Published online by Cambridge University Press: 10 June 2004
Parasite community ecology has recently focused on understanding the forces structuring these communities. There are few surveys, however, designed to study the spatial repeatability and predictability of parasite communities at the local scale in one host. The purpose of our study was to address the relationship between infracommunity and component community richness, and to describe spatial variations on the local scale, of helminth parasite communities in an avian host, the red-legged partridge (Alectoris rufa). We sampled 235 wild partridges from 8 separate localities, with different partridge population densities, in the Ciudad Real and Toledo provinces of central Spain, and we determined their overall and intestinal helminth species. We found that habitat variables (mean temperature and land use) were not significantly associated with any component community. The partridge population abundance index was directly correlated with the prevalence and mean intensity of infection but not with component community species richness. There was a curvilinear relationship between infracommunity and component community species richness, as well as negative interspecific associations, for the helminth species assemblage parasitizing the intestine. A nestedness/anti-nestedness pattern, considered as part of a continuum, was associated with prevalence, mean intensity and partridge population abundance index, but not with component community richness. Increases in the partridge population abundance index and the prevalence and mean intensity of infection were associated with increases in helminth community nestedness. Although negative interactions between helminth species could not be ruled out as forces structuring helminth communities, our results suggest that parasite community structure in the red-legged partridge was primarily determined by the extrinsic influence of parasite habitat heterogeneity and its amplification of the differing probabilities of colonization of parasite species.
Many empirical studies of parasite community ecology have been performed in recent years to understand the forces structuring these communities, especially on fish hosts (Kennedy & Guégan, 1996; Worthen & Rohde, 1996; Rohde, 1998; Poulin, 2001). Much of the knowledge available to date, however, comes from studies that have not been replicated in space (Poulin & Valtonen, 2002). Thus, the community structure patterns found in a sample frequently are not compared with other independent samples from other host populations in the same species. In addition, restrictions imposed by sampling complexity usually preclude the control of variables associated with host populations that may influence parasite community structure. Although many studies have described the changing patterns of parasite community structure on a broad scale, studies defining and quantifying factors that influence the structure of parasite communities at the local scale are scarce (Carney & Dick, 2000; Behnke et al. 2001; Poulin & Valtonen, 2002).
Very recently, studies have been focused primarily on local-regional species richness relationships, as well as on two other aspects of parasite community organization: the quantification of associations between pairs of species and the nestedness/anti-nestedness species distribution pattern. Since infracommunities are subsets of the total number of parasite species present in the component community, infracommunity richness should be determined by component community richness. Although in avian hosts, richer component communities seem to consist of richer infracommunities (Poulin, 1996), in mammalian hosts (Poulin, 1996) and in some fish species (Kennedy & Guégan, 1996), component richness is usually a poor predictor of infracommunity richness. In some cases, this lack of association in fish and mammal hosts has been attributed to the saturation of infracommunities (Poulin, 1997). In other cases, however, the number of species in infracommunities may be determined, among other factors, by the probability of successful infection of the different parasite species, as well as by ecological traits of host populations (e.g. density) or habitat (Hartvigsen & Halvorsen, 1994; Rohde, 1998; Halmetoja, Valtonen & Koskenniemi, 2000).
Regarding helminth community organization, pair-wise associations serve to identify non-random patterns of species distribution (Lotz & Font, 1994; Haukisalmi & Henttonen, 1999). Lack of association between a pair of parasite species indicates that they are randomly distributed among hosts, whereas significant positive or negative associations suggest a departure from random co-occurrence. On the other hand, nestedness is a common departure from random association in free-living organisms (Patterson & Atmar, 1986). In the context of parasite communities, a nested subset pattern occurs when the parasite species found in depauperate infracommunities represent non-random subsets of progressively richer ones. In other words, common species are found in infracommunities of varying richness, but rare species are found only in species-rich infracommunities. The distribution pattern opposite to nestedness is called anti-nestedness, in which species are over-dispersed (see Poulin & Guégan, 2000). The existence of one or both patterns indicates that the distribution of parasite species among host individuals is not mutually independent and that the structuring of the infracommunities is occurring (Poulin, 2001). Significant nestedness and/or anti-nestedness patterns have been observed in many parasite communities of fish (Rohde et al. 1998; Poulin & Guégan, 2000; Poulin & Valtonen, 2001). Poulin & Guégan (2000) have suggested that nestedness and anti-nestedness are the extremes in a continuum of parasite organization, and they showed that this pattern was covariant with other characteristics of fish parasite communities. However, few studies have examined the variation of this pattern in space in fish species (Carney & Dick, 2000; Poulin & Valtonen, 2002) and, to our knowledge, no study has been made in any mammalian or avian host.
