INTRODUCTION
Strongyloides sp., like all nematode infections, present unique challenges to the protective immune response. The Strongyloides sp. life cycle is complex, with multiple stages including infective larvae (L3i), parthenogenetic female adult worms, first stage larvae (L1) and autoinfective larvae (L3a), in the definitive host (Viney and Lok, Reference Viney and Lok2015). There is a wide range in the sizes of these stages, all of which are far beyond the capacity of cells to phagocytize. L3 and parasitic adults display both stage specific and shared antigens as potential targets for the immune response (Soblik et al. Reference Soblik, Younis, Mitreva, Renard, Kirchner, Geisinger, Steen and Brattig2011) The various stages reside in specific locations in the host and it is clear that the protective immune response must function differently to expel adult worms from the intestine as compared with killing larvae in the tissues. Therefore, it is hypothesized that a different protective immune response, in terms of specificity and mechanism, is required to control each of the life stages.
Another challenge for the study of protective immune responses during human strongyloidiasis is that the species infecting humans, Strongyloides stercoralis, only infects humans, primates, dogs and gerbils. This host range provides limited opportunities for performing hypothesis-based research. Therefore, several alternative approaches have been utilized to study the development and mechanisms of protective immunity to the various life stages of Strongyloides sp.
Although it was determined that S. stercoralis does not establish patent infections in immune competent mice (Dawkins and Grove, Reference Dawkins and Grove1982a ) it was established that the L3i and L3a of S. stercoralis could be recovered from mice if they were implanted within diffusion chambers (Abraham et al. Reference Abraham, Rotman, Haberstroh, Yutanawiboonchai, Brigandi, Leon, Nolan and Schad1995). After skin penetration, the L3 migrate throughout the body thus making accurate recovery of the parasites and analysis of the parasite microenvironment difficult. Diffusion chambers contain the parasites in vivo in the subcutaneous tissues, a natural habitat for the larvae. Analysis of the contents of the implanted diffusion chamber allows an accurate assessment of parasite survival in mice and provides a unique view of the innate and adaptive immune factors present in the parasite microenvironment. Use of the mouse/diffusion chamber model is limited to the study of larval stages of S. stercoralis and to the single environment in which the diffusion chamber was implanted. It does provide the opportunity of studying protective immunity to the species of Strongyloides that infects humans and in a host for which there are the tools required to dissect the precise mechanism of parasite control by the immune response.
Species of Strongyloides also infect rodents and these models provide the opportunity of studying the immune response in a natural host-parasite relationship including the mechanisms of eradication of parasitic adults from the intestine. A significant difference in the life cycle between S. stercoralis, the human pathogen, and Strongyloides ratti and Strongyloides venezuelensis, the rodent pathogens, is that hyperinfection and extremely chronic infections that are hallmarks of S. stercoralis infection in humans are absent in S. ratti and S. venezuelensis infections. Still, the majority of mouse strains tested, including BALB/c and C57BL/6 mice are fully susceptible to patent infection with S. ratti (Dawkins et al. Reference Dawkins, Grove, Dunsmore and Mitchell1980) and S. venezuelensis (Sato and Toma, Reference Sato and Toma1990). C57BL/6 mice are more susceptible to infection with both S. ratti and S. venezuelensis as compared to BALB/c mice. Strongyloides venezuelensis L3 migrate predominantly via the lung (Takamure, Reference Takamure1995) while S. ratti L3 are present in lungs, cerebrospinal fluid and heads of infected mice (Dawkins and Grove, Reference Dawkins and Grove1981a ; Dawkins et al. Reference Dawkins, Thomason and Grove1982b ; Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010). Both Strongyloides species have similar timing for the migratory path. L3 are detected in lung and head tissue of infected mice from day 1 to day 3 post infection (p.i.) and maximal L3 numbers are recorded at day 2 p.i. L3 arrive in the small intestine from day 3 p.i. onwards and molt within the intestine to the L4 (day 4 p.i.) and to female adults (day 5 p.i.) (Dawkins and Grove, Reference Dawkins and Grove1981a ) that reproduce by parthenogenesis (Viney and Lok, Reference Viney and Lok2015). Either eggs (S. venezuelensis), or predominantly hatched L1 (S. ratti) are released with the feces by day 5 p.i. Adult S. ratti and S. venezuelensis reside in the mucosa of the small intestine. Light and electron microscopic studies suggest that they may move along the intestine creating tunnels between intestinal epithelial cells but never penetrate the basement lamina or enter the lamina propria below (Dawkins et al. Reference Dawkins, Robertson, Papadimitriou and Grove1983).
Immune competent mice terminate primary S. ratti and S. venezuelensis infections with similar kinetics within a month (Dawkins and Grove, Reference Dawkins and Grove1981a ; Sato and Toma, Reference Sato and Toma1990). The numbers of S. ratti and S. venezuelensis adults decline rapidly after day 6–7 p.i., and adults are almost undetectable after day 10 p.i. Egg and larval excretion within the feces, assessed by microscopic analysis, is below detection limit by day 14 p.i., while the more sensitive detection of Strongyloides spp. DNA by quantitative polymerase chain reaction (qPCR) in the feces shows ongoing S. ratti infection in C57BL/6 (Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010) and BALB/c (Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011) mice until 4 weeks p.i.
A resolved primary infection with S. ratti and S. venezuelensis induced partial resistance to a second infection suggesting the establishment of an adaptive immune response (Dawkins et al. Reference Dawkins, Grove, Dunsmore and Mitchell1980; Dawkins and Grove, Reference Dawkins and Grove1981a ; Sato and Toma, Reference Sato and Toma1990). Primary infections of mice with as few as 6 S. ratti L3i, reduced the larval output during a second infection by 97% (Dawkins and Grove, Reference Dawkins and Grove1982b ). Immunity was mediated by both cellular and humoral effectors, as protection could be transferred from immune mice to naïve mice by serum or mesenteric lymph node cells (Dawkins and Grove, Reference Dawkins and Grove1981b ). If immunity was induced by a primary subcutaneous (s.c.) infection with S. ratti or S. venezuelensis L3i, the number of migrating L3 in the tissue was reduced in the secondary infection, suggesting that predominantly the tissue migrating L3 was the target of the adaptive immune response (Dawkins and Grove, Reference Dawkins and Grove1981a ; Sato and Toma, Reference Sato and Toma1990). However, if the primary infection was initiated by oral transfer of S. ratti adults, thus bypassing the tissue migration phase of L3, the number of migrating L3 in the second infection was not reduced. Importantly, oral infection of mice with S. ratti adults reduced the number of adults in the intestine and the release of eggs in the feces from a secondary infection. This observation suggests that there is an intestinal immune response that acts against the adults of S. ratti that functions independently of the L3 specific immune effectors elicited by presence of the tissue migrating L3 (Grove and Northern, Reference Grove and Northern1989).
In summary, the protective immune response to infection with Strongyloides spp. should be studied through two separate lenses, one focused on tissue migrating larvae and the other on the intestine dwelling adults. It is acknowledged that the two responses will have many shared characteristics, especially in the induction phase of the immune response; however, the effector mechanisms will differ radically based on the targets and their locations. Analysis of control of tissue migrating larvae, including L3i and L3a, will be the focus of the section on S. stercoralis and analysis of elimination of adult parasites from the intestine will be the focus of the section on S. ratti and S. venezuelensis.
CONTROL AND ERADICATION OF MIGRATING L3 IN THE TISSUE – S. STERCORALIS
Innate immunity
Innate immunity in mice to larval S. stercoralis is highly efficient, with parasite elimination occurring within 5–7 days p.i. (Abraham et al. Reference Abraham, Rotman, Haberstroh, Yutanawiboonchai, Brigandi, Leon, Nolan and Schad1995). Analysis of the parasite microenvironment, within the implanted diffusion chamber, revealed the presence of infiltrated eosinophils, neutrophils and macrophages. If parasites were implanted in mice in diffusion chambers that prevented cell ingress, parasite killing did not occur. This suggested that contact between cells and the worms was required for parasite killing and that soluble factors from the host were insufficient to kill the worms (Abraham et al. Reference Abraham, Rotman, Haberstroh, Yutanawiboonchai, Brigandi, Leon, Nolan and Schad1995). Elimination of neutrophils and eosinophils from naïve mice by monoclonal antibody treatment resulted in an increase in parasite survival (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ), suggesting that these cells are active participants in the protective innate immune response. Similarly, S. venezuelensis induced an increase of eosinophils and mononuclear cells in the blood, peritoneal cavity fluid and bronchoalveolar lavage fluid (Machado et al. Reference Machado, Ueta, Lourenco, Anibal, Roque-Barreira and Faccioli2007, Reference Machado, Carlos, Lourenco, Souza, Sorgi, Silva, Ueta, Ramos, Aronoff and Faccioli2010). Studies with S. ratti have revealed that cellular infiltrates, consisting of neutrophils, eosinophils and macrophages, were observed in the skin in response to the invading larvae in rats and mice during both primary and secondary responses (Dawkins et al. Reference Dawkins, Muir and Grove1981; McHugh et al. Reference McHugh, Jenkins and McLaren1989) and that granulocytes are crucial in the early defence against migrating larvae of S. ratti in mice (Watanabe et al. Reference Watanabe, Noda, Hamano, Koga, Kishihara, Nomoto and Tada2000).
Eosinophils
Eosinophils are directly recruited to larval S. stercoralis without the need for other host cell assistance. Chemoattractants derived from the larvae stimulate the same receptors and second messenger signals to induce eosinophil chemotaxis as used by host derived chemokines. A soluble parasite extract stimulates multiple receptors on the eosinophil surface, including CCR3, CXCR2 and CXCR4, and multiple factors from the parasite recruit eosinophils including both proteins and chitin. The redundancy of the chemotactic factors produced by the parasite and the multiple responding receptors on the eosinophils suggests that chemotactic receptors on eosinophils may have evolved to ensure a robust protective response to this infection (Stein et al. Reference Stein, Redding, Lee, Nolan, Schad, Lok and Abraham2009). Alternatively, the parasite may recruit eosinophils for its own benefit as has been reported with other nematodes (Gebreselassie et al. Reference Gebreselassie, Moorhead, Fabre, Gagliardo, Lee, Lee and Appleton2012; Huang et al. Reference Huang, Gebreselassie, Gagliardo, Ruyechan, Lee, Lee and Appleton2014).
A direct role for eosinophils in killing larvae was suggested by the observation that survival of S. stercoralis was increased in naïve mice deficient in IL-5 and survival of the larvae was diminished in mice overexpressing IL-5 (Herbert et al. Reference Herbert, Lee, Lee, Nolan, Schad and Abraham2000). Similarly, treatment of mice with a monoclonal antibody to IL-5 reduced eosinophils in mice and concomitantly reduced the capacity to control the larvae of S. ratti in primary infections (Watanabe et al. Reference Watanabe, Sasaki, Hamano, Kishihara, Nomoto, Tada and Aoki2003). Treatment of naïve mice with an anti-CCR3 monoclonal antibody specifically eliminated eosinophils and blocked innate protective immunity to infection with S. stercoralis (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006; O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). Surprisingly, larval killing was not diminished in naïve PHIL mice, that constitutively lack eosinophils. Treatment of PHIL mice with a monoclonal antibody to eliminate neutrophils resulted in a diminished protective innate immune response, indicating that in the complete absence of eosinophils, neutrophils were capable of controlling the infection (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). Therefore, both eosinophils and neutrophils can kill the larvae in naïve mice (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006) and it was inferred that neutrophils are sufficient to compensate for the loss of eosinophils and kill the larvae in mice with a genetic deficiency in eosinophils. Eosinophil killing of the larvae was shown to be dependent on the granular protein major basic protein (MBP) and not eosinophil peroxidase (EPO) (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). MBP, EPO, eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN), purified from human eosinophils, were tested in vitro for their toxicity to the larvae of S. stercoralis. Only MBP and ECP were toxic to the host adapted larvae (L3+), while survival of infective larvae remained unaffected (Rotman et al. Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Gleich, Nolan, Schad and Abraham1996).
