar, Sangioni and Vogel2018). Resistance to MNT was also reported in Paraná against T. colubriformis and Oesophagostomum columbianum (Cintra et al., Reference Cintra, Teixeira, Nascimento and Sotomaior2016) but it was not observed in H. contortus or T. axei. Moreover, the above worldwide resistance reports did not measure the drug frequency of MNT use. Nevertheless, no molecular marker has been assigned to MNT resistance.
Even though treatments with highly effective anthelmintic drugs have been recommended in short intervals with the objective to eliminate adult worms, preventing the accumulation of parasites (Leathwick, Reference Leathwick2014), drug resistance depends on how intense the selection pressure is, the number of genes involved and the nature of the alleles (Sargison, Reference Sargison2012). Therefore, the dose and the frequency of anthelmintic use are the most important risk factors that influence the rate of selection (Chartier et al., Reference Chartier, Pors, Hubert, Rocheteau, Benoit and Bernard1998). The objective of this study was to determine parasite selection using a therapeutic and a suppressive dose of MNT (Zolvix, Zoetis Animal Health, Holland) after short-interval treatments in naturally infected sheep.
Material and methods
The study was carried out at the Sheep and Goat Production and Research Center of the Federal University of Paraná, LAPOC/UFPR, Brazil (2524′ S; 4907′ L, 900 m a.s.l). The climate is classified as Cfb – humid subtropical with cold winters (Peel et al., Reference Peel, Finlayson and McMahon2007). The study was authorized by the Ethics Committee on Animal Use of the Sector of Agricultural Sciences of UFPR under the protocol 001/2017.
Forty-five Suffolk and White Dorper mix-breed ewes naturally infected with gastrointestinal nematodes were used in the study from February to August 2017. The animals were about 5 years old (±6 months) and presented an average of 83 kg (±14 kg). The ewes were randomly divided into three groups, G1: control, animals that were not treated, G2: animals treated with MNT at 2.5 mg kgLW−1 PO (therapeutic dose, per os) every 30 days for 6 months, and G3: animals treated with MNT at 4.0 mg kgLW−1 (suppressive treatment) every 30 days PO for 6 months or until determining drug failure (efficacy below 60%). The animals were individually weighed (body weight, BW) and checked for body condition score (BCS) according to Gaias (Reference Gaias2012), and Famacha (FMC) anaemia score (van Wyk and Bath, Reference van Wyk and Bath2002) every 2 weeks. Animals from G1 were treated with MNT at 2.5 mg kgLW−1 when the FMC was ≥3 and BCS was below 2. Haematocrit (Ht) was determined monthly. After group division, all animals were allocated to different areas having Tifton 85 (Cynodon dactylon Cv. Tifton 85) and black oat (Avena strigosa L.). The animals remained on pasture for 8 h during the day and were housed during the evening where they received 1% of BW of concentrate (16% crude protein) daily.
Fecal samples were collected before the treatment directly from the rectum and stored at 4 until fecal egg count (FEC) and larval identification were performed. FEC was done weekly during the entire period (D0, D7, D14, D21 and D28 of every month), according to the modified Gordon and Whitlock (Reference Gordon and Whitlock1939) technique in a series of six FEC reduction tests (FECRT). All animals had not been treated for the previous 60 days and were checked for FEC 2 weeks before the study. Animals from G2 and G3 were monitored for 8 h after treatment to determine possible clinical or pharmacological adverse effects before returning to pasture. Coproculture (Roberts and O'Sullivan, Reference Roberts and O'Sullivan1950) and pasture larval count (PLC) (Molento et al., Reference Molento, Buzatti and Sprenger2016) were carried out monthly from each group and area to determine the species/genus of parasite prevalence and for larval identification, in triplicates.
The data from FEC, FMC, BW, BCS and PLC were submitted to Mauchly's sphericity test for significance (P < 0.05) and were evaluated by univariate statistical analysis with repeated measurements in time through the general linear model, GLM Proc from SAS 14.1 (SAS Institute Inc, 2015). The analysis generated two outputs, the main one being related to the effect of the treatment on the analysed variables, and the second set determined the effect of time and the interaction time vs treatment. The time and treatment effects were independent of each other. The statistical model used was
where Yi is the dependent variable, μ is the general mean, Ti is the effect of treatments and ei is the residue. Spearman's correlation analysis was performed according to the CORR Proc of SAS 14.1.
Results
Monthly profile of FEC for all groups is shown in Fig. 1a and b. Animals from the control group showed an average minimum FEC of 89 and a maximum of 7596 with a peak in May and June (autumn) and a steady reduction in July and August (winter). The FEC for G2 and G3 reflected the changes in MNT inefficacy with a few high outlier counts.

Fig. 1. Monthly profile of fecal egg count of all groups before treatment. Control group: black bar, G1/2.5 mg kgLW−1: dark grey bar, G2/4.0 mg kgLW−1: light grey bar. (a) Months of March, April and May, and (b) June, July and August.