The main purpose of the present study was to look, for the first time, for patterns in parasite community organization in a host other than fish. We were interested in describing spatial variations in structure at the local scale in helminth communities, especially in relation to definitive host density, a task that is difficult to achieve in fish populations. In our study we examined the parasite organization at scales of infracommunities and local component communities to address the relationship between infracommunity richness and component community richness. We also addressed the variation in helminth community organization in a single avian host by studying several spatially independent parasite communities. We quantified species pair-wise associations and examined the variation of the continuum between nestedness and anti-nestedness in relation to other component community factors and host density. The effects of biotic factors (host attributes such as age, sex and body condition) and abiotic factors (climatic conditions, land use and geographical distance) on the parasite community were also evaluated.
As avian host, we selected the red-legged partridge, a bird species native to Spain. It is a non-migratory game species, which facilitated the estimation of population density indices and the sampling of birds by hunting, and ruled out the impact of host migration on parasite community structure. Moreover, the main correlates of helminth communities in this species have been previously studied (Calvete et al. 2003). This study showed that helminth component community richness in the wild red-legged partridge was directly correlated to yearly mean temperature across Spain, suggesting that this variation may be due to the population distribution of both intermediate and definitive hosts. A secondary objective of the study was, therefore, to examine this relationship on the local scale.
We sampled wild red-legged partridge populations in 8 localities in the Ciudad Real and Toledo provinces of central Spain (Fig. 1). Both provinces are located within the core distribution area of the red-legged partridge (Blanco-Aguiar, Virgós & Villafuerte, 2003). In order to get samples across a range of host population densities, the 8 populations were selected on the basis of previous estimations of their abundance. To decrease the effects of regional variability of parasite distribution in local estimation of parasite community structure (Srivastava, 1999), the sampling area of each locality was restricted to a single 5×5 km square. Geographical distances between pairs of localities ranged from 20 to 200 km.

Fig. 1. Geographical distribution of the eight localities sampled in the Ciudad Real and Toledo provinces of Spain. Localities are denominated L1 to L8, according to the increment of their partridge population abundance index, with L1 having the lowest and L8 being having the highest abundance index values.
Localities were characterized by means of 4 variables estimated in a parallel survey designed to study the distribution of the red-legged partridge. The 4 variables were partridge population abundance index, 2 climatic variables (yearly mean temperature and rainfall) and a land-use variable-percentage of soil devoted to agricultural use – frequently related to local structure of helminth communities (Davidson et al. 1991; Abu-Madi et al. 2000; Hulbert & Boag, 2001).
Partridge population abundance in each square was estimated by counting the partridges sighted along a 10 km transect driven by car at a low speed (Ricci, 1989; Duarte & Vargas, 2001). A transect count was performed in August 2000 and another in August 2001. The mean of both transects was used as the abundance index (i.e. number of partridges sighted per 1 km transect) for each locality. To estimate land use four 2-km lineal transects were carried out in each square, and the percentage of soil surface devoted to agricultural use was estimated by eye in circular plots of 25-m radius every 100 m along each transect. Means of estimations were used as land-use values for each square. Yearly mean temperature and rainfall were obtained from 20-year time series data, and interpolated values for each square were used as climatic variables (see Seoane et al. 2003). Mean temperature and rainfall had a narrow range of variation between localities (13·7–14·9 °C and 380–581 mm, respectively), although the percentage of soil devoted to agricultural use showed a wider range of variation, from 33% to 84%. An exploratory matrix correlation performed with the 4 variables detected a high inverse correlation between both climatic variables; therefore rainfall was excluded from the subsequent analyses.
Parasitological terms were used following the recommendations of Bush et al. (1997). We differentiated the term habitat from the term parasite habitat. Habitat comprised the external environment in which definitive host populations develop. Parasite habitat included habitat and individual and population variations of the definitive host.
The 8 localities were denoted L1 to L8, according to the increment of their partridge population abundance index, with L8 being the locality with the highest abundance index value.
During the open hunting season, comprising 7 weeks in October through December 2001, 235 wild red-legged partridges were captured, with the sample size for each locality ranging from 25 to 39 individuals. To avoid biases due to unequal sampling, we limited the number of partridges sampled in each locality, independently of partridge density, due to the restrictions imposed by the size of the sampled area in each locality and the relatively low number of partridges that could be captured in localities with low population densities.
Partridge carcasses were weighed using a 500 g Pesola™ precision scale, and the length of the left tarsus was measured using a calliper (±0·01 mm). We sexed 110 males and 125 females by direct examination of their gonads. Red-legged partridges breed during the spring, and the young birds reach adult size by the start of the open hunting season. Age was determined by plumage analysis for 159 adults (birds older than 1 year) and 76 young (4–7 months old). The latter included birds hatched during the year of capture. Sex and age ratios within each locality were approximately the same as in the overall sample (χ2=27·61, D.F.=22, P=0·189).
