Published online by Cambridge University Press: 28 June 2005
This study established the mechanisms by which Microphallus primas cercariae penetrate the crab Carcinus maenas in which they form metacercarial cysts. Light and electron microscopy were used to investigate cercarial features and to follow the fate of cercariae released in close proximity to crabs. It was shown that cercariae were carried in respiratory currents into crabs' branchial chambers where each enveloped itself in a transparent penetration cyst on the gill lamellae. When cercariae were present the number of respiratory current reversals performed by crabs increased. Using an ‘artificial branchial chamber’ it was possible to observe how cercariae attached to crab gills during breaks in current flow that preceded each current reversal. Inside the penetration cysts the now tail-less larvae used their stylets to pierce holes through which they levered themselves into underlying haemolymph channels in the gills. Histochemical tests demonstrated that the penetration cysts were products of glands in the cercariae and that penetration of the crabs was achieved by mechanical means. The importance of crab respiratory current reversals to the success of cercarial penetration is discussed as it represents the exploitation by a parasite of a host behavioural response to an unrelated stimulus.
Microphallus primas is a digenetic trematode in the family Microphallidae. The adult worm was originally described by Jägerskiöld (1909) and additional detail was provided by Saville and Irwin (1991) who completed its life-cycle in the laboratory. Adult worms are normally found as gut parasites in a number of anseriformes and charadriiformes including Haematopus ostralegus, Somateria mollissima and Larus argentatus (Biquet, Deblock and Capron, 1958). The larval forms (sporocysts and cercariae) develop in a mud snail, Hydrobia ulvae (Pennant, 1777) and metacercarial cysts are found in the hepato-pancreas of the shore crab, Carcinus maenas.
The present study was undertaken to establish the mechanism by which tiny (60 μm body length) M. primas cercariae penetrate the relatively tough exoskeleton of shore crabs. Light microscopy was used to observe cercarial anatomical features that could potentially contribute to the processes of host attachment and penetration. Although it has long been accepted that the stylet of microphallid cercariae has a role to play in penetration of the exoskeleton of intermediate hosts (Lühe, 1909; Sewell, 1922; Cable and Hunninen, 1940) the function of the various glands observed in these organisms has been less well established. In this study histochemical techniques were employed to classify the glandular products of M. primas cercariae in an attempt to establish the function of these glands in the penetration process. Scanning electron microscopy was applied to examine cercarial topographical features and transmission electron microscopy was used to trace glandular secretion.
The interaction between M. primas cercariae and C. maenas was investigated at a number of levels. Initially, observations were made to trace the fate of cercariae that were released in close proximity to crabs. Following this, crab gills were excised and inspected for evidence of cercarial penetration. Finally, a specially devised piece of apparatus was used to provide close observation of cercarial behaviour on the surface of pieces of crab gill under different physical conditions. Together these procedures have provided a description of the complex attachment and penetration process carried out by M. primas cercariae.
To obtain sufficient quantities of M. primas cercariae for experimental purposes naturally infected H. ulvae were crushed and freed cercariae were transferred to fresh filtered seawater maintained at 17 °C for 12 h. Preliminary experimental evidence indicated that cercariae obtained in this manner required a minimum period of 3 h, during which they swam freely, before they would infect their next host, C. maenas. This procedure was adopted because of the intermittent lunar-influenced characteristics of M. primas cercarial release from H. ulvae. Descriptions of M. primas cercariae were compiled from specimens that were either chilled in seawater at 4 °C, vitally stained in 0·1% (w/v) aqueous Nile Blue Sulphate or fixed in Tellyesniczky's Formal-acetic-alcohol.
Free-swimming M. primas cercariae were fixed in 3% (v/v) glutaraldhyde phosphate, buffered to pH 7·2, for scanning microscopy and prepared following the procedure described by Saville et al. (1997). Preparation of cercariae for transmission microscopy followed the procedure adopted by Irwin et al. (1984). Observations were made using JEOL JSM 840 and JEOL 100S electron microscopes.