Neutrophils
Proof that neutrophils have the capacity to kill the larvae of S. stercoralis was obtained from studies in which purified neutrophils were implanted with larvae within diffusion chambers and implanted in mice. Neutrophils can kill the larvae when implanted in naïve mice (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006) through a process that is dependent on the neutrophil specific granular protein myeloperoxidase (MPO) (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). If neutrophil recruitment to the parasites in mice was blocked, either because of a defect in Gαi2 signalling (Padigel et al. Reference Padigel, Stein, Redding, Lee, Nolan, Schad, Birnbaumer and Abraham2007b ) or in the expression of CXCR2 (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006), the capacity of mice to kill S. stercoralis larvae was significantly decreased. CXCR2 dependent recruitment of neutrophils to larvae occurs independently of IL-17 and molecules extracted from S. stercoralis are capable of directly recruiting neutrophils through CXCR2, using signalling pathways similar to those used by host chemokines. In addition, the S. stercoralis soluble extract also induced neutrophils to release the chemokines MIP-2 and KC, which further enhanced the recruitment of neutrophils. The finding that neutrophils produce increased amounts of neutrophil-recruiting chemokines following exposure to S. stercoralis soluble extract suggests an efficiently orchestrated system whereby a primary stimulus from a parasite causes an autocrine amplification of cell recruitment through release of host-derived chemokines. The efficiency of this recruitment strategy is further highlighted by the observation that the CXCR2 receptor has the ability to respond to both parasite- and host-derived factors resulting in highly efficient neutrophil recruitment and control of infection with S. stercoralis (O'Connell et al. Reference O'Connell, Redding, Hess, Lok, Nolan and Abraham2011b ).
One of the challenges to the effector cells of the innate immune response is achieving contact with their target. Although it has been shown that both eosinophils and neutrophils undergo chemotaxis to the soluble worm products, it does not explain how the cells come into contact with moving worms. It has been reported that the S. stercoralis larvae migrate through tissue at the rate of 10 cm h−1 (Napier, Reference Napier1949) whereas neutrophils migrate through tissue at a rate of 0·06 cm h−1 (Chtanova et al. Reference Chtanova, Schaeffer, Han, van Dooren, Nollmann, Herzmark, Chan, Satija, Camfield, Aaron, Striepen and Robey2008; Peters et al. Reference Peters, Egen, Secundino, Debrabant, Kimblin, Kamhawi, Lawyer, Fay, Germain and Sacks2008; Bruns et al. Reference Bruns, Kniemeyer, Hasenberg, Aimanianda, Nietzsche, Thywissen, Jeron, Latge, Brakhage and Gunzer2010). Extracellular traps, a fibrous network of nuclear DNA released from neutrophils after a cell death process called etosis, have been associated with control of several pathogens (Kruger et al. Reference Kruger, Saffarzadeh, Weber, Rieber, Radsak, von Bernuth, Benarafa, Roos, Skokowa and Hartl2015). Mouse neutrophils were induced to release NET's, in vitro and in vivo, after exposure to live S. stercoralis larvae. Worms were seen trapped within the NET's, preventing their movement, yet the parasites were not killed by entrapment within the DNA snare (Bonne-Annee et al. Reference Bonne-Annee, Kerepesi, Hess, Wesolowski, Paumet, Lok, Nolan and Abraham2014). It was hypothesized that the DNA trap restricted the movement of the larvae thereby allowing effector cells to migrate to the immobilized worm to kill it.
Complement
In addition to neutrophils and eosinophils, complement activation is required for innate protective immunity to larval S. stercoralis in mice. Complement component C3 was detected on the surface of the larvae (Brigandi et al. Reference Brigandi, Rotman, Yutanawiboonchai, Leon, Nolan, Schad and Abraham1996) and eosinophils only killed the larvae if a source of complement was provided (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). Mice deficient in C3 did not kill the parasite whereas mice deficient in C3a receptor did eliminate the worms, suggesting that C3b is the active component of C3 that is required to mediate larval killing (Kerepesi et al. Reference Kerepesi, Hess, Nolan, Schad and Abraham2006). C3b may function in the larval killing process as an adherence molecule for cells, or it may facilitate activation and degranulation of the cells. Live S. stercoralis larvae activated complement in vitro through both the classical and alternative pathways which promoted the adherence of human monocytes and neutrophils to the surface of S. stercoralis (de Messias et al. Reference de Messias, Genta and Mohren1994). Complement also promotes the binding of cells to the larvae of S. ratti (Grove et al. Reference Grove, Northern and Dawkins1985).
Antigen presenting cells (APC)
The transition from the innate to the adaptive immune response requires the parasite to be: (1) killed, (2) dissociated into a phagocytosable form and (3) presented by APC to T cells. Eosinophils are efficiently and independently recruited to the parasite (Stein et al. Reference Stein, Redding, Lee, Nolan, Schad, Lok and Abraham2009) where they have the capacity to kill the larvae through the release of MBP (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ), which leads to the complete disintegration of the larvae. Interestingly, eosinophils also have the capacity to act as APC inducing parasite specific Th2 responses, including IL-4 and IL-5, and both IgM and IgG antibody responses in both primary and secondary infections of mice with S. stercoralis (Padigel et al. Reference Padigel, Lee, Nolan, Schad and Abraham2006, Reference Padigel, Hess, Lee, Lok, Nolan, Schad and Abraham2007a ). Therefore, eosinophils have the capacity to migrate to the parasite microenvironment, kill the parasite and then present the antigens to naïve T cells to induce adaptive immunity to the infection.
Innate immunity – summary
The proposed sequence of events leading to protective innate immunity to larval S. stercoralis starts with neutrophils coming into contact with live worms as the worms migrate through the tissues. This contact induces the neutrophils to die through the process of etosis thereby releasing DNA that traps the larvae and prevents their movement. Cells, including neutrophils and eosinophils, undergo chemotaxis to the trapped worms and upon contact release toxic molecules that kill the parasite in collaboration with complement component C3b. Finally, eosinophils have the added capability of acting as APC to initiate the adaptive immune response to the parasite (Fig. 1).
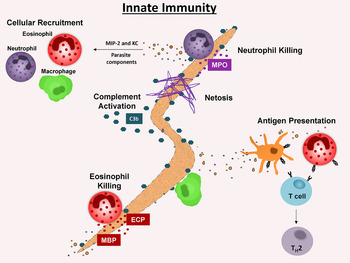
Fig. 1. Innate immunity to Strongyloides stercoralis L3 in mice. Neutrophil recruitment to the L3 microenvironment is parasite specific and results in neutrophil production of additional chemokines MIP-2 and KC. Eosinophils are also preferentially recruited by parasite components and are required for the innate but not adaptive immune response. Eosinophil granular proteins, human and mouse MBP and human ECP are toxic to L3. Eosinophils also act as APC, presenting parasitic antigens, resulting in the induction of Th2 cells. Neutrophils die by etosis and release NETs to trap larvae. Neutrophils utilize MPO to kill L3 during the innate and adaptive immune responses. L3 killing by granulocytes is C3 dependent. C3b potentiates adherence of effector cells to L3. Abbreviations: APC, antigen presenting cells; ECP, eosinophil cationic protein; MBP, major basic protein; MPO, myeloperoxidase.
Adaptive immunity
The adaptive immune response in mice, induced by immunization with live larvae, is highly effective with greater than 90% of the challenge larvae killed within 24 h (Abraham et al. Reference Abraham, Rotman, Haberstroh, Yutanawiboonchai, Brigandi, Leon, Nolan and Schad1995). Several components have been identified that are integral to the protective adaptive immune response.
T cells
Attempts to infect mice with S. stercoralis have revealed that immunologically intact mice and mice deficient in T cells killed the infections within several days (Dawkins and Grove, Reference Dawkins and Grove1982a ) However, SCID mice, which have a deficiency in both T and B cells, could be infected with S. stercoralis, with low numbers of adult worms and first stage larvae developing in the mice after infection (Rotman et al. Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Nolan, Schad and Abraham1995). This indicates that S. stercoralis can develop in mice and that lymphocytes are part of the immune response involved in eliminating the infection. Protective adaptive immunity to S. stercoralis larvae in mice requires CD4+ but not CD8+ T cells (Rotman et al. Reference Rotman, Schnyder-Candrian, Scott, Nolan, Schad and Abraham1997). Immunized mice treated with recombinant IL-12 demonstrated a pronounced shift from a Th2 to a Th1 response that blocked mice from developing protective adaptive immunity. Depletion of the Th2 associated cytokines IL-4 or IL-5 from immunized mice using monoclonal antibodies impaired larval killing (Rotman et al. Reference Rotman, Schnyder-Candrian, Scott, Nolan, Schad and Abraham1997). Therefore, CD4+ T cells, and in particular Th2 cells, are required for the protective adaptive immune response.
B cells and Antibody
Protective innate immunity in μMT mice, that lack mature B cells, was sufficient to eliminate all parasites, whereas immunized μMT mice with no detectable antibody did not kill challenge infections in the adaptive immune response. Xid mice, which lack B-1 cells, developed a modest level of parasite specific IgG with little IgM following immunization and did not kill worms in the adaptive immune response. These studies demonstrates that B cells, and specifically B-1 cells, are required for adaptive immunity, but not innate immunity, to S. stercoralis and suggest that IgM is required for adaptive immunity (Herbert et al. Reference Herbert, Nolan, Schad and Abraham2002a ).
Mice immunized with live larvae of S. stercoralis have elevated parasite-specific IgA, IgG1 and IgM levels in the serum (Abraham et al. Reference Abraham, Rotman, Haberstroh, Yutanawiboonchai, Brigandi, Leon, Nolan and Schad1995). IgM, recovered from mice 1 week after initial immunization, passively transferred protective immunity to naive mice through a mechanism dependent on granulocytes and complement (Brigandi et al. Reference Brigandi, Rotman, Yutanawiboonchai, Leon, Nolan, Schad and Abraham1996). Both IgM and IgG recovered at three and 5 weeks post immunization could passively transfer immunity. IgG requires complement and neutrophils to kill the worms and functions through antibody-dependent cellular cytotoxicity (ADCC) based on studies in the Fc receptor gamma (FcRγ)−/− mice. This is in contrast to IgM from mice immunized with live larvae where protective immunity is ADCC-independent. Western blots were performed to determine what antigens the protective IgM and IgG recognized and it was determined that both antibody isotypes recognized some shared antigens, whereas other antigens were recognized independently by either protective IgG or IgM. Furthermore, IgM bound to the surface of the cuticle, basal cuticle-hypodermis, coelomic cavity and glandular oesophagus, whereas the IgG bound only to the basal cuticle-hypodermis and the coelomic cavity (Ligas et al. Reference Ligas, Kerepesi, Galioto, Lustigman, Nolan, Schad and Abraham2003). It therefore was concluded that while IgM and IgG antibodies are both protective against larval S. stercoralis, they recognize different antigens and utilize different killing mechanisms. Similarly, a role for antibody and cells in adaptive protective immunity to S. stercoralis in jirds has been observed (Nolan et al. Reference Nolan, Rotman, Bhopale, Schad and Abraham1995). IgG is required for antibody-dependent immunity to S. ratti (Murrell, Reference Murrell1981), and a correlation was observed between protective immunity to S. ratti and IgG in mice and rats (Dawkins and Grove, Reference Dawkins and Grove1981b ; Uchikawa et al. Reference Uchikawa, Ichiki and Komaki1991; Bleay et al. Reference Bleay, Wilkes, Paterson and Viney2007). Finally, passive immunization of mice with a IgM monoclonal antibody to HSP60 led to reduced numbers of migrating S. ratti larvae in lung and head (Ben Nouir et al. Reference Ben Nouir, Piedavent, Osterloh and Breloer2012).