For each bird, the liver and alimentary tract, comprising the glandular stomach, gizzard and intestine, were extracted, sealed in plastic bags and frozen until processing in the laboratory. Glandular stomach and gizzard were dissected and examined using a 10–30× binocular microscope. The gall bladder and main bile ducts were opened and examined for trematodes. The liver was immersed in a saline solution, and any remaining trematodes were obtained by consecutive compression-decompression of the hepatic parenchyma. All freshly obtained helminths were fixed in 70% (v/v) alcohol. Formalin (5%) was injected into the small intestine and caecum to fix the helminths prior to extraction and handling. The small intestine and caecum were dissected and examined for helminths, both in the gut and in scrapings of the mucosa (Calvete et al. 2003).
A body condition index for each host was estimated by ANCOVA, with body mass as dependent variable, sex and age as fixed effect factors, and tarsus length as covariable. The standardized residuals were utilized as body condition index independent of sex and age. This index is a measure of relative body mass corrected for differences in structural body size and has been extensively used in birds (Johnson et al. 1985; Blem, 1990).
Prior to analysing the parasite communities, we evaluated the heterogeneity of parasite infection among hosts by determining variations associated with host factors, including the prevalence and abundance of each species, the combined prevalence and abundance of all helminth species, and species richness. We used the Mixed Procedure of the SAS system to fit mixed models to the data using maximum likelihood estimations of the parameter vector through an iterative process (Little et al. 1996). Logit and negative binomial error distributions with log-link function were used to analyse prevalence and abundance, respectively. To analyse species richness, we used a Poisson distribution with log-link. Sex, age, body condition index and their interactions of second grade were the fixed-effect independent variables. To control the effects of localities, we fitted this variable as a random term in GLMMs using the SAS macro program GLIMMIX (Little et al. 1996). The final models were obtained by a backward elimination procedure. When helminth species were analysed separately, only data of localities where each species was present were considered.
These and all subsequent analyses were carried out at both the overall and intestinal levels. Overall level consisted of all helminth species found in the partridges, whereas intestinal level consisted only of helminth species parasitizing the intestines, with the parasite species of the liver, glandular stomach and gizzard excluded. We made this distinction because intestinal species form a spatially cohesive assemblage that is more likely to be a true community than the ensemble of all parasite species found anywhere in a bird (Poulin & Valtonen, 2002).
As the geographical distance between areas may be an important source of similarity, both in terms of component community and infra-community structure, a permutational regression approach was employed to assess the independence of parasite assemblages sampled in each locality in relation to geographical distance. This method, based on the permutations of distance matrices to perform a multivariate analysis, was originally described by Legendre, Lapointe & Casgrain (1994) and extended to the study of parasite communities by Feliu et al. (1997) and Poulin & Morand (1999). In a first analysis we verified that variations in the host abundance index were independent of geographical distance. In the subsequent analyses we used the abundance index, mean temperature, land use (percentage of agricultural soil) and geographical distance as independent variables, and we performed this multivariate analysis with three dependent variables, component community richness, Sorenson's index of qualitative similarity, and Morisita-Horn's index of quantitative similarity between helminth communities (Magurran, 1988). In these analyses, similarity indices and geographical distances represented all possible pair-wise comparisons between localities, while the other variables were transformed into distance matrices by computing the Euclidean distance (i.e. the absolute value of the difference) between values for all possible pairs. Multiple regression analyses were performed using the PERMUTE 3.4 program (Legendre et al. 1994) on values in the matrices, and repeated after each of the 999 random permutations of the dependent variable matrix. The significance of each partial regression coefficient obtained was calculated as the probability of obtaining, in the 999 random permutations, a regression coefficient greater than or equal to the one observed. A stepwise regression approach with a backward elimination procedure was used to enter only independent variables that contribute significantly to the explanation of the dependent variable in the regression.
At the component community structure level, species richness, prevalence (proportion of birds infected by at least 1 helminth species) and mean intensity of infection (mean number of helminths per infected partridge) were estimated for each helminth community. Since no statistically significant, or nearly significant, correlation was found between sample size and component community species richness, this was not corrected by sample size. To describe the structure of each community, we calculated the Simpson's diversity index expressed as 1/D (Magurran, 1988) and the modified Hill's ratio (F), since this last measure of evenness is relatively unaffected by species richness (Lande, 1996). We then tested correlations between component community parameters and habitat variables using Spearman's rank correlation coefficient.
We used the local-regional richness plots to test for species saturation (Srivastava, 1999). Local species richness was defined as the maximum infracommunity richness observed in each locality, whereas regional richness was defined as the component community parasite species richness of each locality. A limit to infracommunity parasite species richness with increasing component community richness does not necessarily indicate saturation (Rohde, 1998), but proportional sampling indicates non-saturation, i.e. dependence of infracommunity richness on component community richness.