M. primas cercariae and crab gills fixed in 4% (v/v) phosphate-buffered formaldehyde were washed in distilled water for 1 h before being dehydrated through an ascending series of alcohols to 95% ethanol. Specimens were infiltrated with glycol methacrylate (GMA) over several hours. They were placed into 100% GMA and left overnight on a rotatory mixer to complete infiltration. Polymerization was achieved in a final mixture of GMA under nitrogen. Sections, 0·1mm thick, were cut on glass knives using an ultramictrome. Each was flattened in a drop of distilled water on a glass slide, dried on a hot plate, and stored for staining. Histochemical tests were performed according to Pearce (1968). Periodic-Acid-Schiffs (PAS) and Schiffs reaction without oxidation were adopted to localized carbohydrates. Acid mucosubstances were stained by an alcian blue method and an azure A method was employed to identify other mucosubstances. Basic proteins were stained by the mercuric bromo-phenol blue (HgBPB) technique and with toluidine blue.
Parasite-free young crabs were collected from a rocky shore where it had previously been established that M. primas did not occur in the crab population. Young crabs, consisting mainly of newly settled megazoea, were maintained in 150L tanks until required for experimentation. Barnacles were provided as a food source each week.
Crabs (10–15 mm carapace width) were placed individually in glass beakers containing 10 ml of filtered seawater. A small quantity of seawater containing approximately 50 M. primas cercariae was introduced. To follow the fate of the cercariae, crabs were sacrificed at 5 min intervals for a period of 30 min following exposure to the cercariae and the crab gills were excised, examined and photographed. Control experiments were carried out in which similar sized crabs were placed in identical containers with only filtered seawater. All crabs (experimental and controls) were maintained at room temperature (17 °C) and frequently monitored during the experimental period and their behaviour recorded. To restrict the movement of each crab, a 10 ml beaker was placed into each 25 ml beaker, allowing the crab's respiration currents to be observed with comparative ease through a low-powered binocular microscope. In an attempt to determine if the presence of cercariae in the respired seawater influenced the frequency of respiratory current reversals in crabs, the following experiment was developed and carried out.
Several young crabs, maintained in 25 ml beakers, were individually subjected to a fixed number of cercariae in their immediate vicinity. To start with, 2 crabs each had 50 cercariae introduced into their beakers. Additional pairs of crabs were exposed to larger numbers of cercariae, increasing by increments of 50 cercariae, until each of the final pair was exposed to approximately 300 cercariae (the maximum number released at any one time from a naturally infected H. ulvae). At the end of this observation period, 1 crab from each pair was sacrificed and the gills excised and fixed in 4% (v/v) phosphate-buffered formaldehyde. The remaining crab of each pairing was placed in fresh seawater devoid of cercariae and further monitored at regular intervals until the frequency of its respiratory reversals returned to that of control crabs which were not subjected to cercariae.
Crabs with a carapace width greater than 50 mm were collected from the same locality as the young crabs mentioned above and their gills were dissected out and placed in fresh filtered seawater. Final selection of the crab depended on the diameter of its gills. Selected gill size was dictated by the internal bore (6–7 mm) of the glass Pasteur pipettes adopted for this experiment (see Fig. 1A). The drawn-out tip of each pipette was removed and a rubber bulb was placed over the newly cut (narrower) end. Individual gills were wedged, tapered end first, into the wide end and a restricted waist in each pipette prevented further movement of the gill towards the bulb end. Actively swimming cercariae could be drawn into and gently expelled from these modified pipettes by alternating positive and negative pressure on the bulb with thumb and forefinger. This process attempted to imitate the respiratory current of seawater over the gill of a crab. The apparatus was called the Artificial Brachial Chamber (ABC). The procedure was repeated for each phyllobranchiate gill from each side of a crab's branchial chamber. There are 9 pairs of phyllobranchiate gills in C. maenas but only 3 were suitable for this experiment. In the first experiment each gill (nos. 4, 5 and 6) from the ‘left-hand side’ of a branchial chamber was subjected to 10 intakes/expulsions of seawater containing cercariae. On the last sequence all seawater was expelled from the ABC. Gills were then removed and examined under a compound microscope. This experiment was repeated with gills number 4, 5 and 6 from the ‘right-hand side’ of the same crab. However, in this case a break of 5, 10 or 15 sec was introduced into the middle of the intake sequence, leaving the seawater containing the cercariae surrounding the gill in a stationary state for the specified time before it was expelled. The interruptions in seawater movement imitated breaks in current flow that we had observed in C. maenas activity just before the onset of each respiratory current reversal. Observations were made throughout the duration of these experiments using a low-powered binocular microscope.