Complement
The protective adaptive immune response in mice to larval S. stercoralis is dependent on complement activation. In the initial studies, immunized mice treated with Cobra venom factor to deplete C3, were shown to be unable to kill the larvae (Brigandi et al. Reference Brigandi, Rotman, Yutanawiboonchai, Leon, Nolan, Schad and Abraham1996). The necessity of complement was confirmed in immunized C3−/−mice, where larval killing also did not occur. C3a receptor−/− mice killed larvae during the adaptive immune response as efficiently as wild-type mice thereby suggesting that C3b is the active complement component (Kerepesi et al. Reference Kerepesi, Hess, Nolan, Schad and Abraham2006). C3 has been observed to be on the surface of larvae recovered from immunized mice (Brigandi et al. Reference Brigandi, Rotman, Yutanawiboonchai, Leon, Nolan, Schad and Abraham1996), and possibly serves as an anchor for cells to attach to the larvae in order to mediate killing of the parasite, as has been seen with human complement and cells in response to S. stercoralis (de Messias et al. Reference de Messias, Genta and Mohren1994). Thus complement activation is an integral component of both protective innate and adaptive immunity to S. stercoralis in mice.
Eosinophils
Depleting IL-5 from mice immunized against infection with S. stercoralis, either by monoclonal antibody treatment (Rotman et al. Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Gleich, Nolan, Schad and Abraham1996, Reference Rotman, Schnyder-Candrian, Scott, Nolan, Schad and Abraham1997) or by genetically knocking out IL-5 (Herbert et al. Reference Herbert, Lee, Lee, Nolan, Schad and Abraham2000) resulted in decreased numbers of eosinophils and an absence of protective adaptive immunity. However, when eosinophils were specifically absent, either due to elimination by monoclonal antibody treatment (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006) or the use of PHIL mice which are genetically deficient in eosinophils (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ), it was determined that eosinophils were not required as effector cells in the adaptive immune response. Interestingly, immunized IL-5 deficient mice, that had severely reduced numbers of eosinophils, failed to establish protective immunity and had lower levels of parasite-specific IgM (Herbert et al. Reference Herbert, Lee, Lee, Nolan, Schad and Abraham2000). Reconstitution of immunized IL-5 deficient mice with wild-type eosinophils elevated the parasite-specific IgM levels and the mice were then able to eliminate challenge infections (Herbert et al. Reference Herbert, Lee, Lee, Nolan, Schad and Abraham2000). Similarly it has been reported that IgM induced by the adjuvant alum is compromised in mice genetically deficient in eosinophils and that transfer of IL-4 expressing eosinophils restored the production of antigen specific IgM (Wang and Weller, Reference Wang and Weller2008), thereby confirming a role for eosinophils in IgM production. Immunized PHIL mice, which have no eosinophils but do have intact cytokine levels, did not have reduced IgM levels (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). The immunized PHIL mice appear to have an alternative source for molecules required for the induction of IgM production that IL-5−/− mice do not have. Therefore, eosinophils function as effector cells in the innate immune response, antigen presenting cells and as sources of cytokines required for IgM production in the adaptive immune response.
Neutrophils
Studies were performed to determine the role of neutrophils in protective adaptive immunity to S. stercoralis. Using CXCR2−/− mice it was demonstrated that a reduction in recruitment of neutrophils resulted in significantly reduced adaptive protective immunity. Protective antibody developed in immunized CXCR2−/− mice, thereby demonstrating that neutrophils are not required for the induction of the adaptive protective immune response. Moreover, neutrophils from wild type and CXCR2−/− mice killed the larvae of S. stercoralis at the same rate, thus demonstrating that the defect in the CXCR2−/− mice was in recruitment of neutrophils and not in their ability to kill larvae (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006). Mice deficient in Gαi2 also failed to kill the larvae in a challenge infection with S. stercoralis despite developing an antigen-specific Th2 response characterized by increased IL-4, IL-5, IgM and IgG. Neutrophils from Gαi2−/− mice were competent in killing larvae; however, immunized Gαi2−/− mice had significantly reduced recruitment of neutrophils to the parasite microenvironment, as seen within the diffusion chamber (Padigel et al. Reference Padigel, Stein, Redding, Lee, Nolan, Schad, Birnbaumer and Abraham2007b ). These data demonstrate that CXCR2 and Gαi2 are not required for the development of the protective immune responses against S. stercoralis; however, they are essential for the recruitment of neutrophils required for killing of larvae.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize pathogen associated molecular patterns on pathogens and alert the immune response to the presence of invading pathogens. C3H/HeJ mice, which have a point mutation in the Tlr4 gene, were used to determine the role of TLR4 in protective immunity to S. stercoralis. TLR4 was not required for killing the larvae during the innate immune response, but was required for killing the parasites during the adaptive immune response. No differences were seen in the T cell responses, antibody responses or cell recruitment to the parasite between wild type and C3H/HeJ mice after immunization. However, it was determined that neutrophils from the C3H/HeJ mice could not participate in killing the worms in the adaptive immune response. The Tlr4 mutation severely alters the effector function, but not recruitment, of cells to the parasite microenvironment (Kerepesi et al. Reference Kerepesi, Hess, Leon, Nolan, Schad and Abraham2007). Finally, as in the innate immune response, neutrophils deficient in MPO had significantly decreased larval killing capacity (O'Connell et al. Reference O'Connell, Hess, Santiago, Nolan, Lok, Lee and Abraham2011a ). Therefore, neutrophils require both MPO and TLR4 to kill the larvae of S. stercoralis in the adaptive immune response.
Macrophages
Macrophages can be separated into subsets, with classically activated macrophages associated with Th1 responses and alternatively activated macrophages associated with Th2 responses (Murray et al. Reference Murray, Allen, Biswas, Fisher, Gilroy, Goerdt, Gordon, Hamilton, Ivashkiv, Lawrence, Locati, Mantovani, Martinez, Mege, Mosser, Natoli, Saeij, Schultze, Shirey, Sica, Suttles, Udalova, van Ginderachter, Vogel and Wynn2014). Infection of mice with the larvae of S. stercoralis resulted in the induction of alternatively activated macrophages within the peritoneal cavity. Induction of this subset of macrophages required a Th2 response and specifically the production of IL-4. Alternatively activated macrophages, but not classically activated macrophages, kill the larvae with neutrophils and complement in vitro. Using transwell culture plates, it was determined that both neutrophils and macrophages can kill the parasite with soluble help from the reciprocal cell. In vivo studies have demonstrated that purified neutrophils implanted with larvae within diffusion chambers can kill the larvae when implanted in naïve mice (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006). It is hypothesized that the neutrophils killed the worms in the in vivo studies with soluble help from macrophages located on the outside of the diffusion chamber. Finally, macrophages kill efficiently in the adaptive immune response in collaboration with parasite-specific IgM (Bonne-Annee et al. Reference Bonne-Annee, Kerepesi, Hess, O'Connell, Lok, Nolan and Abraham2013).
Adaptive immunity – summary
In summary, the adaptive immune response is initiated by eosinophils functioning as APC stimulating CD4+ T cells to produce the Th2 cytokines, IL-4 and IL-5. B cells produce IgM, which collaborates with macrophages, and IgG, which collaborates with neutrophils, to kill the larvae. Eosinophils do not participate in killing the parasites in the adaptive immune response, but are required for inducing the production of IgM. Complement activation, specifically C3b, is required for the accelerated killing in the adaptive immune response. Neutrophils kill through a process dependent on TLR4 and MPO. In addition to the antibody dependent killing, macrophages that have been alternatively activated by IL-4 also kill the parasites as part of a triad composed of macrophages, neutrophils and complement (Fig. 2).

Fig. 2. Adaptive immunity to Strongyloides stercoralis L3 in mice. Immunization of mice with L3i results in Th2 cell derived secretion of IL-5 and IL-4. IL-5 is required for eosinophils, which collaborate with B cells to produce parasite specific IgM. IL-4 is required for the development of alternatively activated macrophages that interact with neutrophils, complement and IgM to kill during the adaptive immune response. Accelerated L3 killing by neutrophils during the adaptive response is mediated via MPO and complement component C3 and is IgG dependent. Abbreviation: MPO, myeloperoxidase.
Vaccine development
Studies were performed to identify antigens from S. stercoralis that would induce protective immunity and thereby be components of a vaccine against the infection. Mice immunized with soluble antigens derived from S. stercoralis larvae, administered with alum as the adjuvant, had a 50% reduction in larval survival. Purified IgG from mice immunized with the soluble antigens passively transferred immunity to naïve mice and was ADCC independent. Immunization of mice with the small pool of antigens specifically recognized by the protective IgG induced a level of parasite killing comparable with live larval immunization (Herbert et al. Reference Herbert, Nolan, Schad, Lustigman and Abraham2002b ). These studies demonstrated that a limited pool of native antigens, identified by mouse protective antibody, were capable of inducing a high level of protective immunity to S. stercoralis in mice.
Passive transfer studies were performed using human serum with antibodies to diagnostic S. stercoralis antigens. Protective immunity developed in mice receiving the human serum. Using protective purified human IgG, seven proteins were recognized in the pool of soluble S. stercoralis antigens, but only three were identified in the S. stercoralis EST database. The three proteins, tropomyosin (Sstmy-1), Na+-K+ATPase (Sseat-6) and LEC-5 (Sslec-5) were constructed into DNA plasmids. Sseat-6 was the only plasmid that induced a limited, but statistically significant, level of protective immunity against the S. stercoralis larvae (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005), showing that the DNA encoding a single antigen could induce the development of protective immunity.
Single recombinant purified protein antigens were tested for efficacy as a vaccine against S. stercoralis. Ss-EAT-6, Ss-TMY-1 and Ss-LEC-5 were selected as they were recognized by human IgG and there was success with Ss-eat-6 using DNA immunization (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005). In addition, the recombinant diagnostic antigens Ss-NIE-1 and Ss-IR (Krolewiecki et al. Reference Krolewiecki, Ramanathan, Fink, McAuliffe, Cajal, Won, Juarez, Di Paolo, Tapia, Acosta, Lee, Lammie, Abraham and Nutman2010) were included in the study. Immunization with the recombinant antigens in alum revealed that only immunization with the diagnostic antigen Ss-IR stimulated high and reproducible levels of protective immunity to infection. IgG from mice immunized with Ss-IR could transfer protective immunity and was found to bind to the larval surface and to the granules in the glandular esophagus. Interestingly, this is the same location that the protective human IgG bound to the worms (Kerepesi et al. Reference Kerepesi, Nolan, Schad, Lustigman, Herbert, Keiser, Nutman, Krolewiecki and Abraham2004; Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011).