However, probability of sampling infracommunities with the highest species richness is directly related to sampling effort and inversely related to component community richness and prevalences of helminth species. Since our sample sizes were limited by partridge population density and since there were marked differences in helminth species distribution between localities, the pattern shown by the local-regional plot may be confounded by these factors, thus we carried out a second approximation to local-regional richness plotting. Using the algorithm of Janovy et al. (1995), we estimated a null model comprising the expected probabilities of sampling the maximum infracommunity richness observed in each locality. For every helminth community, the difference between observed and expected probability was divided by expected probability, and these standardized values were plotted against the component community richness. The use of this null model accounted for the influence of sample size, component community richness and distribution of helminth species on sampling probabilities of infracommunities, with the maximum species richness observed within each locality. Therefore, proportional sampling, in addition to random distribution of species, should produce a distribution of standardized values independent of component community richness (i.e. parallel to the horizontal axis) and with random variations around zero value of the vertical axis.
To test for potential sampling biases due to differential probability of partridges being hunted as a function of parasitization, we applied the same null model to the uninfected birds and compared the distribution of standardized values with the distribution of standardized values in infracommunities of maximum richness. If highly parasitized birds were more prone to be hunted that non-infected ones, then, standardized values of non-infected birds would show an opposite distribution than standardized values of birds with maximum infracommunity richness.
For each parasite community sampled, we computed the index of nestedness, N (Patterson & Atmar, 1986) and compared it with the expected values under the hypothesis that parasite species are allocated at random among hosts, according to their prevalence. Using the algorithm RANDOM1 of Patterson & Atmar (1986), we generated a series of expected N values from 1000 randomly generated presence/absence matrices. The proportion of simulated N values that were lower than or equal to the observed value gave the P-value, which was used as a measure of departure from the structure expected from random assembly (Hugueny & Guégan, 1997). When the P-value is [les ]0·05, the infracommunities are significantly nested; when the P-value is [ges ]0·95, most are significantly anti-nested (Poulin & Guégan, 2000; Poulin & Valtonen, 2001). Although the significance of individual P-values was established after Bonferroni correction (Morrison, 2002), we considered the P-value as a continuous variable in order to assess its correlation with other factors.
We computed interspecific pairwise associations among the infection intensities of the most common helminth species within each sample using Spearman's rank correlation coefficient. We excluded birds that did not harbour at least 1 of the 2 species in a pair (Lotz & Font, 1994), and we analysed only for those helminth species harboured by at least 5 birds in a sample.
Of the 235 partridges examined, 161 (69%) were infected by at least 1 species of helminth at the overall level, whereas 98 (42%) were infected by at least 1 species of helminth at the intestinal level. We identified a total of 15 helminth species parasitizing the wild red-legged partridges, including 1 species of hepatic trematode (Dicrocoelium sp.), 1 species of nematode in the gizzard (Cheilospirura gruweli) and in the intestine, 7 nematode species (Ascaridia sp., Capillaria caudinflata, Capillaria obsignata, Heterakis gallinarum, Heterakis tenuicaudata, Subulura suctoria and Trichostrongylus tenuis) and 6 cestode species (Choanotaenia infundibulum, Lyruteina nigropunctata, Raillietina cesticillus, Raillietina echinobothrida, Raillietina tetragona and Skryabinia bolivari). The species with the widest ranges were Dicrocoelium sp., C. gruweli, H. tenuicaudata, T. tenuis and S. bolivari, which were found in 5 or more localities, whereas Ascaridia sp., H. gallinarum, R. cesticillus, R. echinobothrida and R. tetragona were each found in only 1 locality. Spatial distribution of species across localities (i.e. prevalence) showed a positive relationship with mean helminth abundance for all helminth species found in 2 or more localities (Fig. 2). The highest prevalence and mean abundance in a locality were shown by Dicrocoelium sp. and C. gruweli, whereas, at the intestinal level, L. nigropunctata and H. tenuicaudata exhibited the highest prevalence and mean abundance values in a single locality.

Fig. 2. Relationship between prevalence (i.e. spatial distribution) and mean helminth abundance for each species. Only helminth species found in two or more localities have been represented. Horizontal axis: prevalence (%); vertical axis: mean helminth abundance.
Species analysis could be performed only for Dicrocoelium sp., C. gruweli, H. tenuicaudata, T. tenuis, L. nigropunctata and S. bolivari. Sex and age were not significantly (P>0·05) associated with variation in prevalence or abundance of these species within localities. Body condition index was inversely associated with the prevalence of both S. bolivari (B=−0·015, F[1,161]=5·92, P=0·016) and C. gruweli (B=−0·012, F[1,165]=4·82, P=0·029) and directly associated with the prevalence of T. tenuis (B=0·032, F[1,111]=9·73, P=0·002).