Fig. 1. (A) Drawing of the Artificial Branchial Chamber apparatus used to observe the behaviour of Microphallus primas cercariae (C) during simulated respiratory currents over an excised gill (G) removed from Carcinus maenas. (B) Diagrammatic representation of the anterior tip of M. primas cercaria displaying features likely to be involved in the penetration of a second intermediate host. Each cercaria has a well-defined stylet (S) that protrudes from the centre of the oral sucker (OS). A distended portion (DP) of a fore-duct (FD), extending from a gland in the mid-region of the body is clearly visible just posterior to the oral sucker. The anterior portion (D) of the fore-duct projects forward and ventrally to an opening (O) on the ventral surface posterior to the oral sucker. (C) Scanning electron photomicrograph of the anterior ventral surface of a free-swimming M. primas cercaria. In this oral region the duct openings (O) are located posterior to the stylet opening (S). Note possible secretions from the lateral duct openings (arrowheads). (D) Transmission electron photomicrograph of a duct opening on the ventral surface a free-swimming M. primas cercaria. Musculature (M) in close association with tegumental flaps (F) indicates the presence of a sphincter. Note the secretion (S) in the duct opening appears to be continuous with the dense-vacuolated vesicles (V). (E) Light photomicrograph (LM) of gill number 6 dissected from C. maenas that was exposed to M. primas cercariae. Several tail-less larvae (arrowheads) can be identified deep within the folds of the gill lamellae. (F) LM at high magnification, of the protective membrane or cyst (arrowheads) surrounding a tail-less larva attached to a gill. (G) LM of a tail-less larva within a penetration cyst. The distinctive stylet (S) is in a position to pierce the lamella cuticle. (H) LM of a live post-penetration larva (L) migrating towards the main haemolymph channel. Note the aperture (arrowhead) through which the larva entered the gill. The empty transparent penetration cyst (C) has partially collapsed.
The major features at the anterior tip of M. primas cercariae are represented in Fig. 1B. They include an oral sucker, a stylet with a sharp point, and four gland cell ducts. Each of the ducts has a distinct distended portion that, in the lateral pair, is clearly continuous with gland cell bodies situated some distance towards the posterior of the head region. The distended portions of the inner ducts do not appear to be connected to posteriorly situated gland cells in fully mature cercariae. There was, however, some evidence of their attachment to gland cells in cercariae at earlier stages of development. The ducts extending in the anterior direction from the distended portions were narrow and they curled ventrally before opening on the ventral surface of the organism posterior to the oral sucker. Scanning electron microscopy confirmed the location of the duct openings (Fig. 1C). A semicircular ridge of tegument, with a distinct gap in its posterior portion, extended around the lateral and anterior edges of each duct opening. Transmission electron microscopy demonstrated the presence of musculature and tegumental flaps indicative of a sphincter at each duct opening. Secretions appeared to be continuous with dense vesicles deeper within the duct (Fig. 1D).
Preliminary observations of young crabs exposed to actively swimming cercariae revealed that the cercariae were drawn into each crab's branchial chamber by the respiratory current. The inhalant flow entered the crab's carapace through openings at the base of the legs and at the lower edges of the branchiostegites. The largest of these apertures, ‘Milne-Edwards openings’, are found at the bases of the chelipeds and presented the best location to observe the ingress of cercariae carried on the respiratory current. The exhalant openings lie on either side of the epistome just above the mouth. Again, close observation revealed that substantial numbers of the trematode larvae could be seen being emitted from the crab by way of these openings.