AUTOINFECTION AND HYPERINFECTION
Strongyloides stercoralis infections in humans are extremely long-lived (Robson et al. Reference Robson, Beeching and Gill2009; Prendki et al. Reference Prendki, Fenaux, Durand, Thellier and Bouchaud2011), through a process of autoinfection, whereby L1 develop in the intestine into small, morphologically distinct infective larvae termed L3a. L3a penetrate the wall of the lower ileum or colon or the skin of the perianal region, enter the circulation again, travel to the lungs as well as other routes, and then to the small intestine, thus repeating the cycle. This maintains the life cycle for decades in the human host with infection levels moderated to limit pathogenicity. However, if infected individuals develop changes in the immune system secondary to diabetes, hematologic malignancies, malnutrition, hypogammaglobulinemia, use of immunosuppressive drugs such as corticosteroids, chronic obstructive pulmonary disease or malignancy, organ transplantation and HTLV-1, there is an increased risk of hyperinfection with S. stercoralis (Weatherhead and Mejia, Reference Weatherhead and Mejia2014). This results in uncontrolled over-proliferation of larvae with spread to organs, including the lungs, liver and brain. Systemic sepsis is a common complication owing to translocation of enteric bacteria accompanying larval invasion of the gut wall (Greaves et al. Reference Greaves, Coggle, Pollard, Aliyu and Moore2013).
It is interesting that S. stercoralis infected SCID mice, lacking T and B cells, do not develop hyperinfection which suggests that control of hyperinfection is not T or B cell dependent in mice (Rotman et al. Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Nolan, Schad and Abraham1995). Treatment of S. venezuelensis infected BALB/c mice with the immune suppressive glucocorticoid Dexamethasone resulted in a generalized increase in tissue and intestinal parasite burden and prolongation of infection (Machado et al. Reference Machado, Carlos, Sorgi, Ramos, Souza, Soares, Costa-Cruz, Ueta, Aronoff and Faccioli2011). However, although limited numbers of larvae were observed in the tissue up to day 37 p.i., this model may not resemble the uncontrolled and often lethal dissemination of large larval numbers observed in the human hyperinfection syndrome. Although mice are not susceptible to infection with S. stercoralis, Mongolian gerbils are susceptible, supporting development of all life stages. Treatment of infected gerbils with methylprednisolone acetate resulted in the development of hyperinfection characterized by the presence of large numbers of L3a and the death of the animals (Nolan et al. Reference Nolan, Megyeri, Bhopale and Schad1993). Neonatally infected gerbils produce a burst of autoinfection that amplifies the adult worm population but eventually control the production of L3a in most cases. This short burst of autoinfection in naive hosts suggests that the production of L3a is downregulated by the host's immune responses and exposure to L3i during the initial infection may prime the host and prevent development of autoinfection under normal circumstances (Nolan et al. Reference Nolan, Bhopale and Schad1999a ). To test this hypothesis, parasitic adult worms were orally transmitted to naïve gerbils to determine if L3a would develop in the absence of an immune response directed at the L3i. Oral transfer of parasitic adult worms produced autoinfection in gerbils. If the transplanted adult worms were derived from infected gerbils treated with methylprednisolone acetate, L3a were observed in low numbers from day 5–9. If the adult worms came from untreated gerbils the number of L3a was negligible. Mice, infected orally with the adult worms, did not have any L3a. SCID mice were also infected orally with adult worms and did not develop L3a. This suggests that the development of L3a is not under the control of T and B cells in mice (Nolan et al. Reference Nolan, Bhopale and Schad1999b ). Finally, gerbils infected with a large number of L3i developed high numbers of adult worms. L3a were seen in these animals for as long as 7 months and although a mechanism was not defined, it was suggested that the large number of L3a was a reflection of the large numbers of the precursor stage, the L1. Finally it was determined that these gerbils had a strong anti-L3i immune response, while allowing the L3a to remain alive in the tissues (Nolan et al. Reference Nolan, Bhopale, Rotman, Abraham and Schad2002).
The observation in gerbils, suggesting that the immune response that is effective at killing the L3i was not effective against the L3a was tested in mice. Immune responses generated by immunizing mice with live L3i were directed at the host activated, tissue migrating third stage larvae L3+ (Brigandi et al. Reference Brigandi, Rotman, Leon, Nolan, Schad and Abraham1998). Combining this observation with the susceptibility of the L3+ to MBP (Rotman et al. Reference Rotman, Yutanawiboonchai, Brigandi, Leon, Gleich, Nolan, Schad and Abraham1996), suggests that L3i are resistant to immune attack whereas the L3+ is the susceptible stage. Antigenic differences were seen between the L3i, L3+ and L3a. Immunity generated with L3i and directed at L3+ did not kill the L3a (Brigandi et al. Reference Brigandi, Rotman, Nolan, Schad and Abraham1997). This might explain how infections persist in human hosts for decades. Incoming L3i would be targeted by the adaptive immune response and this immunity would prevent overwhelming infection with the parasite. The L3a would survive in the face of this immune response, thereby perpetuating the parasite within the host. Furthermore, production of L3a is apparently controlled by the immune response, based on their uncontrolled development in immunosuppressed individuals. The net result is that the immune response controls L3i and L3a through different mechanisms which results in infections with S. stercoralis persisting for the lifetime of the host, yet causing only minor pathological effects in most cases.
CONTROL AND ERADICATION OF PARASITIC ADULTS FROM THE INTESTINE – S. RATTI AND S. VENEZUELENSIS
Surprisingly little is known about the nature of effector cells and mechanisms mediating intestinal control and eradication of Strongyloides spp. parasites compared with the detailed information regarding eradication of tissue migrating L3 that was reviewed in the section “Control and eradication of migrating L3 in the tissue – S. stercoralis”. One problem lies in the differentiation of immune mechanisms relevant for control of migrating L3 vs adults. Any change in intestinal parasite burden of mice that were infected by s.c. injection of L3i could be the result of changed immunity to tissue migrating L3 alone, changed immunity to the parasitic adult in the intestine alone or a combination of both. Although this issue can be addressed either by performing oral infections thus bypassing the tissue migration phase or by quantification of tissue migrating L3 and adults in the intestine, the differentiation between tissue and intestinal immunity is not always clear and relevant immune effectors certainly overlap.
Innate intestinal effector cells
Several lines of evidence suggest that mucosal mast cells are the central effector cells mediating expulsion of S. ratti and S. venezuelensis from the intestine. Mast cells were induced in the small intestine during S. ratti and S. venezuelensis infection in mice (Khan et al. Reference Khan, Horii, Tiuria, Sato and Nawa1993) and rats (Shintoku et al. Reference Shintoku, Kadosaka, Kimura, Takagi, Kondo and Itoh2013). Mouse mast cell protease 1 (mMCP1), a protease that is specific for mucosal mast cells (Reynolds et al. Reference Reynolds, Stevens, Lane, Carr, Austen and Serafin1990), was elevated in the serum of S. ratti and S. venezuelensis infected mice (Sasaki et al. Reference Sasaki, Yoshimoto, Maruyama, Tegoshi, Ohta, Arizono and Nakanishi2005; Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010). Similarly rat mast cell protease II was elevated in intestinal tissue of S. ratti infected rats (Wilkes et al. Reference Wilkes, Bleay, Paterson and Viney2007).
WBB6F1-KitW/W−v (W/Wv) mice carry a mutation in the stem cell factor (SCF) receptor Kit that results in mast cell deficiency. Release of S. ratti (Nawa et al. Reference Nawa, Kiyota, Korenaga and Kotani1985) and S. venezuelensis (Khan et al. Reference Khan, Horii, Tiuria, Sato and Nawa1993) eggs and L1 in the feces was prolonged in W/Wv mice. Reconstitution with wild type bone marrow cells partially reverted the phenotype suggesting a contribution of mast cells to intestinal immunity. Nawa et al. also described increased numbers of L3 in the head of S. ratti infected W/Wv mice during first infection (Nawa et al. Reference Nawa, Kiyota, Korenaga and Kotani1985) and numbers of migrating S. venezuelensis L3 in W/Wv mice were not recorded (Khan et al. Reference Khan, Horii, Tiuria, Sato and Nawa1993). Therefore a formal differentiation between mast cell function in immunity to migrating larvae and parasitic adults in W/Wv mice is missing. Since W/Wv mice suffer from several other haematological and non-haematological deficiencies including a drastic reduction in the number of basophils, it is difficult to definitively attribute their phenotype to mast cell deficiency alone (Reber et al. Reference Reber, Marichal and Galli2012).
Support for a role of intestinal mast cells in expulsion of parasitic adults originates from two studies using Notch 2 and phosphatidylinositol-3 kinase (PI3K) subunit p85α deficient mice. Conditional Notch 2 deficient mice show a changed intestinal mast cell distribution (Sakata-Yanagimoto and Chiba, Reference Sakata-Yanagimoto and Chiba2015). Accumulation of mast cells predominantly in the lamina propria instead of the intestinal epithelial layer was associated with increased fecal egg release during S. venezuelensis infection (Sakata-Yanagimoto et al. Reference Sakata-Yanagimoto, Sakai, Miyake, Saito, Maruyama, Morishita, Nakagami-Yamaguchi, Kumano, Yagita, Fukayama, Ogawa, Kurokawa, Yasutomo and Chiba2011). PI3Kp85α/− mice lack gastrointestinal and peritoneal mast cells while still containing dermal mast cells. These mice displayed prolonged fecal release of S. venezuelensis eggs and prolonged presence of parasitic adults in the intestine (Fukao et al. Reference Fukao, Yamada, Tanabe, Terauchi, Ota, Takayama, Asano, Takeuchi, Kadowaki, Hata Ji and Koyasu2002). Still, as PI3Kp85α deficiency results in impaired Kit signalling that was most likely causing their mast cell deficiency, these mice do not represent a Kit independent mouse model for mast cell deficiency either.
Kit independent mast cell deficient mice were generated recently by heterozygous transgenic expression of Cre recombinase under the control of the mast cell specific carboxypeptidase A3 promoter (Cpa3Cre/wt) that led to deletion of mast cells in mucosal and connective tissues via genotoxic mechanisms (Feyerabend et al. Reference Feyerabend, Weiser, Tietz, Stassen, Harris, Kopf, Radermacher, Moller, Benoist, Mathis, Fehling and Rodewald2011). Cpa3Cre mice have reduced basophil numbers while other features of the immune system are normal. In line with the former studies using the mixed background WBB6F1-Kit W/W−v mice, mast cell deficient S. ratti infected Cpa3Cre/wt mice displayed higher parasite burden and prolonged fecal release of eggs and L1 compared with their mast cell competent Cpa3wt/wt littermates (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). Using mice that lack mucosal and connective tissue mast cells (Feyerabend et al. Reference Feyerabend, Weiser, Tietz, Stassen, Harris, Kopf, Radermacher, Moller, Benoist, Mathis, Fehling and Rodewald2011) or selectively connective tissue mast cells (Dudeck et al. Reference Dudeck, Dudeck, Scholten, Petzold, Surianarayanan, Kohler, Peschke, Vohringer, Waskow, Krieg, Muller, Waisman, Hartmann, Gunzer and Roers2011) or basophils (Ohnmacht et al. Reference Ohnmacht, Schwartz, Panzer, Schiedewitz, Naumann and Voehringer2010), we are currently re-evaluating overlapping and separate roles of mast cells and basophils during S. ratti infection in mice. Mice lacking either connective tissue mast cells or basophils displayed an initial elevation in intestinal parasite burden but eventually terminated infection with wild type kinetics whereas mice that lacked both connective tissue mast cells and mucosal mast cells were unable to terminate S. ratti infection for more than 150 days. Here, immunity to tissue migrating L3 was not affected since differences in parasite burden were restricted to the intestinal life stage of S. ratti. These results show that basophils and connective tissue mast cells contribute to early intestinal immunity but are dispensable for final expulsion of parasitic adults whereas mucosal mast cells represent essential terminal effector cells that mediate the final expulsion of S. ratti from the intestine (Reitz and Breloer unpublished results).