Combined abundance for all species was not significantly associated with any host factor at the overall or intestinal level. Body condition, however, was found to be inversely related to combined prevalence both at the overall (B=−0·012, F[1,208]=3·93, P=0·049) and intestinal (B=−0·012, F[1,208]=5·51, P=0·02) level.
In a similar way, sex or age was not significantly related to infracommunity species richness, but body condition index was inversely associated with this parameter, both at the overall (B=−0·004, F[1,188]=4·94, P=0·027) and intestinal (B=−0·007, F[1,188]=7·27, P=0·008) level.
We found that between-locality variations in component community richness, Sorenson's index and Morisita–Horn's index were not related to geographical distance between localities. The models we utilized did not detect significant associations between variation of dependent variables and the other independent variables, i.e., abundance index, mean temperature and land use.
At the overall level, the variation between communities in the prevalence of birds infected by at least one helminth species ranged between 33% and 93%, and component community richness ranged between 4 and 10 helminth species (Table 1). Spearman's rank correlation test detected a significant, direct correlation between the partridge abundance index and prevalence, and a direct correlation, which was nearly statistically significant, between mean intensity of infection and prevalence. No correlation was found between component community richness and prevalence or mean intensity (Table 2).
Table 1. P-nestedness values and component community parameters of helminth assemblages at overall and intestinal level (Prevalence in %; S.E. between parentheses. Localities are denominated L1 to L8, according to their partridge population abundance index.)

Table 2. Matrix of correlations between community parameters and P-nestedness values (Significant r-Spearman values are in bold. P-values between parentheses.)

At the intestinal level, the prevalence of infected birds by locality ranged between 9% and 79%, and component community richness ranged between 2 and 8 helminth species (Table 1). At this level the correlation test showed a significant, direct correlation between the partridge abundance index and mean intensity of infection, and a direct correlation, which was nearly statistically significant, between the partridge abundance index and prevalence. Mean intensity and prevalence were directly and significantly correlated, and there was a direct correlation between component community richness and prevalence (Table 2).
No significant correlation was observed between prevalence or mean intensity and habitat variables such as mean temperature and land use or between component community richness and mean temperature. However, a direct correlation, which was nearly statistically significant, was observed between component community richness and percentage of soil devoted to agricultural use, both at the overall (r-Spearman=0·65, P=0·078) and intestinal (r-Spearman=0·64, P=0·088) levels. Simpson's diversity index and F-Hill ratio did not significantly correlate with any habitat variable or any of the other component community parameters.
Maximum infracommunity richness ranged between 2 and 5 helminth species at the overall level and between 1 and 4 helminth species at the intestinal level (Table 1). When plotted against component community richness, a ceiling for infracommunity richness was suggested at high component community richness values, especially at the intestinal level. A curvilinear function fit the data better than a linear function at the intestinal level, although the difference was small (Fig. 3). At the overall level, a curvilinear relationship was slightly better than a linear relationship, although both lacked statistical significance.

Fig. 3. Relationships between component helminth species richness and maximum parasite species richness at overall and intestinal level. The curvilinear function fit the data better than the linear function at the intestinal level (Curvilinear: Y=−0·311+1·941ln(X); P=0·007, R2=0·73; Linear: Y=0·373+0·448X; P=0·013, R2=0·67). At the overall level, both functions lacked statistical fitness to the data (Curvilinear: Y=−0·101+2·092ln(X); P=0·066, R2=0·46; Linear: Y=1·619+0·321X; P=0·074, R2=0·44).
A plot of standardized differences between observed and expected sampling probability of maximum infracommunity richness against component community richness (Fig. 4) showed a deviation of observed sampling probabilities from the null model probabilities. The distribution of values suggested that observed maximum infracommunity richness was sampled at a higher frequency than expected at increasing values of component community richness. The slope of the linear distribution adjusted to the data differed significantly from 0 at the intestinal level (B=0·727, P=0·041, R2=0·53), but not at the overall level (P=0·26). Since this trend may be due to sampling bias, in that partridges infected with a high number of helminth species may be more prone to be hunted that those not infected, we estimated standardized differences for this last class of birds. In the case of sampling bias we would expect an opposite distribution of standardized differences of non-infected birds in relation to the distribution of maximum infracommunity richness. However, this was not the pattern (Fig. 4), since distribution of standardized differences of non-infected birds did not show significant deviations from expected probabilities accounted for by the null model. This suggests that sampling biases did not cause the high frequencies estimated in infracommunities with maximum species richness.