The first FECRT showed a high efficacy of MNT for G2 (97%) and G3 (98%). The comparison of the FEC values related to D7 after treatment each month revealed that there was no statistical difference between the doses (P > 0.05), indicating a similar selection pressure from MNT (Fig. 2). FECRT data for the subsequent months showed a constant drop in MNT's efficacy attesting a fast-phenotypic selection for resistance, with clear inefficacy data for both treatment regimens. The results obtained in June showed the highest FEC for both groups after treatment. The efficacy of MNT showed a small increase in July; however, the values were not statistically different (P > 0.05) from the month before. It was observed that MNT at a therapeutic and suppressive dose had a non-linear polynomial efficacy regression (R 2) of 0.988 and 0.992, respectively. The efficacy at the end of the 6-month period was substantially reduced by 3.4-fold for G2 and 2.5-fold for G3.

Fig. 2. Efficacy of monepantel considering fecal egg count in ewes on day 7 post-treatment of each month for the 2.5 mg kgLW−1 (dashed line/G2) and 4.0 mg kgLW−1 (solid line/G3).
FMC, BCS and BW of each experimental group on D7 after treatment are described in Table 1. The mean FMC values for the animals in all groups were close to 1 (G1 = 2, G2 = 1.5, G3 = 1.16) evidencing an adequate condition and a possible resilience to parasitosis even in the onset of MNT failure. The mean Ht was of 27, 29 and 32% for G1, G2 and G3, respectively. BCS showed no statistical difference between the groups (G1 = 3.25, G2 = 3.4, G3 = 3.5), showing a small decrease in June and July.
Table 1. Famacha, body condition score (BCS) and body weight (kg) in untreated (G1) and after monepantel at 2.5 mg kgLW−1 (G2) and 4.0 mg kgLW−1 (G3) at the end of the 6-month selection process
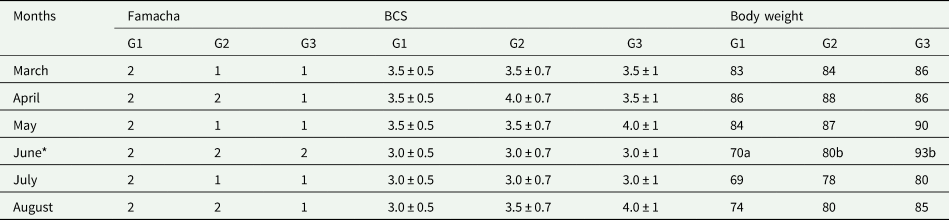
* The values with different letters in June indicate statistical differences in the row.
Although performance measurements were not affected by the treatments, there was an effect of the period (month) and the interaction of treatment vs period on BW values (P < 0.0001) (Table 1). Moreover, the average weight of the animals of the different groups was similar during the first 3 months, the period when the ewes were at the end of their pregnancy. The animals in G1 presented the lowest mean BW among the groups in June (P < 0.05) (Table 1). The data may have been affected by parturition, which occurred in June and may relate to the high incidence of twin births, which was 32, 12 and 20% for G1, G2 and G3.
Parasite prevalence did not differ (P > 0.05) between the groups. Table 2 shows the identification of larvae found in each group. Haemonchus spp., Trichostrongylus sp. and Cooperia spp. were present at the end of the experimental period, exhibiting for the first time the inefficacy/resistance of MNT as shown in Figs 1 and 2.
Table 2. Parasite larval identification and distribution (%) in untreated (G1), and after monepantel at 2.5 mg kgLW−1 (G2) and 4.0 mg kgLW−1 (G3) at the end of the 6-month selection process

In the present study, the presence of clinical signs (FMC < 3) in ewes from G1 coincided with an increase in their FEC and a small decrease in Ht values (data not shown). MNT treatment was necessary in 11 animals (three animals in May and July, and five in June) to maintain the necessary health status of the control group. The average PLC was approximately 2000 larvae kg−1 DM−1 (dry matter) with the presence of >70% of Haemonchus spp. The occurrence of the highest FEC in June also coincided with the highest PLC values (3850 larvae kg−1 DM−1, data not shown). There was no treatment effect on PLC, as MNT failed to provide reasonable efficacy.
Discussion
The present data demonstrate a sharp reduction in efficacy after the suppressive use of MNT in the first months and for 6 months. The data are consistent with the most predicted parasite selection models that discuss the existence of three kinds of genotypes derived from a unique gene of resistance (homozygous susceptible, SS; heterozygote, RS; and homozygous resistant, RR) that may be phenotypically different in the presence of a drug (Leathwick, Reference Leathwick2013). The efficacy of an anthelmintic against these genotypes may vary according to the dose rate chosen or the length of time that the worm is exposed to the chemical, inhibiting parasites with susceptible alleles to reproduce, reducing the refugia (Barnes et al., Reference Barnes, Dobson and Barger1995). As pointed out by Sargison (Reference Sargison2012), selection for parasite resistance depends on the number of genes, the dominant and recessive nature of the alleles and the intensity of the selection pressure. Moreover, aspects such as prior exposure to anthelmintic compounds, inherent sensitivity to compounds in functional dose-limiting species, the initial frequency of potential resistance genes within a population, the nature of the genetic heritage and the impact of non-specific mechanisms of resistance on the survivability of certain isolated individuals hinder the estimates of what may occur within certain parasite populations after an anthelmintic is administered (Bartley et al., Reference Bartley, Devin, Nath and Morrison2015).