Eosinophils and neutrophils predominantly mediate immunity to tissue migrating L3 as reviewed in the section “Control and eradication of migrating L3 in the tissue- S. stercoralis” and are dispensable for intestinal immunity to S. ratti and S. venezuelensis. Although eosinophils expanded in the intestinal tissue of S. ratti and S. venezuelensis infected mice, abrogation of this eosinophilia by anti-IL-5 treatment or in IL-5-deficient mice did not abrogate intestinal immunity (Korenaga et al. Reference Korenaga, Hitoshi, Yamaguchi, Sato, Takatsu and Tada1991a ; Ovington et al. Reference Ovington, McKie, Matthaei, Young and Behm1998; Watanabe et al. Reference Watanabe, Sasaki, Hamano, Kishihara, Nomoto, Tada and Aoki2003). Increased eosinophilia in IL-5 transgenic mice did not accelerate termination of infection but reduced numbers of parasitic adults after oral infection (El-Malky et al. Reference El-Malky, Maruyama, Hirabayashi, Shimada, Yoshida, Amano, Tominaga, Takatsu and Ohta2003). However, it should be noted that analysis of intestinal immunity to S. ratti and S. venezuelensis infection in eosinophil deficient mice has not been performed. Depletion of Ly6G+ neutrophils and Ly6C+ monocytes by anti-Gr1 (RB6–85C) treatment increased numbers of S. ratti L3 in the tissue and subsequent intestinal parasite burden (Watanabe et al. Reference Watanabe, Noda, Hamano, Koga, Kishihara, Nomoto and Tada2000). If Gr1+ cells were depleted after the completion of the tissue migrating phase i.e. at day 3 p.i., the intestinal parasite burden did not change but fecal egg release increased. Thus, granulocytes are more important in controlling migrating L3 in the tissue as demonstrated for S. stercoralis (Galioto et al. Reference Galioto, Hess, Nolan, Schad, Lee and Abraham2006) than for eradication of adults from the intestine although they may contribute to parasite control by affecting fecundity.
Adaptive immune response
RAG1−/− mice that lack T and B cells displayed comparable numbers of parasitic adults in the intestine at day 6 p.i. (Breloer et al. Reference Breloer, Hartmann, Blankenhaus, Eschbach, Pfeffer and Jacobs2015) demonstrating that initial control of S. ratti can be maintained in the absence of adaptive immunity. However, termination of infection clearly depends on a concerted action of innate effectors and the adaptive immune system, as infection was drastically prolonged for up to 1 year in T cell deficient nude mice (Dawkins et al. Reference Dawkins, Mitchell and Grove1982a ). CD4+ T cells were the dominant cells contributing to timely termination of infection, since MHC-I−/− mice which lack CD8+ T cells displayed an unchanged course of S. venezuelensis infection whereas MHC-II−/− mice, which lack CD4+ T cells, displayed a delay in clearance of infection by 1 week (Goncalves et al. Reference Goncalves, Rodrigues, Silva, Goncalves, Cardoso, Beletti, Ueta, Silva and Costa-Cruz2008; Rodrigues et al. Reference Rodrigues, Silva, Goncalves, Cardoso, Alves, Goncalves, Beletti, Ueta, Silva and Costa-Cruz2009, Reference Rodrigues, Cardoso, Goncalves, Silva, Massa, Alves, Ueta, Silva and Costa-Cruz2013). As the authors did not quantify tissue migrating larvae, the net effect of absent CD4+ T cells could reflect specifically a less efficient eradication of migrating L3 as shown before in CD4+ T cell depleted mice for S. stercoralis (Rotman et al. Reference Rotman, Schnyder-Candrian, Scott, Nolan, Schad and Abraham1997) or a combination of less efficient eradication of tissue migrating L3 and expulsion of parasites from the intestine.
The canonical T cell response to helminth infection is the Th2 response that is characterized by production of the cytokines IL-3, IL-4, IL-5, IL-9 IL-10 and IL-13 and the subsequent induction of antibody isotype switch to IgG1 and IgE (Allen and Maizels, Reference Allen and Maizels2011). Infection of rats (Wilkes et al. Reference Wilkes, Bleay, Paterson and Viney2007; Chiuso-Minicucci et al. Reference Chiuso-Minicucci, Marra, Zorzella-Pezavento, Franca, Ishikawa, Amarante, Amarante and Sartori2010) or mice (Machado et al. Reference Machado, Ueta, Lourenco, Anibal, Roque-Barreira and Faccioli2007; Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010; Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014) with S. ratti and S. venezuelensis provoked all features of this canonical Th2 response. Thereby the magnitude of Th2 cytokine production was positively correlated to the infection dose in S. venezuelensis and S. ratti infected mice (Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010; Schilter et al. Reference Schilter, Pereira, Eschenazi, Fernandes, Shim, Sousa, Teixeira and Negrao-Correa2010) and rats (Bleay et al. Reference Bleay, Wilkes, Paterson and Viney2007) while single or repeated infection doses both elicited comparable Th2 responses (Paterson et al. Reference Paterson, Wilkes, Bleay and Viney2008).
Evidence for a contribution of the Th2 immune response to anti-Strongyloides immunity is supported by several studies using mice with defective Th2 polarization. Signal transducer and activator of transcription (STAT) 6−/− mice showed prolonged fecal egg release by 11 days compared with wild type mice (Sasaki et al. Reference Sasaki, Yoshimoto, Maruyama, Tegoshi, Ohta, Arizono and Nakanishi2005). Injection of IL-27 or transgenic overexpression of IL-27 in S. venezuelensis infected mice resulted in reduced IgE and IL-4 production and a 10 day prolongation of egg release in the feces (Yoshimoto et al. Reference Yoshimoto, Yasuda, Mizuguchi and Nakanishi2007).
Transgenic overexpression of IL-12 was shown to abrogate immunity against S. stercoralis L3 in the tissue (Rotman et al. Reference Rotman, Schnyder-Candrian, Scott, Nolan, Schad and Abraham1997). The reciprocal promotion of Th2 polarization in IL-12−/− C57BL/6 mice elevated several aspects of S. venezuelensis specific Th2 response such as eosinophilia, the production of IL-13, IL-10, IL-3, IL-5 as well as IgG1 and IgE response but resulted only in a slight decrease in parasitic adult numbers in the intestine day 7 p.i. in one study (Machado et al. Reference Machado, Carlos, Lourenco, Sorgi, Silva, Ramos, Ueta, Aronoff and Faccioli2009). Unchanged parasite burden in the intestine on days 7, 10 and 14 p.i. and unchanged fecal egg release were reported in another study where IL-12−/− mice were infected with a lower dose of S. venezuelensis L3i (Negrao-Correa et al. Reference Negrao-Correa, Pinho, Souza, Pereira, Fernandes, Scheuermann, Souza and Teixeira2006).
Interestingly, the induction of a Th1 driven anti-S.ratti HSP60 response induced by vaccination with SrHSP60 and characterized by antigen-specific production of IFNγ and elevation of S. ratti specific IgG2b and IgG2c with simultaneous absence of IL-13 production, did not protect mice from subsequent infections (Nouir et al. Reference Nouir, Eschbach, Piedavent, Osterloh, Kingsley, Erttmann, Brattig, Liebau, Fleischer and Breloer2012). By contrast, susceptibility of vaccinated mice was increased leading to elevated numbers of adults in the intestine and increased fecal release of S. ratti DNA. However, if the anti-SrHSP60 response was biased towards Th2 by vaccination with alum precipitated SrHSP60, antigen-specific IgG1 and IL-13 were induced, IFNγ was absent and mice were protected from subsequent infection thus highlighting the importance of intact Th2 response for control of S. ratti infection.
Type 2 innate lymphoid cells (ILC2) that are activated in response to tissue derived alarmins play an important role in the initiation of Th2 immune responses (Licona-Limon et al. Reference Licona-Limon, Kim, Palm and Flavell2013b ). Strongyloides venezuelensis infection triggered production of the alarmin IL-33 by alveolar epithelial type II cells (ATII) in the lung day 5 and 7 p.i. (Yasuda et al. Reference Yasuda, Muto, Kawagoe, Matsumoto, Sasaki, Matsushita, Taki, Futatsugi-Yumikura, Tsutsui, Ishii, Yoshimoto, Akira and Nakanishi2012). The authors provide evidence that IL-33 induced expansion of IL-5 and IL-13 producing ILC2 in the lungs of infected mice days 7–15 p.i. IL-33−/− mice showed no ILC2 induction, reduced eosinophilia and reduced local induction of IL-5 and IL-13 mRNA upon S. venezuelensis infection. Parasite burden recorded as fecal release of eggs was increased, clearance of infection was delayed by 1 day and intestinal mMCP1 concentrations at days 7–14 were reduced. Increased S. venezuelensis fecal egg release in IL-33−/− mice was partially restored by intranasal treatment with IL-33. Interestingly, nasal application of chitin also induced IL-33 in ATII cells, suggesting that presence of nematode parasites such as S. venezuelensis can be sensed by conserved “pathogen associated molecular patterns”.
As parasite burden was quantified by fecal egg release and the number of migrating L3 in the lung was not assessed, discrimination between IL-33 mediated eradication of L3 and adults remains difficult. On the one hand IL-33 mediated eosinophilia occurred in the lung, suggesting that immunity to L3 in the lung was improved. On the other hand IL-33 was not detected during the tissue migration phase of S. venezuelensis day 1–3 but was elevated after day 4, when S. venezuelensis has already reached the intestine. Thus IL-33 induced ILC2 that promote type 2 immunity may affect the intestinal life stage as well.
Cytokines
Mice lacking the IL-4Rα chain, part of the IL-4 and IL-13 receptor, showed delayed eradication of S. venezuelensis, although initial parasite burden were alike (Negrao-Correa et al. Reference Negrao-Correa, Pinho, Souza, Pereira, Fernandes, Scheuermann, Souza and Teixeira2006). Using bone marrow chimeras, the authors provide evidence that IL-4Rα expressed on lymphocytes was dispensable for timely termination of S. venezuelensis infection while mice that were deficient for IL-4Rα selectively on non-hematopoietic cells displayed the same delay in S. venezuelensis expulsion as complete IL-4Rα−/− mice. A comparable impact of IL-4Rα signalling on non-hematopoietic cells was shown for control of Nippostrongylus brasiliensis infection in the intestine, possibly via activation of intestinal smooth muscle cells or intestinal epithelial cells that express IL-4Rα (Finkelman et al. Reference Finkelman, Shea-Donohue, Morris, Gildea, Strait, Madden, Schopf and Urban2004). It is conceivable that IL-4 and IL-13 trigger IL-4Rα mediated signalling redundantly since both cytokines are induced with comparable kinetics during S. ratti infection (Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010) and neither IL-13 neutralization nor IL-4 deficiency delayed parasite expulsion from the intestine (Watanabe et al. Reference Watanabe, Hamano, Yada, Noda, Kishihara, Nomoto and Tada2001; Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). A significant elevation of S. ratti eggs passed with feces in IL-4−/− mice was recorded at day 6 p.i. only, while fecal egg release was unchanged at day 5 and at days 7–13.
IL-5 neutralization or deficiency predominantly compromised immunity to migrating L3 in tissues as reviewed in the section “Control and eradication of migrating L3 in the tissue- S. stercoralis”, but neither changed kinetics of parasite expulsion during first infection nor interfered with immunity to the intestinal life stage in immune mice during second infection (Korenaga et al. Reference Korenaga, Hitoshi, Yamaguchi, Sato, Takatsu and Tada1991a ; Ovington et al. Reference Ovington, McKie, Matthaei, Young and Behm1998; Watanabe et al. Reference Watanabe, Sasaki, Hamano, Kishihara, Nomoto, Tada and Aoki2003). Since overexpression of IL-5 promoted expulsion of adults 1 day or 3 days after implantation (El-Malky et al. Reference El-Malky, Maruyama, Hirabayashi, Shimada, Yoshida, Amano, Tominaga, Takatsu and Ohta2003), IL-5 may contribute to intestinal immunity but apparently is not essential.