Fig. 4. Standardized difference between observed and expected sampling probability of maximum infracommunity richness and non-infected birds in each locality plotted against component community richness. Expected probability of sampling accounted for variations due to sample size, component community richness and helminth species prevalence distribution. Observed maximum infracommunity richness was sampled at a higher frequency than expected at increasing values of component community richness whereas distribution of standardized differences of non-infected birds did not show significant deviations from expected probabilities accounted for by the null model. Filled circles: maximum infracommunity richness. Open circles: Uninfected red-legged partridges. Continuous line: regression line for maximum infracommunity richness. Dropped line: regression line for uninfected birds.
At the overall level, only 1 helminth community, sampled in locality L8 (Table 1), showed a statistically significant nested structure (P=0·016), but this significance was lost when the Bonferroni correction was applied. No other nested or anti-nested community structure was detected. Interestingly, P-nestedness values were significantly and inversely correlated with the partridge abundance index, prevalence and mean intensity, both at the overall and intestinal levels, but not with component community richness (Table 2). P-nestedness values showed no significant or nearly significant correlation with habitat variables such as mean temperature and land use.
Only 11 of the 56 species pairs analysed at the overall level and four of the 22 analysed at the intestinal level showed a positive association. Moreover, statistical significance was found only in negative correlations, 13 at the overall level and 6 at the intestinal level (Table 3). This number of significantly correlated species pairs was higher than expected by chance in all helminth communities. However, the set of significant interspecific correlations lacked a consistent pattern across helminth communities, except for the cestode L. nigropunctata, which showed significant negative correlations with all other helminth species with which it was identified.
Table 3. Statistically significant pair-wise associations of helminth species in red-legged partridges (Number pairs between parentheses were tested at overall and intestinal levels. Birds not harbouring at least 1 of the 2 species of worms in each pair were excluded. N, actual sample sizes. Localities are sorted by increasing abundance index.)

Spatial pseudoreplication, yearly season, unequal sampling area size or sampling effort, and individual host heterogeneity against parasite infection can be confounding variables in studies designed to test the spatial repeatability or predictability of parasite community structure and to identify the shaping processes. The helminth communities described here were not spatially correlated, since the species richness, both similarity indices, and population abundance of the definitive host did not show covariance with distance between localities. Thus, they can be considered as independent samples of different helminth communities. Season of the year, size of the area in which the definitive host is sampled, and number of hosts sampled can have a major effect on the estimation of structure and, especially, component community species richness. For this reason, in order to avoid biases among parasite communities we endeavoured to ensure that the time of sampling, the area size, and the sampling effort were approximately the same for all localities.
Regarding host heterogeneity, the low density of the partridge populations sampled precluded the possibility of sampling a single class of sex and/or age, thus increasing host homogeneity. To obtain a reasonable sample size, we had to include birds of both sexes and age classes simultaneously. No correction for these factors was necessary, however, because we observed no difference in abundance, prevalence or species richness related to sex or age. Body condition of the birds was the only factor related to abundance and prevalence of any helminth species and to the combined species prevalence and species richness. This relation may suggest the possibility of a sampling bias due to the method used to capture partridges. In this sense, highly parasitized birds may be more prone to be hunted that non-infected birds, and the observed structure of parasite communities may be affected to a great extent by this confounding effect. This possibility was rejected, however, since the observed frequency of highly parasitized birds did not increase in proportion to a decrease in the frequency of non-infected birds.
In addition, although the red-legged partridge is a non-migratory species and the impact of host migration on parasite community structure was ruled out, many wild populations of this bird are usually restocked for the purpose of sport hunting, which can also affect parasite populations. We lessened the potential effects of local interference caused by supplementations by sampling squares in which no recent supplementation was carried out. Further, no difference related to bird age was found in helminth community structure. Since supplementations are usually carried out by releasing young birds near the start of the hunting season, this finding suggested that any effects of local recent supplementation were avoided. Nevertheless, given that the extent to which wild partridge populations have been supplemented has not usually been well-recorded by sport hunter associations, it is still possible that previous re-stockings have altered the distribution of helminths.
In general, our study showed that, on the local scale, there was a low spatial repeatability and predictability of composition and structure of parasite helminth communities in this avian host, at least from the factors quantified in this study. Although all localities were in the same geographical region, low repeatability was expected, since they differed by habitat variables. This lack of spatial repeatability is the most common pattern found in other surveys. Variations in characteristics of host populations or their habitat seem to determine the composition and richness of component communities at a fine scale, playing, in some instances, more important roles than large-scale processes (Hartvigsen & Halvorsen, 1994; Haukisalmi & Henttonen, 1999; Behnke et al. 2001; Poulin & Valtonen, 2002). For example, changes in diversity and abundance of intermediate hosts in relation to characteristics of the habitat have been frequently suggested as the main cause of differences among parasite communities (Valtonen, Holmes & Koskivaara, 1997; Carney & Dick, 2000; Halmetoja et al. 2000).