All ewes received a supplement containing 16% of crude protein for the maintenance of proper BCS of 2.5, and although untreated animals showed high FEC in certain periods, the infection did not impose a major health risk to the control animals. It is known that animals that are maintained in adequate nutritional conditions may show low FEC and higher levels of packed cell volume and total plasma protein, causing a reduction in parasite survival and fecundity (Kyriazakis and Houdijk, Reference Kyriazakis and Houdijk2006; Hoste et al., Reference Hoste, Torres-Acosta, Quijada, Chan-Perez, Dakheel, Kommuru, Mueller-Harvey and Terrill2016; Lopez-Leyva et al., Reference Lopez-Leyva, Gonzalez-Garduno, Huerta-Bravo, Ramírez-Valverde, Glafiro Torres-Hernandez, Javier Arece-García and Lopez-Arellano2020). Moreover, BW and BCS can also be used as predictors for identifying animals that need treatment (Laurenson et al., Reference Laurenson, Kahn, Bishop and Kyriazakis2016). Ewes from all groups had high Ht (27–32%) values equivalent to FMC 1 and 2 according to van Wyk (Reference van Wyk2001). Wallace et al. (Reference Wallace, Bairden, Duncan, Eckersall, Fishwick, Holmes, Mckellar, Mitchell, Murray, Parkins and Stear1999) stated that factors such as the period in which the animals remain exposed to parasitic infections and the nutritional status of animals can contribute to the level of infection, influencing the FMC score.
The present data showed no treatment effect on FEC regardless of MNT dose. Only a small number (n = 11) of animals from G1 received MNT after presenting clinical signs of anaemia and high FEC during the period. Haemonchus spp. and Trichostrongylus spp. were determined for the first time to show resistance to MNT in Parana, Brazil, being the most prevalent parasites with similar distribution among groups. Kaplan et al. (Reference Kaplan, Burke, Terrill, Miller, Getz, Mobini, Valencia, Williams, Williamson, Larsen and Vatta2004) demonstrated that high prevalence of H. contortus in sheep was associated with clinical signs of anaemia, high FEC and low Ht, indicating a negative correlation between FEC and Ht count in the USA. Molento et al. (Reference Molento, Tasca, Ferreira, Bononi and Stecca2004) reported that even though some Corriedale sheep presented high FEC, their performance was maintained, attesting the resilience status of these individuals withstanding parasite infections with no major signs of anaemia in Brazil.
As observed, the two suppressive drug regimens using short-interval and high drug concentrations of MNT led to a rapid phenotypic selection of independent nematode populations. Niciura et al. (Reference Niciura, Cruvinel, Moraes, Chagas, Esteves, Benavides and Amarante2019) stated that the dosage of anthelmintics is one of the most important risk factors for the induction of MNT resistance, influencing the selection pressure and contributing to the increase of resistance. As mentioned above, in the case of MNT, little is known about the genes that are responsible for resistance. Molecular mechanisms associated with MNT resistance in H. contortus have been reported for the target genes mptl-1, des-2 and deg-3 (Kaminski et al., Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Mäser2008; Rufener et al., Reference Rufener, Mäser, Roditi and Kaminsky2009; Bagnall et al., Reference Bagnall, Rufell, Raza, Elliott, Lamb, Hunt and Kotze2017). Thus, it is important to continue to investigate if these or other genes would contribute to the phenotype of resistance as seen in the present study. It is noteworthy to mention that all larvae from the coproculture and PLC were preserved for future genetic studies.
Even though considerable efforts have been dedicated to the development of new anthelmintic classes, suppressive and unassisted use of these products is still a continuing cause for the increase of parasite resistance (Fortes and Molento, Reference Fortes and Molento2013). More than a decade ago, the arrival and the availability of MNT bringing a new mechanism of action was considered critical to efficient parasite control worldwide but unfortunately, and as it has happened to all major parasiticides, the compound had a short lifespan in most of the small ruminant-producing countries as producers and veterinarians advocated similar and inappropriate control practices (i.e. short treatment intervals). The data demonstrate for the first time that MNT has a working life of only a few months when used in a suppressive frequency rapidly selecting H. contortus. This practice also reveals a sharp susceptible-resistance phenotypic population replacement. We recommend that MNT be used in rotation with other parasiticides alongside other management strategies (i.e. FMC, BCS, FEC) to determine a transient threshold abundance level for target treatments to reduce treatment frequency and the selection process for resistance.
Data
All information is available and can be accessed by request to replicate the findings of the article.
Author contributions
All authors contributed to the work from conception and elaboration, data collection, statistical analysis and writing of the article.
Financial support
The authors gratefully acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES) of the Ministry of Education of Brazil for the M.Sc. fellowship to Karla Duarte.
Conflict of interest
None.
Ethical standards
All ethical standards have been carefully applied and the methodological procedures were approved by the Ethics Committee on the Use of Animals of the Agricultural Science Sector of the Federal University of Paraná, UFPR number 001/2017.