When crabs were placed in the restricted space of the experimental apparatus they became very agitated for a short time. After 5 min, by which time they had become placid, cercariae were added in 5 ml of seawater to the experimental crabs and a similar volume of seawater, devoid of cercariae, was added to the control crabs. Both experimental and control crabs again became agitated but the control crabs quickly reverted to being placid. The experimental crabs, on the other hand, became increasingly agitated over a period of 10 min and their respiratory activity appeared to be influenced. Close inspection revealed that they displayed a higher number of respiratory directional changes than appeared in the control crabs. Respiratory current reversal is a well-known feature of crab's respiration and each episode is preceded by a discernable period of ‘still water’. The experiments showed that control crabs displayed approximately one 5-sec reverse flow each minute at room temperature (17 °C). In experimental crabs this was increased and the increase was in proportion to the number of cercariae present in the inhaled seawater. As cercarial numbers were increased from 50 to 300, in steps of approximately 50 cercariae, the frequency of the respiratory reversals increased from 1/min to a maximum of 9/min. Control crabs maintained a regular respiratory pattern of approximately 1/min throughout this period. The frequency of respiratory current reversals was observed to return to that of control crabs within 20–30 min for crabs that had been exposed to low numbers (<100) of cercariae. In contrast, crabs exposed to higher numbers (>200) of cercariae, continued at a peak of 9/min for 10–15 min before reducing respiratory current reversals to 5/min. The frequency of respiratory current reversals fluctuated around this value (5/min) for approximately 1 h more and did not return to that of the control crabs for 2 h post-exposure.
Inspection of gills excised from crabs exposed to 50 cercariae for 5 min under a low power microscope revealed the presence of small ovoid cysts (Fig. 1E). Closer examination showed that these were M. primas cercariae that had lost their tails and were now enclosed in a protective membrane or cyst (Fig. 1F and G). The cysts appeared to be on the outer surface of individual gill lamellae and features such as the diagnostically shaped stylet and the oral sucker could be observed inside. However, when crabs exposed to free-swimming cercariae were left for 30 min the cysts became increasingly difficult to identify. Close inspection revealed that only empty cysts remained on the gill surfaces and the tail-less larvae could now be observed inside the gill lamellae (Fig. 1H). Observations on the gills of 3 crabs sacrificed after 5 min exposure to 50 cercariae are presented in Table 1. The number of cysts containing tail-less larvae decreased from an initial mean of 19 per crab to less than half of that (8·3 per crab) by 15 min. By 30 min all but one of the larvae had penetrated the gills.

Microscopical examination of sections cut through crab gills that had been exposed to large numbers of cercariae (>100), and stained with toluidine blue (Fig. 2A–E) confirmed the observations made on living material. The majority of penetration cysts were observed on lamellae deep within the gills. In general, the penetration cysts were located below the peripheral dilation of each lamella and the inter-lamellar space at that point could just accommodate their dimensions (Fig. 2A–E). The penetration cysts were most frequently located between the terminal bulbous knob of each lamella plate and the raphe (central stem of gill) (Fig. 2A, B, D and E). However, occasionally, a penetration cyst was found at the raphe/lamella junction (Fig. 2C). Of each of the 9 pairs of phyllobranchiate gills removed and fixed from individual experimentally infected crabs, penetration cysts were most frequently located on gills 3, 4, 5 and 6. The shortest gills were the first pair (4 mm) while the longest pair of gills were number 6 (20 mm). Gill pairs 1, 2, 7, 8 and 9 were devoid of penetration cysts. Two important features of this penetration process were that each empty cyst had remained intact, protecting a puncture hole that had been left in the lamella by the penetrating larva. The post-penetration larva had subsequently entered and completely filled the haemolymph channel within the lamella (Fig. 2E). Post-penetration larvae were observed in a number of sections examined and appeared to be migrating in the direction of the main efferent vessel of the gill.

Fig. 2. (A) Light photomicrograph (LM) of section through a penetration cyst on gill lamella from Carcinus maenas that had been exposed to free-swimming M. primas cercariae. The penetration cyst is attachment below the peripheral dilation of the lamella and the larva has orientated itself in a position to puncture the gill cuticle. (B) LM of section through a penetration cyst located close to the raphe, the larva has partially breached the lamella cuticle. (C) LM of section through a penetration cyst located on the raphe. The larva is halfway into the gill. Note the constriction of the body of the larva as it forces its way through the hole into the haemolymph channel. (D) LM of section near the tip of a gill lamella. Note the collapsed penetration cyst that has been almost completely vacated by the larva. (E) LM of section through a gill lamella inside which a larva can be seen completely filling the haemolymph channel. Note the stylet-formed hole (arrowhead) and the empty oval penetration cyst. (F) LM of a single lamella plate dissected from a gill used in the ABC apparatus. Note the position of the two penetration cysts (arrowheads) at the peripheral dilated region. (G) LM of two tail-less larvae in cysts (arrows) on the surface of a gill removed from the ABC apparatus. (H) LM of a larva (L) inside a penetration cyst on a gill lamella (G) removed from the ABC apparatus. The section was cut through the anterior portion of the oval penetration cyst (arrowheads) demonstrating the space available for the larvae to orientate it stylet. Note the position of the stylet in relation to the gill lamella cuticle.