Repeated injection of IL-3 reduced parasite burden and reciprocally increased the number of intestinal mast cells and mMCP1 concentration in serum and intestinal tissue of nude mice and immune competent C57BL/6 mice (Abe and Nawa, Reference Abe and Nawa1988; Abe et al. Reference Abe, Sugaya, Ishida, Khan, Tasdemir and Yoshimura1993). As IL-3 injection did not reduce parasite burden in W/Wv mice, IL-3 apparently acted via activation of mast cells or other effector cells that are absent or dysfunctional in W/Wv mice. The numbers of L3 recovered from heads of infected mice were unchanged in the presence or absence of IL-3 while numbers of parasitic adults were reduced in IL-3 treated mice even after oral infection with adult worms. Thus IL-3 activated effector cells attacked the adult parasites in the intestine rather than the migrating L3 in the tissues. The accelerated expulsion of adults in orally infected mice was observed as early as 6 h p.i., suggesting that initial establishment and not survival of adults in the intestine was compromised by IL-3 treatment (Abe et al. Reference Abe, Sugaya, Ishida, Khan, Tasdemir and Yoshimura1993). Supporting these findings, IL-3 deficient mice displayed delayed expulsion of S. venezuelensis that was correlated to reduction in mast cell and basophil expansion during infection (Lantz et al. Reference Lantz, Boesiger, Song, Mach, Kobayashi, Mulligan, Nawa, Dranoff and Galli1998, Reference Lantz, Min, Tsai, Chatterjea, Dranoff and Galli2008). In summary, these studies provide direct evidence that IL-3 supports expulsion of S. ratti and S. venezuelensis from the intestine. Interestingly, W/Wv mice and IL-3−/− mice terminated infection with a delay of maximal 8 days whereas W/Wv mice, that additionally lacked IL-3, displayed prolongation of egg release for more than 50 days, suggesting a certain level of redundancy in IL-3 mediated effects.
Several lines of evidence suggest that IL-9 acts in concert with IL-3 to induce mast cell activation and S. ratti expulsion from the intestine. IL-9 is a cytokine with pleiotropic function that was shown to promote mast cell expansion and recruitment (Noelle and Nowak, Reference Noelle and Nowak2010). Injection of recombinant IL-9 reduced numbers of S. ratti adults in the small intestine of BALB/c and C57BL/6 mice whereas neutralization of endogenous IL-9 increased intestinal parasite burden (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). Increased parasite burden in anti-IL-9 treated mice was correlated to reduced mMCP1 concentrations in the serum, indicating reduced activation of mucosal mast cells in the absence of endogenous IL-9. Injection of recombinant IL-9 alone did not trigger mMCP1 release as shown for IL-3 (Abe et al. Reference Abe, Sugaya, Ishida, Khan, Tasdemir and Yoshimura1993) but synergistically increased the mMCP1 release induced by recombinant IL-3 if both cytokines were injected together (Sasaki et al. Reference Sasaki, Yoshimoto, Maruyama, Tegoshi, Ohta, Arizono and Nakanishi2005).
IL-9 production was further elevated in S. ratti infected mice that were depleted of Foxp3+ regulatory T cells (Treg) (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014) or were deficient for a negative co-regulatory receptor (B and T lymphocyte attenuator, BTLA) or its ligand (herpes virus entry mediator, HVEM) (Breloer et al. Reference Breloer, Hartmann, Blankenhaus, Eschbach, Pfeffer and Jacobs2015). Increased IL-9 production was correlated to reduced parasite burden in the intestine and reduced release of S. ratti L1 in the feces, while numbers of migrating L3 in head and lung were not reduced. Thus elevated IL-9 in Treg depleted or BTLA deficient mice improved immunity to the intestinal life stage of S. ratti. As only mast cell competent Cpa3wt/wt but not mast cell deficient Cpa3Cre/wt littermates benefited from elevated IL-9 in Treg depleted mice, this study provides direct evidence that IL-9 functions as additional activator of mast cells during S. ratti infection (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). It should be noted that an additional putative function for IL-9 as enhancer of smooth muscle contractility that was shown to promote expulsion of Trichuris muris (Klementowicz et al. Reference Klementowicz, Travis and Grencis2012) has not been investigated so far.
IL-9 is part of the canonical Th2 response (Allen and Maizels, Reference Allen and Maizels2011) but the existence of a specialized Th9 cell is currently debated (Schmitt et al. Reference Schmitt, Klein and Bopp2014) and specifically Th9 and not Th2 cells were shown to protect mice against N. brasiliensis infection (Licona-Limon et al. Reference Licona-Limon, Henao-Mejia, Temann, Gagliani, Licona-Limon, Ishigame, Hao, Herbert and Flavell2013a ). T cells derived from S. ratti-infected mice clearly secreted IL-9 upon antigen-specific stimulation ex vivo, but flow cytometric analysis revealed that IL-9 was produced by a low frequency of both CD4+ T cells and CD4− cells in vivo (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). As several non-T cells, including ILC2 and mast cells can principally secrete IL-9, the possible sources of IL-9 during S. ratti infection still need to be elucidated.
An unexpected role in promoting intestinal immunity was demonstrated for IL-18 in concert with IL-2. Daily injections with a combination of IL-18 and IL-2 induced intestinal mastocytosis and elevation of serum concentrations of mMCP1, IL-3, IL-4, IL-9 and IL-13 (Sasaki et al. Reference Sasaki, Yoshimoto, Maruyama, Tegoshi, Ohta, Arizono and Nakanishi2005). As mMCP1 induction by IL-18/IL-2 treatment was CD4+ T cell dependent and could be replaced by injection of IL-3 and IL-9, the authors suggested that IL-3 and IL-9 producing CD4+ T cells were induced and subsequently caused the elevation of mMCP1. IL-2/IL-18 treated mice rapidly expulsed S. venezuelensis adults that were implanted in the intestine showing that specifically intestinal immunity was increased. Accelerated expulsion in IL-2/IL-18 treated mice was correlated with increased mastocytosis and not observed in W/Wv mice. Supporting these findings, IL-18−/− mice and mice deficient for IL-18 receptor displayed a transient reduction of mMCP1 release at days 7 and 10 p.i. and a delay in termination of S. venezuelensis by 4 days. Induction of mMCP1 by IL-18 and IL-2 treatment was independent of functional STAT6, i.e. independent of a classic Th2 polarization. STAT6−/− mice also displayed a delayed termination of S. venezuelensis infection by 11 days but additional neutralization of IL-18 and IL-2 in STAT6−/− mice prolonged infection even further and reduced mMCP1 in serum beyond day 14. Thus it was hypothesized that two synergistic pathways for mast cell activation exist, one that depends on STAT6 mediated classic Th2 polarization and one STAT6 independent that functions via IL-18/IL-2.
Antibodies
A central function of S. ratti-specific IgM and IgG is promoting the eradication of migrating L3 in the tissue during first and second infection and in vaccinated mice, as reviewed in the section “Control and eradication of migrating L3 in the tissue- S. stercoralis”. Still, evidence for additional contribution of B cells to intestinal immunity was provided using B cell deficient JHD mice (El-Malky et al. Reference El-Malky, Maruyama, Al-Harthi, El-Beshbishi and Ohta2013). Strongyloides venezuelensis adults that were directly implanted in the intestine, thus bypassing the tissue migration phase, established better within B cell deficient mice compared with wild type mice. The number of remaining adults 5 days after implantation was increased and egg release was prolonged. Numbers of intestinal eosinophils and mucosal mast cells were comparable, suggesting that activation and not recruitment of effector cells was compromised in B cell deficient mice. However, since indicators of mast cell degranulation such as serum concentration of mMCP1 were not recorded, this hypothesis was not formally tested.
The FcRγ subunit chain is associated with the function of several Fc receptors such as the high affinity receptor for IgG, FcγRI and the high affinity receptor for IgE, FcεRI. Mice lacking the FcRγ chain displayed no IgE mediated anaphylaxis in vivo and cross-linking surface associated IgE did not induce mast cell degranulation in vitro (Takai et al. Reference Takai, Li, Sylvestre, Clynes and Ravetch1994). Strongyloides venezuelensis expulsion from the intestine was delayed in FcRγ−/− mice upon s.c infection. (Onah et al. Reference Onah, Uchiyama, Nagakui, Ono, Takai and Nawa2000). Since oral infection also led to prolonged egg release in FcRγ−/− mice compared with wild type mice during first and second infection, intestinal immunity was impaired (Onah and Nawa, Reference Onah and Nawa2004). Nevertheless mast cell numbers increased in the intestine at day 13 p.i. and mMCP1 concentrations were even elevated in FcRγ−/− mice (Onah et al. Reference Onah, Uchiyama, Nagakui, Ono, Takai and Nawa2000). The authors concluded that protective immunity during first infection depended on IgE/FcεR mediated activation of intestinal effector cells, while recruitment of mast cells to the intestine of S. venezuelensis infected mice was independent of functional FcεR. However, it should be noted that the FcRγ chain is involved in signal transduction via several Fc receptors as well as C-type lectin receptors (Takai et al. Reference Takai, Li, Sylvestre, Clynes and Ravetch1994), thus the phenotype of the FcRγ−/− mice may not be caused by defective FcεR alone.
Direct evidence for contribution of both FcεR and FcγRIII in expulsion of S. venezuelensis from the small intestine was provided using mice deficient in activation-induced cytidine deaminase (AID) (Matsumoto et al. Reference Matsumoto, Sasaki, Yasuda, Takai, Muramatsu, Yoshimoto and Nakanishi2013). B cells cannot undergo affinity maturation and subsequent class switch in AID−/− mice resulting in intact S. venezuelensis specific IgM but defective IgG1 and IgE responses. Initial parasite burdens were comparable in AID−/− and wild type mice but final expulsion of S. venezuelensis was 8 days delayed. Transfer of wild type immune serum to AID−/− mice accelerated expulsion of S. venezuelensis. Thereby the serum was transferred after completion of the tissue migration phase at day 6/7 p.i., showing that antibodies targeted the parasitic adult in the intestine. IgG and IgE acted redundantly since both isotypes, purified from immune serum, promoted expulsion. Depletion of either isotype reduced the protective effect of the serum, while only simultaneous depletion of IgE and IgG abrogated protective efficiency. Furthermore, transfer of immune serum in mice lacking either FcγRIII or FcεR1α still promoted expulsion while purified S. venezuelensis specific IgG or IgE did not accelerate parasite expulsion in mice lacking the respective Fc receptor. Supporting these findings, timing of S. venezuelensis infections was unchanged in mice lacking either FcγRIII or FcεR1α (Matsumoto et al. Reference Matsumoto, Sasaki, Yasuda, Takai, Muramatsu, Yoshimoto and Nakanishi2013). Also neutralization of IgE by application of anti-IgE mAb did not prolong larval output during S. ratti infection although IgE depletion from the serum of infected mice was controlled to be complete from day 0 to day 28 p.i. (Korenaga et al. Reference Korenaga, Watanabe and Tada1991b ), thus emphasizing the redundant functions of IgE and IgG.
As transfer of S. venezuelensis specific IgG and IgE did not promote expulsion of S. venezuelensis adults in W/Wv mice Ig function was dependent on mast cells or other effector cells absent in W/Wv mice (Matsumoto et al. Reference Matsumoto, Sasaki, Yasuda, Takai, Muramatsu, Yoshimoto and Nakanishi2013). It should be noted that the authors did not compare the effect of S. venezuelensis specific Ig transfer in WBB6F1-W/Wv mice to WBB6F1+/+ mice but to C57BL/6 mice as WBB6F1+/+ mice displayed ten times lower parasite burden without Ig treatment highlighting the importance of the genetic background on susceptibility.