A previous survey showed that some helminth component community features in the red-legged partridge exhibit large-scale, predictable variation in Spain (Calvete et al. 2003), with the species richness of helminth parasite component community (and Simpson's diversity index) directly correlated with mean temperature. However, we failed to find any association between this habitat variable and the component community richness or diversity indices on the local scale. Although the low statistical power derived from the small number of communities studied may preclude detecting statistically significant associations, this fact supports the idea that local factors mask the effects of factors operating on a large scale. Our observation, of a nearly significant correlation between species richness and the percentage of agricultural soil, suggests that land use may be one of the factors shaping partridge helminth communities on the local scale (mainly species richness), a correlation suggested for other avian and mammalian hosts (Davidson et al. 1991; Abu-Madi et al. 2000; Hulbert & Boag, 2001). In our study, however, only host population abundance correlated significantly with the structure of parasite communities, namely prevalence, intensity of infection and distribution of helminth species across hosts, but not with species richness.
When local-regional richness plots are used to test for species saturation, a linear relationship suggests that the number of species in an infracommunity is generally proportional to the number of available species. Unsaturated local-regional plot will be supported by evidence of the non-interactive nature of the communities, since all non-interactive community models yield unsaturated communities. A curvilinear relationship may be due to interspecific interactions and the species saturation of infracommunities. Saturated local-regional plots are not predicted by any of the non-interactive community models; thus strong interspecific interactions are necessary but not sufficient to support saturation (Srivastava, 1999).
In our study we found that a curvilinear function fitted the relationship between infracommunity and component community richness better than a linear function. This was especially true at the intestinal level, where helminth species form a spatially cohesive assemblage, with higher probabilities for interspecific interactions in the definitive host, than at the overall level. In addition, the finding of significant negative interactions between helminth species suggests that a community has become saturated. This is of special concern, since non-saturation seems to be the most common pattern in avian hosts (Poulin, 1996). Almost all significant pair-wise associations, and most of the non-significant associations, were negative, suggesting that some form of antagonistic interaction, possibly competition, may be occurring between pairs of helminth species. However, Lotz & Font (1994) showed that a high proportion of rare parasite species, with low prevalence in the component community (as in our data set), can lead to an excess of spurious negative associations. It is also likely that associations between helminth species may simply be inherited, with amplification, from intermediate hosts, and that they have no relationship to interspecific interactions in the definitive host (Forbes et al. 1999; Poulin & Valtonen, 2002; Vickery & Poulin, 2002). For these reasons, the associations between parasite species reported here should be used cautiously as evidence supporting the saturated nature of helminth communities.
In addition, there are many additional ways in which a curvilinear relationship can occur without the need for species saturation, including variable sampling effort or helminth communities comprising different prevalence distribution of species. The comparison of observed frequencies of maximum infracommunity richness against a null model corrected by sample size and the prevalence distribution of species confirmed that the curvilinear relationship was not caused by these confounding factors. In fact, we found that infracommunities with maximum species richness were represented in the sample at higher frequencies than expected from a random distribution. However, processes operating at the infracommunity level are not still necessary to explain this curvilinear relationship, since Rohde (1998) showed that it may be the consequence of the differential likelihoods of parasite species to appear in an infracommunity, as determined by transmission rates and intrinsic life-spans.
Another departure from random assembly in helminth community structure assessed in our study was nestedness. Nestedness and its counterpart, anti-nestedness, have been widely studied in ecto- and endo-parasites of fish hosts. Whether an assemblage is nested or not appears to be more usually linked with the overall abundance and commonness of parasites than with the richness of the parasite species pool locally available. That is, nestedness is usually directly associated with the prevalence and mean intensity of parasites in the community, but it lacks correlation with component community richness (Rohde et al. 1998; Poulin & Guégan, 2000; Poulin & Valtonen, 2001). Our results agree with these observations. If nestedness and anti-nestedness are considered along a continuum (Poulin & Guégan, 2000), our findings suggest that P-nestedness values were inversely correlated with prevalence and mean intensity. That is, the structure of helminth communities in this avian host was nearer to nestedness when prevalence and mean intensity increased. In addition, the partridge population abundance index was also correlated in the same way. Thus, helminth communities from red-legged partridge populations with high density exhibited a structure nearer to nestedness than helminth communities from partridge populations with low density.