In the first set of experiments using the ABC the cercariae were maintained in constant motion over the gill surfaces and no penetration cysts containing tail-less cercariae were observed on the lamellae. In contrast, in the second set of experiments during which interruptions in the flow of seawater over the gill were introduced, a number of these cysts were observed adhering to the surface of each gill tested (Fig. 2F). During observations of cyst formation and subsequent penetration of the gill cuticle in the ABC, the behaviour of individual cercariae was recorded. As each cercaria collided with the surface of the gill lamellae, it appeared to adhere briefly to this surface when the anterior region was in contact, but was quickly swept off by the respiratory current. If the flow of seawater was interrupted during this contact period, as in the second experiment, a sequence of events ensued in rapid succession. Initially each cercaria collided with the surface of the gill and this impact was consistently received in a region adjacent to the swollen penetration ducts. (This may have been a consequence of the folded swimming posture adopted by this species of microphallid). A small amount of secretion appeared to be discharged from duct openings on the ventral surface of the cercaria. The cercaria stopped swimming briefly, during which time the body extended and contracted once or twice. As each cercaria, with secretions around its oral sucker, was rolled over the gill surface by the current it adhered briefly by its anterior region to this surface. When the artificially induced ‘respiratory current’ stopped, as occurs in respiratory current reversals in crabs, the cercaria discharged a larger amount of secretion from the duct openings around the oral sucker and simultaneously discarded its tail. The now tail-less larva flexed its body violently whilst remaining attached to the gill surface by the oral sucker. This action appeared to smear its body with the discharged secretions (except at the interface between the parasite's oral sucker and the crab's gill) and the secretions developed into a cyst-like structure or penetration cyst around the larva (Fig. 2G). The tail-less larva then proceeded to penetrate the tissue barrier presented by the gill surface. The stylet, which was clearly visible (Fig. 2H), was used in a spearing action by the muscular oral sucker and eventually a hole was pierced in the gill (this process could take several minutes). The larva then maneuvered its body into a position from which it could use the distal portion of the inner surface of the penetration cyst as a launching platform from which it propelled itself through the stylet-formed aperture into the crab's haemolymph. Although the cysts produced by cercariae on gills inside the ABC were found on the outer margins of the gill lamellae (Fig. 2G) they had the same dimensions (60×30 μm) and appearance as penetration cysts produced by M. primas cercariae in experimentally infected crabs.
The results of histochemical tests on sections through free-swimming cercariae, penetration cyst material and the enclosed tail-less larvae are summarized in Table 2. The distended portions of the ducts in free-swimming cercariae gave a strong positive PAS reaction that was subsequently shown to be inhibited by diastase treatment and they also produced a weak reaction with toluidine blue. These results indicated the presence of a glyco-protein compound. A similar reaction was observed in the penetration cyst material. The content of the lateral distended ducts of the encysted tail-less larvae retained only a weak positive PAS reaction. The weak reaction to toluidine blue, observed in all 4 ducts of free-swimming cercariae, was not detected following encystment. Both the tegument of free-swimming cercariae and that of encysted tail-less larvae stained moderately for mercuric bromo-phenol blue and toluidine blue. These results demonstrated that the content of the distended portions of the ducts of free-swimming cercariae and penetration cyst wall material had very similar histochemical properties suggesting that the former had given rise to the latter. As the tegument of free-swimming cercariae and that of encysted larvae displayed the same reactions it would appear that the tegument did not play a part in cyst formation.

This work has demonstrated that M. primas cercariae, like most ‘locate and penetrate’ cercariae, used glands to assist their entry into a host. In this case, the glandular secretions were used to form a penetration cyst on the surface of crab gill lamellae. The 4 distended ducts in the anterior tip of free-swimming cercariae would appear to store secretions produced from cystogenous glands deeper in the cercaria's body. When contact is made with a crab's gill surface these secretions are discharged to give rise to a penetration cyst around each tail-less larva. The semicircular ridges around the duct opening may well play a part in directing the secretions in a posterior direction ensuring that they eventually form a layer over the entire cercarial body. Although entry into the gill was achieved through a hole produced by stylet activity, the cyst wall provided the all-important buttress from which the cercaria could use its muscular strength to push itself into the lamella. All the evidence obtained in this study would suggest that the organism relied on purely physical rather than chemical means to penetrate the gill cuticle.