Mechanism of S. ratti and S. venezuelensis expulsion from the intestine
The data accumulated so far strongly suggest that mast cells, probably alongside with additional yet poorly characterized intestinal effector cells, promote final expulsion of S. ratti and S. venezuelensis from the intestine. Thereby IL-3, IL-9 and Strongyloides spp. specific antibodies of IgG and IgE isotype trigger mast cell activation. Mast cells contain a plethora of preformed effector molecules including proteases, lysosomal enzymes, cytokines, biogenic amines and proteoglycans that are released upon activation. Additionally the de novo synthesis of effector molecules such as lipid mediator prostaglandin and leukotriene and platelet activation factor is induced (Wernersson and Pejler, Reference Wernersson and Pejler2014).
Prostaglandin E2 (PGE2) (Machado et al. Reference Machado, Carlos, Lourenco, Souza, Sorgi, Silva, Ueta, Ramos, Aronoff and Faccioli2010) and Leukotriene B4 (LTB4) (Machado et al. Reference Machado, Ueta, Lourenco, Anibal, Sorgi, Soares, Roque-Barreira, Medeiros and Faccioli2005) were elevated in the lung and in the intestine of S. venezuelensis infected mice. Inhibition of LTB4 synthesis using either an inhibitor (MK886) or mice lacking an enzyme involved in LTB synthesis (5-lipoxygenase) increased parasite burden in small intestines and fecal egg release suggesting a contribution of LTB4 to parasite control. The phospholipid mediator platelet activation factor (PAF) has also been implicated in intestinal immunity since PAF receptor deficient mice displayed delayed parasite expulsion leading to increased adult numbers at day 12 p.i. (Negrao-Correa et al. Reference Negrao-Correa, Souza, Pinho, Barsante, Souza and Teixeira2004).
mMCP1 is a protease specifically expressed by mucosal mast cells (Reynolds et al. Reference Reynolds, Stevens, Lane, Carr, Austen and Serafin1990) and elevation of mMCP1 concentration in the serum indicates mast cell activation. Elevated mMCP1 concentrations in the serum of S. ratti and S. venezuelensis infected mice were associated with reduced intestinal parasite burden in several studies (Abe et al. Reference Abe, Sugaya, Ishida, Khan, Tasdemir and Yoshimura1993; Sasaki et al. Reference Sasaki, Yoshimoto, Maruyama, Tegoshi, Ohta, Arizono and Nakanishi2005; Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011, Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014; Breloer et al. Reference Breloer, Hartmann, Blankenhaus, Eschbach, Pfeffer and Jacobs2015). Reciprocal reduction in systemic mMCP1 concentration upon IL-9 neutralization was correlated with increased parasite burden (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014), suggesting a causative link between mMCP1 and parasite expulsion. FcRγ deficient mice, by contrast, displayed a clear delay in S. venezuelensis expulsion while both mastocytosis and mMCP1 release were comparable or even increased in the absence of the FcRγ chain at later time points (Onah et al. Reference Onah, Uchiyama, Nagakui, Ono, Takai and Nawa2000). Since mast cells can be partially activated, leading to secretion of some effector molecules without complete degranulation (Wernersson and Pejler, Reference Wernersson and Pejler2014), mMCP1 may be an indicator of mast cell activation that does not necessarily indicate complete degranulation and may not contribute to protection in all settings. It should be noted that the course of S. ratti or S. venezuelensis infection in mMCP1 deficient mice that display delayed expulsion of another gastrointestinal parasite, Trichinella spiralis (Knight et al. Reference Knight, Wright, Lawrence, Paterson and Miller2000), has not been investigated so far, thus direct evidence regarding the contribution of mMCP1 to expulsion of Strongyloides spp. is missing.
Strongyloides ratti does not invade the intestinal wall but rather embeds itself in the mucosa of the intestine tightly attached to the layer of intestinal epithelial cells (Dawkins et al. Reference Dawkins, Robertson, Papadimitriou and Grove1983). Oral implantation resulted in quick and stable adherence to the intestine after 4 h in naïve but not in immune mice. This intestinal immunity was inversely correlated to mast cell numbers and was compromised in W/Wv mice (Maruyama et al. Reference Maruyama, Yabu, Yoshida, Nawa and Ohta2000). In vitro incubation of S. venezuelensis adults with the mast cell products chondroitin sulphate (CHS) A and E or heparin reduced their ability to invade the mucosa after subsequent intestinal implantation. It is conceivable that CHS contributes to expulsion of parasitic adults by preventing adhesion to the intestinal mucosa. In support of this hypothesis, the increased intestinal parasite burden in FcRγ−/− mice upon oral infection was correlated with a reduction in free CHS present in intestinal washings (Onah and Nawa, Reference Onah and Nawa2004). Thereby, the number of mast cells in the intestine and release of mMCP1 were not changed (Onah et al. Reference Onah, Uchiyama, Nagakui, Ono, Takai and Nawa2000) and total content of CHS in gut homogenates was actually increased, suggesting that mast cells were recruited but not fully activated in the absence of FcRγ chain. Differences in CHS release were also implicated in the reduced intestinal susceptibility of 129/SvJ mice to S. venezuelensis infection compared with C57BL/6 mice (Nakamura-Uchiyama et al. Reference Nakamura-Uchiyama, Nagao, Obara, Ishiwata and Nawa2001). Therefore, the release of CHS by fully activated mast cells apparently promotes expulsion of parasitic adults from the intestine by preventing their adhesion to the intestinal epithelial cell layer.
Nitric oxide (NO) an inflammatory mediator that is produced by macrophages and granulocytes was induced in macrophages exposed to antigens derived from S. venezuelensis L3 and parasitic adults in vitro and during the tissue migration phase of S. venezuelensis infection in vivo (Ruano et al. Reference Ruano, Lopez-Aban, Gajate, Mollinedo, De Melo and Muro2012). Treatment of BALB/c mice with NO donors reduced numbers of L3 in the lung and in the intestine in normal mice whereas treatment with an inhibitor of the inducible NO synthase reciprocally increased parasite burden (Ruano et al. Reference Ruano, Lopez-Aban, Fernandez-Soto, de Melo and Muro2015). Of note, treatment with NO donors also reduced the drastically increased parasite burden and fecal egg release in immune suppressed mice. Since mice were infected by s.c. injection of S. venezuelensis L3i, the reduced number of parasites in the intestine could reflect the already reduced number of migrating L3. Thus the target of NO, L3 or adult, remains to be elucidated.
In light of the many effector molecules, including cytokines that are produced by intestinal effector cells or are present in mast cell granules (Wernersson and Pejler, Reference Wernersson and Pejler2014), the analysis of potential effector molecules that contribute to expulsion of S. ratti and S. venezuelensis from the intestine is rather fragmented to date. The data accumulated so far regarding the pathways contributing to parasite expulsion from the intestine are summarized in Fig. 3.

Fig. 3. Control and eradication of parasitic S. venezuelensis and S. ratti adults from the small intestine. Migrating S. venezuelensis L3 induce tissue-derived IL-33 in the lung. IL-33 triggers the expansion of IL-5 and IL-13 producing ILC2 that subsequently promote parasite control and initiation of Th2 response. Classical STAT6 dependent Th2 response results in production of IL-13, IL-4, IL-5, IL-3 and IL-9. Additional IL-9 may be produced by IL-18/IL-2 activated CD4+ T cells and other undefined sources. IL-27 and IL-12 antagonize initiation of Th2. Foxp3+ Treg and BTLA-HVEM mediated signalling dampens IL-9 production during S. ratti infection. IL-4 triggers B cell differentiation to IgG1 and IgE producing plasma cells. IgG and IgE as well as IL-3 and IL-9 activate mast cells and probably other intestinal effector cells. Mast cell activation results in mMCP1 release with unknown contribution to parasite expulsion. The mast cell product CHS interferes with attachment of S. venezuelensis to IEC. Other potential mast cell products LTB4, PGE2 and PAF as well as IL-4 and IL-13 receptor engagement on somatic cells promotes parasite expulsion via unknown mechanisms. Abbreviations: BTLA, B and T lymphocyte attenuator; CHS, chondroitin sulphate; HVEM, herpes virus entry mediator; IEC, intestinal epithelial cells; LTB4, Leukotriene B4; mMCP1, mouse mast cell protease 1;PAF, platelet activation factor; PGE2, Prostaglandin E2; STAT, signal transducer and activator of transcription.
IMMUNE MODULATION
Immune evasion
Helminth parasites manage to establish chronic infections by actively dampening the immune response of their hosts (McSorley and Maizels, Reference McSorley and Maizels2012). Rodent specific S. ratti and S. venezuelensis, by contrast, cause transient infections that are terminated after 3–4 weeks (Dawkins et al. Reference Dawkins, Grove, Dunsmore and Mitchell1980; Sato and Toma, Reference Sato and Toma1990). Since parasitic females have the capacity to live for more than 1 year in the absence of adaptive immunity (Gardner et al. Reference Gardner, Gems and Viney2006) the timely termination of S. ratti and S. venezuelensis infection in wild type mice and rats apparently reflects efficient and unsuppressed function of the immune system. In spite of this, recent evidence suggests that active suppression of the host's immune response is required for S. ratti to survive even the first week within the intestine of immune competent mice (Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011, Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014; Breloer et al. Reference Breloer, Hartmann, Blankenhaus, Eschbach, Pfeffer and Jacobs2015).
Infection of BALB/c and C57BL/6 mice induced transient expansion of Foxp3+ Treg numbers first at day 2 p.i. in popliteal and inguinal lymph nodes and later on day 7–14 p.i. in MLN. The Foxp3+ Treg population contracted to naïve levels by day 36 p.i., thus kinetics of Foxp3+ Treg expansion and contraction resembled the parasites migration path through the host, with 2 days tissue migration and prolonged presence in the intestine until complete clearance after 1 month (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). The transient depletion of Foxp3+ Treg in BALB/c mice before s.c. infection with S. ratti L3i reduced parasite burden in the intestine and fecal release of eggs and L1 at any time point of infection until clearance. As Treg depletion did not affect eradication of migrating L3 in lung and head tissue, specifically intestinal immunity was improved (Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011). Treg depletion led to a generalized increase in Th2 associated IL-3, IL-5, IL-13 and IL-10 production by MLN cells and elevated IgE and mMCP1 concentration in the serum. However, the improved intestinal immunity in Treg depleted mice was not due to increased Th2 response in general but was caused by increased IL-9 production and subsequent IL-9 driven accelerated activation of mucosal mast cells. Improved resistance and accelerated mMCP1 release in Treg depleted BALB/c mice were abrogated by IL-9 neutralization while IL-13 neutralization had no effect (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). Additional deficiency of mast cells in Treg depleted Cpa3Cre/wt mice increased parasite burden to wild type levels while IL-9 production was still elevated. Thus mast cells apparently represent one effector cell population that was first activated by IL-9 and subsequently contributed to improved S. ratti expulsion in Treg depleted mice.
The depletion of Treg (DEREG) mouse model used for these studies causes only transient Treg depletion (Lahl et al. Reference Lahl, Loddenkemper, Drouin, Freyer, Arnason, Eberl, Hamann, Wagner, Huehn and Sparwasser2007). Treg numbers returned to normal levels day 6 p.i. and Treg depletion at later time points after infection i.e. at day 4 p.i. did not reduce parasite burden (Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011) suggesting that suppression of IL-9 mediated mast cell activation by Foxp3+ Treg was achieved during the first days of infection.