The two hypotheses generally accepted to explain nested/anti-nested parasite species patterns are extinction-colonization processes and parasite habitat heterogeneity (Guég0an & Hugueny, 1994; Worthen & Rohde, 1996; Rohde et al. 1998; Poulin & Guégan, 2000). In this regard, parasite habitat heterogeneity would not only consist of the way in which infective stages of parasite species are distributed, but also the variations in dietary specialization or feeding rates among individual hosts and how they amplify differences among parasite species in probabilities of colonization or extinction (Poulin & Valtonen, 2001). In agreement with these hypotheses, our results suggest that an increase in partridge population density amplified the positive interassemblage relationship between intensity and prevalence. That is, the local proportion of hosts harbouring infracommunities and the actual numbers of parasites tend to co-vary, aside from the intrinsic differences among parasite species in colonizing capabilities. Thus, although changes in colonization processes associated with partridge density may cause variations in nestedness, this does not preclude other effects of parasite habitat heterogeneity. Since red-legged partridges exhibit an aggregated distribution and their home range in the study area varies from 25 to 55 ha (F. Buenestado, unpublished results), a relatively small surface compared with the 5×5 km area sampled in each locality, the structuring of a nested/anti-nested pattern may be favoured from a non-random distribution of the infective stages of parasites (mainly their intermediate hosts for most species identified) across the habitat. Moreover, a non-random distribution of infective stages of parasites may also have given rise to the curvilinear relationship between infracommunity and component community species richness due to ‘pseudosaturation’, or the overestimation of component community richness with respect to the true size of the species pool available for single infracommunities (Rohde, 1998). In addition, non-random distribution of infective stages may also have given rise to the pattern of negative interspecific associations we observed.
Given the multivariate nature of parasite community composition, it is not possible to use observational data to identify all the factors shaping communities. Our results suggest, however, that parasite community structure in the red-legged partridge may not be determined from within the parasite community but rather mainly by the extrinsic influence of parasite habitat heterogeneity and its ability to amplify differences among parasite species in probabilities of colonization. In this case, parasite habitat heterogeneity would comprise not only non-random distribution of infective stages of parasites or differential individual feeding rates and host dietary specializations, but also variations in definitive host population density.
We are deeply grateful to owners of game estates in the municipalities Méntrida, Moral de Calatrava, Argamasilla del Alba, Poblete, Ciudad Real, Huecas, Corral de Almaguer, Terrinches, and Santa Cruz de Mudela, for the provision of partridge samples for this study. We thank Rosa Estrada for her assistance in the laboratory and Jordi Figuerola for the algorithm to test negative binomial error structure. This research is a contribution to the project FEDER-UE FD 1997-2299 and the project PBI-02-004 funded by Consejería de Ciencia y Tecnología from Junta de Comunidades de Castilla la Mancha (JCCM). We acknowledge support from FEDENCA agreement. J.A.B.A. was supported by J.C.C.M. fellowship and European Social Fund as part of a Ph.D. thesis.

Fig. 1. Geographical distribution of the eight localities sampled in the Ciudad Real and Toledo provinces of Spain. Localities are denominated L1 to L8, according to the increment of their partridge population abundance index, with L1 having the lowest and L8 being having the highest abundance index values.
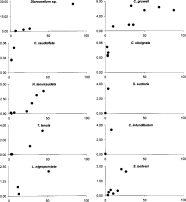
Fig. 2. Relationship between prevalence (i.e. spatial distribution) and mean helminth abundance for each species. Only helminth species found in two or more localities have been represented. Horizontal axis: prevalence (%); vertical axis: mean helminth abundance.

Table 1. P-nestedness values and component community parameters of helminth assemblages at overall and intestinal level

Table 2. Matrix of correlations between community parameters and P-nestedness values
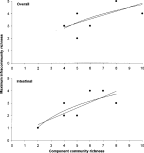
Fig. 3. Relationships between component helminth species richness and maximum parasite species richness at overall and intestinal level. The curvilinear function fit the data better than the linear function at the intestinal level (Curvilinear: Y=−0·311+1·941ln(X); P=0·007, R2=0·73; Linear: Y=0·373+0·448X; P=0·013, R2=0·67). At the overall level, both functions lacked statistical fitness to the data (Curvilinear: Y=−0·101+2·092ln(X); P=0·066, R2=0·46; Linear: Y=1·619+0·321X; P=0·074, R2=0·44).
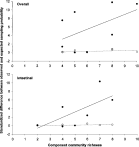
Fig. 4. Standardized difference between observed and expected sampling probability of maximum infracommunity richness and non-infected birds in each locality plotted against component community richness. Expected probability of sampling accounted for variations due to sample size, component community richness and helminth species prevalence distribution. Observed maximum infracommunity richness was sampled at a higher frequency than expected at increasing values of component community richness whereas distribution of standardized differences of non-infected birds did not show significant deviations from expected probabilities accounted for by the null model. Filled circles: maximum infracommunity richness. Open circles: Uninfected red-legged partridges. Continuous line: regression line for maximum infracommunity richness. Dropped line: regression line for uninfected birds.

Table 3. Statistically significant pair-wise associations of helminth species in red-legged partridges