Production of a penetration cyst to assist in the invasion of a second intermediate host would appear to be a feature of a number of other microphallid species. M. basodactylophallus (Bridgman, 1969) and M. turgidus (Bridgman, 1969) cercariae gain entry into the blue crab, Callinectes sapidus (Rathbun, 1896) and the grass shrimp, Palaemonetes pugio (Holthuis, 1949) respectively by forming cysts on the gill surfaces of those decapod crustaceans (Heard and Overstreet, 1983). M. similis (Jägerskiöld, 1900) Baer, 1943 cercariae have been observed to penetrate the gill cuticle of C. maenas by way of a stylet-formed perforation while protected inside a penetration cyst (unpublished observations). Levinseniella brachysoma (Créplin, 1837) cercariae are found attached all over the body of the amphipod, Gammarus oceanicus (Segerstråle, 1947), each protected by a ‘membranous’ cyst (Galaktionov, 1988). M. claviformis (Brandes, 1888) cercariae penetrate 2 amphipods, Corophium volutator (Pallas, 1776) and C. arenarium (Crawford, 1937) in a similar manner (Jensen, Jensen and Mouritsen, 1998). All of the above-mentioned species possess a stylet, and in all cases their respective second intermediate hosts possess gills. In order to function, gills must have a relatively thin exoskeleton in comparison to that which occurs elsewhere on the body. It would appear that when a parasite of this type attempts to gain entry into such a host via these appendages, they must overcome the twin obstacles of the gill cuticle and the effects of a respiratory current. As far as can be ascertained no other investigations have observed and described the mechanisms involved in penetration cyst formation and host penetration that have been observed in this case. Helluy (1982) observed the penetration by M. papillorobustus (Rankin, 1940) Baer, 1943 cercariae into its second intermediate host G. insensibilis (Stock, 1966). This amphipod's translucent exoskeleton permitted her to directly observe this larval trematode's ‘cyste de penetration’ on the gill plates, but she did not describe the mechanisms involved in its formation.
The fact that the presence of penetrating M. primas cercariae affected the frequency of respiratory current reversals in young C. maenas represents an interesting observation. This may simply be a stress-related response by the crab to a large numbers of invading parasites but the extended duration of this reaction would indicate a more complex interaction between the attacking organisms and the response of the crab. Arudprugasam and Naylor (1964a) suggested that the frequency of reversals in crabs may be influenced by the presence of particulate matter on gills, while Rajashekhar and Wilkins (1991) placed more emphasis on reduced oxygen levels in respired seawater. However, these investigators agree that the current reversal phenomenon observed in crabs appears to be a spontaneous natural rhythm of gill ventilation. The physical impacts of cercariae on crab gills, being similar to those of any particulate matter, could initiate the increase in reversal frequency. After penetration into the gill vasculature, the parasites would reduce haemolymph flow in the lamellae resulting in progressive hypoxia, leading to further reversal frequency. This, in turn, would provide more opportunities for cercariae to attach and produce penetration cysts on the gill surfaces, resulting in greater infection success. A process such as this would be expected to lead to an over-dispersed population of M. primas metacercariae in crabs where the parasite is prevalent. This, in fact, is the case. At the site where material was collected for this research the authors have observed that most crabs are uninfected or possess only a small number of cysts whereas a few individuals have in the region of 100 metacercarial cysts in the hepato-pancreas.
The fact that penetration cysts were frequently located deep within the folds of gill lamellae in experimentally infected crabs and were only found on the outer margin of lamellae on gills inside the ABC is not surprising. The direction of the seawater circulation over the gill in the ABC was from the base rather than from a lateral direction, as is the normal path in the branchial chamber (Arudprugasam and Naylor, 1964b). In addition, the cleaning activity of the branchial flabella (gill rakers) in the branchial chamber, described by Caveny, Modi and Wilkens (1992), could not have been a factor influencing cyst distribution on gills in the ABS.