Comparison of BALB/c and C57BL/6 mice showed that Treg expansion and phenotype during S. ratti infection was similar in both mouse strains. Treg function was apparent in both strains as Treg depletion elevated Th2 associated cytokine production and IgE concentration in C57BL/6 mice and BALB/c mice to the same extent (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014). Also efficiency of Treg depletion and repopulation kinetics was comparable in BALB/c and C57BL/6 DEREG mice. Strikingly, Treg depletion in C57BL/6 mice did not reduce parasite burden in high dose or low dose infections and did not significantly increase mMCP1 release and IL-9 production. Thus, expanding Foxp3+ Treg mediate control of IL-9 production and subsequent mast cell activation in S. ratti infected BALB/c mice in a non-redundant manner while this is not the case in C57BL/6 mice. As immunity to S. ratti in C57BL/6 mice also depends on IL-9 (Blankenhaus et al. Reference Blankenhaus, Reitz, Brenz, Eschbach, Hartmann, Haben, Sparwasser, Huehn, Kuhl, Feyerabend, Rodewald and Breloer2014) and C57BL/6 are even more susceptible to S. ratti infection than BALB/c mice, it was hypothesized that additional regulatory elements of the immune system maintain control of IL-9 production and subsequent mast cell activation in S. ratti infected C57BL/6 mice in the absence of Treg.
Expression of the regulatory receptor BTLA (Murphy and Murphy, Reference Murphy and Murphy2010) increased predominantly on CD4+ T lymphocytes in MLN of day 7 S. ratti infected C57BL/6 mice (Breloer et al. Reference Breloer, Hartmann, Blankenhaus, Eschbach, Pfeffer and Jacobs2015). Absence of either BTLA or its ligand HVEM reduced parasite burden in the intestine and fecal release of eggs and L1 throughout infection. Numbers of migrating L3 in lungs and heads of BTLA−/− and HVEM−/− mice were not reduced suggesting that intestinal immunity improved in the absence of BTLA-HVEM mediated signalling. Thereby specifically IL-9 production by MLN increased and mMCP1 release in the serum was accelerated in BTLA−/− and HVEM−/− mice. Thus BTLA-HVEM mediated signalling interfered with IL-9 driven mast cell activation and promoted S. ratti survival in wild type C57BL/6 mice.
Although expression of the regulatory receptor cytotoxic T lymphocyte attenuator 4 (CTLA4) increased on Foxp3− effector T cells in MLN of S. ratti infected BALB/c and C57BL/6 mice (Blankenhaus et al. Reference Blankenhaus, Klemm, Eschbach, Sparwasser, Huehn, Kuhl, Loddenkemper, Jacobs and Breloer2011; and Blankenhaus unpublished results) it played only a minor role during immune evasion. Blockade of CTLA4 mediated signalling elevated S. ratti specific and polyclonal Th2 response in both mouse strains, but did not reduce fecal release of S. ratti eggs and L1. Intestinal parasite burden was significantly reduced in anti-CTLA4 treated BALB/c mice at day 7 p.i but not at day 6 or day 8 p.i., thus providing no consistent increase in resistance.
In summary these studies show that suppression of IL-9 driven mast cell activation via induction of Foxp3+ Treg predominantly in BALB/c mice or BTLA expression predominantly in C57BL/6 mice was central for establishment of even transient intestinal infection by S. ratti in immune competent mice.
Bystander suppression
Helminth-induced immune suppression spills over to bystander antigens and may change the outcome of allergy, vaccination or co-infections (McSorley and Maizels, Reference McSorley and Maizels2012). In this context, the suppression of some aspects of Ovalbumin (OVA) induced allergy were reported in S. stercoralis infected mice (Wang et al. Reference Wang, Nolan, Schad and Abraham2001). Implantation of L3 prior to immunization with OVA decreased eotaxin concentration in the lung as well as OVA-specific IgE production to intranasal challenge OVA exposure, although S. stercoralis did not induce a patent infection in BALB/c mice and L3 were never present in the lung. S. venezuelensis infection induced lung airway hyporesponsiveness (AHR) in the absence of other allergic stimuli (Silveira et al. Reference Silveira, Nunes, Cara, Souza, Correa, Teixeira and Negrao-Correa2002) but the more dramatic increase in AHR elicited by experimental OVA induced asthma was ameliorated by S. venezuelensis infection during OVA challenge in sensitized mice (Negrao-Correa et al. Reference Negrao-Correa, Silveira, Borges, Souza and Teixeira2003).
Still the direct comparison of the immune modulatory effects induced by transient S. ratti infection to long lasting Litomosoides sigmodontis infection showed that chronic nematode infection caused a stable suppression of immune responses to bystander antigens, whereas transient S. ratti infection predominantly polarized bystander immune responses towards a Th2 phenotype. Mice that carried a chronic L. sigmodontis infection displayed drastically reduced IgG responses to model antigen vaccination affecting both Th1 associated IgG2 and Th2 associated IgG1 responses (Hartmann et al. Reference Hartmann, Haben, Fleischer and Breloer2011; Haben et al. Reference Haben, Hartmann and Breloer2014). By contrast, transient S. ratti infection suppressed Th1 associated IgG2 responses to model antigen vaccination more pronounced than Th2 associated IgG1 responses (Hartmann et al. Reference Hartmann, Eschbach and Breloer2012). Modulation of vaccination response was only observed if vaccination was performed during maximal S. ratti parasite burden. Also production of Th1 associated IFNγ by anti-CD3 stimulated spleen cells and MLN was only transiently suppressed during S. ratti infection while anti-CD3 engagement induced production of Th2 associated cytokines at the same time (Eschbach et al. Reference Eschbach, Klemm, Kolbaum, Blankenhaus, Brattig and Breloer2010). Furthermore, concurrent infection with L. sigmodontis interfered with the induction of Plasmodium berghei specific cytotoxic lymphocytes by experimental vaccination whereas concurrent S. ratti infection did not abrogate vaccination efficacy under similar conditions (Kolbaum et al. Reference Kolbaum, Tartz, Hartmann, Helm, Nagel, Heussler, Sebo, Fleischer, Jacobs and Breloer2012b ).
In line with these findings, multiple infections with S. venezuelensis did not change the course of experimental autoimmune encephalomyelitis in Lewis rats (Chiuso-Minicucci et al. Reference Chiuso-Minicucci, Van, Zorzella-Pezavento, Peres, Ishikawa, Rosa, Franca, Turato, Amarante and Sartori2011) and the pathology induced in a model of type I diabetes resulting in weight loss, hyperglycemia and islet infiltration was only mildly ameliorated by a regimen of previous S. venezuelensis immunization and infection (Peres et al. Reference Peres, Chiuso-Minicucci, da Rosa, Domingues, Zorzella-Pezavento, Franca, Ishikawa, do Amarante and Sartori2013). The beneficial effect of S. venezuelensis infection was correlated to transient induction of Th2 associated IL-5 and IL-10 suggesting that Th1 associated islet inflammation was attenuated by the pre-existing Th2 polarization rather than by active suppression. Chronic L. sigmodontis infection, by contrast, clearly prevented autoimmune diabetes in non-obese mice independent of a Th2 polarization by TGFβ mediated immune suppression (Hubner et al. Reference Hubner, Shi, Torrero, Mueller, Larson, Soloviova, Gondorf, Hoerauf, Killoran, Stocker, Davies, Tarbell and Mitre2012).
Co-infection
It is conceivable that the transient Th2 polarization observed in Strongyloides spp. infected mice would interfere with the initiation of protective immune responses to infection with parasites that are predominantly controlled by Th1 associated mechanism. However, Plasmodium yoelii and Leishmania major were efficiently controlled in S. ratti co-infected mice (Kolbaum et al. Reference Kolbaum, Ritter, Zimara, Brewig, Eschbach and Breloer2011, Reference Kolbaum, Eschbach, Steeg, Jacobs, Fleischer and Breloer2012a ). Thereby the S. ratti induced Th2 response was outcompeted by the P. yoelii and L. major induced Th1 polarization although mice were first infected with S. ratti for 6 days to establish Th2 polarization before co-infection was performed. Despite the suppressed Th2 polarization in co-infected mice, the S. ratti infection itself was also efficiently controlled and terminated, even if L. major infections were performed 14 days before S. ratti infection to allow full establishment of Th1 polarization (Kolbaum et al. Reference Kolbaum, Ritter, Zimara, Brewig, Eschbach and Breloer2011). These studies suggest that the immune system is sufficiently elastic to cope with two infections requiring opposing types of immune response. Still, pre-existing S. venezuelensis infection interfered with an efficient response to intravenous Mycobacterium bovis co-infection 3 days later. Thereby bacterial load in lung increased at day 28 of M. bovis infection and lung concentration of IL-17 but not of IFNγ decreased (Dias et al. Reference Dias, de Castro, Alves, Rezende, Rodrigues, Machado, Fernandes, Negrao-Correa, Teixeira and Ferreira2011). Also numbers of S. venezuelensis adults increased in co-infected mice and Th2 response recorded as concentration of IL-4 and IL-13 in lung and intestinal tissues decreased (Carmo et al. Reference Carmo, Vicentini, Dias, Alves, Alves, Brandi, De Paula, Fernandes, Barsante, Souza, Teixeira, Negrao-Correa and Ferreira2009). It should be noted that the course of Mycobacterium tuberculosis aerosol infection was not changed by pre-existing S. ratti infection (Stubbe, Breloer and Hölscher, unpublished results).
In summary these findings suggest that transient S. ratti and S. venezuelensis infections in mice predominantly polarize the immune system towards a Th2 phenotype and suppress IL-9 driven mast cell activation to delay their own expulsion from the intestine but do not display the long lasting and systemic suppression of bystander immune responses observed in chronic helminth infections. Still, some bystander suppression may occur in selected settings and modulation of IL-9 driven pathology by pre-existing Strongyloides spp. infections has not been investigated.
CONCLUDING REMARKS
Analysis of the immune response against a helminth parasite that displays both, tissue migrating and intestinal life stages reveals the flexibility of the mammalian immune system. While the initiation of a canonical Th2 immune response is a shared prerequisite of protective immunity to both stages the response culminates with the activation of different effector cells in tissue and intestine reflecting the different strategies of trapping and killing vs expulsion that evolved for eradication of parasites at these different locations.
Strongyloides spp. L3 are first sensed by conserved structures such as chitin and provoke tissue derived alarmin production and ILC2 induction. L3 are predominantly opsonized by complement, trapped by NETs and eradicated by neutrophils and eosinophils via MPO and MBP during first contact (Fig. 1). Eosinophils participate in induction of adaptive immune response by acting as APC. Strongyloides spp. specific IgM and IgG opsonize tissue migrating L3 in immune mice and lead to highly efficient killing by neutrophils and macrophages that reduces numbers of L3 by more than 90% (Fig. 2).
The expulsion of parasitic female adults from the intestine is dependent on mast cells that are activated via the cytokines IL-3 and IL-9 and via Strongyloides spp. specific IgE and IgG in association with FcεR and FcγR, but the exact mechanism of parasite expulsion still needs to be elucidated (Fig. 3). Although immune competent mice completely terminate infection after 1 month, the immune system is not working with maximal efficiency. Recent evidence shows S. ratti delays its own expulsion by dampening IL-9 driven mast cell activation via induction of Foxp3+ Treg and the regulatory receptor BTLA.
ACKNOWLEDGEMENTS
We thank Tom Nolan for critical reading of the manuscript and Martina Reitz for preparing the figures. We would like to apologize in advance to all authors whose work we did not include in this review due to space limitations.
FINANCIAL SUPPORT
Support to M. B. from the German Research Association (DFG) Grant BR 3754/2-1 and 2-2 is gratefully acknowledged.