Now that penetration cyst formation has been observed and described for M. primas perhaps it will be shown to be common to other gill penetrating microphallids. It represents an effective means of overcoming this most hazardous hurdle in the life-cycle of these parasites.
The authors wish to thank Mr T. Fennan for assistance with digitizing photomicrographs. This work contributes to the INTAS funded project 011.0210.

Fig. 1. (A) Drawing of the Artificial Branchial Chamber apparatus used to observe the behaviour of Microphallus primas cercariae (C) during simulated respiratory currents over an excised gill (G) removed from Carcinus maenas. (B) Diagrammatic representation of the anterior tip of M. primas cercaria displaying features likely to be involved in the penetration of a second intermediate host. Each cercaria has a well-defined stylet (S) that protrudes from the centre of the oral sucker (OS). A distended portion (DP) of a fore-duct (FD), extending from a gland in the mid-region of the body is clearly visible just posterior to the oral sucker. The anterior portion (D) of the fore-duct projects forward and ventrally to an opening (O) on the ventral surface posterior to the oral sucker. (C) Scanning electron photomicrograph of the anterior ventral surface of a free-swimming M. primas cercaria. In this oral region the duct openings (O) are located posterior to the stylet opening (S). Note possible secretions from the lateral duct openings (arrowheads). (D) Transmission electron photomicrograph of a duct opening on the ventral surface a free-swimming M. primas cercaria. Musculature (M) in close association with tegumental flaps (F) indicates the presence of a sphincter. Note the secretion (S) in the duct opening appears to be continuous with the dense-vacuolated vesicles (V). (E) Light photomicrograph (LM) of gill number 6 dissected from C. maenas that was exposed to M. primas cercariae. Several tail-less larvae (arrowheads) can be identified deep within the folds of the gill lamellae. (F) LM at high magnification, of the protective membrane or cyst (arrowheads) surrounding a tail-less larva attached to a gill. (G) LM of a tail-less larva within a penetration cyst. The distinctive stylet (S) is in a position to pierce the lamella cuticle. (H) LM of a live post-penetration larva (L) migrating towards the main haemolymph channel. Note the aperture (arrowhead) through which the larva entered the gill. The empty transparent penetration cyst (C) has partially collapsed.

Table 1. Number of cysts containing tail-less larvae observed on the surface of gills excised from 3 crabs (A, B, C) following their exposure to 50 free-swimming Microphallus primas cercariae

Fig. 2. (A) Light photomicrograph (LM) of section through a penetration cyst on gill lamella from Carcinus maenas that had been exposed to free-swimming M. primas cercariae. The penetration cyst is attachment below the peripheral dilation of the lamella and the larva has orientated itself in a position to puncture the gill cuticle. (B) LM of section through a penetration cyst located close to the raphe, the larva has partially breached the lamella cuticle. (C) LM of section through a penetration cyst located on the raphe. The larva is halfway into the gill. Note the constriction of the body of the larva as it forces its way through the hole into the haemolymph channel. (D) LM of section near the tip of a gill lamella. Note the collapsed penetration cyst that has been almost completely vacated by the larva. (E) LM of section through a gill lamella inside which a larva can be seen completely filling the haemolymph channel. Note the stylet-formed hole (arrowhead) and the empty oval penetration cyst. (F) LM of a single lamella plate dissected from a gill used in the ABC apparatus. Note the position of the two penetration cysts (arrowheads) at the peripheral dilated region. (G) LM of two tail-less larvae in cysts (arrows) on the surface of a gill removed from the ABC apparatus. (H) LM of a larva (L) inside a penetration cyst on a gill lamella (G) removed from the ABC apparatus. The section was cut through the anterior portion of the oval penetration cyst (arrowheads) demonstrating the space available for the larvae to orientate it stylet. Note the position of the stylet in relation to the gill lamella cuticle.
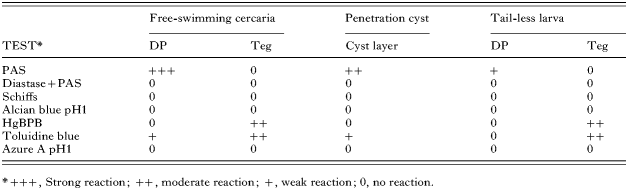
Table 2. Results of histochemical tests conducted on sections of free-swimming Microphallus primas cercariae and penetration cysts containing larvae