Published online by Cambridge University Press: 11 July 2005
The capsalid monogenean Neobenedenia girellae, a parasite of seawater fishes, was found to express an antigen that elicits antibodies in rabbits, and these antibodies had agglutination/immobilization activity against N. girellae larvae (oncomiracidia) in vitro. Indirect immunofluorescence staining of N. girellae oncomiracidia showed that this agglutination/immobilization antigen was expressed on the surface of cilia. An intraperitoneal injection of ciliary proteins (either sonicated or intact) with adjuvant also elicited agglutinizing/immobilizing antibodies in sera from Japanese flounder, Paralichthys olivaceus. These antisera showed a clear correlation between anti-ciliary antibody levels (measured by enzyme-linked immunosorbent assays) and their agglutination/immobilization activity. Anti-ciliary antibody levels in Japanese flounder reached a plateau at 39 days after booster immunization and were significantly higher in the two immunized groups (injection of sonicated or intact cilia) as compared with control fish (injection of bovine serum albumin; ANOVA, Tukey's test, P<0·01). Anti-ciliary antibodies were also found in fish mucus; however, there was no correlation between fish serum anti-ciliary antibody levels and mucus antibody levels. A Triton X-114-soluble 8 kDa glycoprotein of the ciliary integral membrane fraction is a plausible candidate for the agglutination/immobilization antigen based on SDS-polyacrylamide gel electrophoresis and immunoblot analyses with rabbit and fish antisera.
At 25 °C, Neobenedenia girellae (Monogenea: Monopisthocotylea: Capsalidae) (Hargis, 1955; Yamaguchi, 1963) emerge from 5- to 6-day-old eggs as free-living ciliate larvae, or oncomiracidia. Oncomiracidia swim around host fish and attach to the fin or skin surfaces of their host and continue to grow for 10–11 days before reaching sexual maturity (Bondad-Reantaso et al. 1995). These parasites cause the host fish to weaken or die from secondary bacterial infection. In recent years, infection of valuable commercial fish species with N. girellae has become a serious problem in Japan. The fish species affected include Japanese flounder (Paralichthys olivaceus), amberjack (Seliola dumerili), yellowtail (Seriola quinqueradiata) and tiger puffer (Takifugu rubripes) (Ogawa et al. 1995). Among host fish species in Japan, Japanese flounder is thought to be the most susceptible (K. Ogawa, personal communication). Japanese flounder inhabit the coastal waters of the northwestern Pacific Ocean, which is an important source of commercial aquaculture fish stocks for Japan. The repertoire of chemotherapeutic agents available for treatment of fish against N. girellae is limited because of human consumption. On the other hand, immunoprophylaxis is thought to be a safer for fish and humans than chemotherapeutic treatment.
Some free-living ciliates, such as the protozoan ciliate Tetrahymena thermophila and Paramecium aurelia have immobilization antigens (i-antigens) which are surface proteins that, when injected into rabbits, elicit the production of antibodies with immobilizing activities in vitro (Jones, 1965; Bruns, 1971). Of the protozoan ciliates that infect fish, Ichthyophthirius multifiliis and Philasterides dicentrarchi have been shown to express surface i-antigens, and their host fishes (the channel catfish Ictalurus punctatus and the turbot Scophthalmus maximus, respectively) produce immunoglobulin M (IgM) directed against i-antigens, thereby immobilizing these ciliates in vitro (Dickerson, Clarke and Findly, 1989; Iglesias et al. 2002). By the use of several immunological analyses, the i-antigen of I. multifiliis was found to localize to the surface of cilia. Vaccination of naïve channel catfish with i-antigen elicits immunity in fish, prevents fish from infection by I. multifiliis, and improves the survival rate against a lethal challenge of infectious parasite (Wang and Dickerson, 2002).
Here we show that live N. girellae oncomiracidia are immobilized by antisera from rabbits that had been immunized with an insoluble fraction of the oncomiracidia. Ciliary proteins of the oncomiracidia contained an immobilization antigen, as shown by indirect immune microscopy with rabbit antisera and by immobilization assays with antisera from Japanese flounder that were immunized with cilia isolated from the oncomiracidia. An abundant 8 kDa glycoprotein from the ciliary surface, extracted by phase separation in solutions of Triton X-114, is a plausible candidate for the immobilization antigen of the oncomiracidia based on immunoblotting with antiserum from immunized rabbits and fish.
A population of Neobenedenia girellae was maintained using the spotted halibut (Verasper variegatus) as a host in a 100 litre polycarbonate tank (water temperature, 25±1 °C) in our laboratory (Umeda and Hirazawa, 2004). Laid eggs were collected by putting 5 cm2 nylon nets (mesh size, 5 mm) into the tank. The eggs, which were entangled in the nets, were collected and incubated in a 300 ml plastic beaker at 20 °C for 8–10 days. Within 12 h after hatching, the oncomiracidia were used for experiments. Fixed samples of the parasites, which were collected from the body surface of the spotted halibut, have been deposited in the South Australian Museum as voucher specimens: SAMAAHC32859 and SAMAAHC32860 (Hirazawa et al. 2004).
The oncomiracidia (body length: approximately 200 μm) were concentrated by using a cell strainer (Becton Dickinson Labware, USA), 2·7 cm in diameter and 40 μm in mesh opening, and the oncomiracidia were trapped when seawater containing the hatched parasites was passed through the cell strainer. The strainer containing collected oncomiracidia was transferred to a culture dish (diameter 9 cm) with 25 ml of PBS for 1 h. The captured oncomiracidia shed their ciliated epidermal cells and only ciliated epidermal cells passed through the mesh of the cell strainer. The PBS containing the ciliated epidermal cells, as confirmed by observation under the microscope, was transferred to a 2 ml microcentrifuge tube and was centrifuged at 16000 g for 5 min at 4 °C to remove the PBS. The ciliated epidermal cells were stored at −80 °C until used for the experiments.
Japanese flounder were obtained from a local juvenile flounder producer in Oita prefecture, Japan, and were maintained in a 200 litre polycarbonate tank. The tank was supplied with seawater at a flow rate of 1·2 l/min; the seawater was sand-filtered and UV-irradiated (Flonlizer 4-L unit; ~50000 μws/cm2; Chiyoda Kosan Ltd, Japan). The fish were fed commercial extruded 3-mm pellets (Nippon Suisan Kaisha Ltd, Japan) twice a day, at a feeding rate of 3% body weight. The saline content, pH and chemical oxygen demand of seawater used at our laboratory was approximately 34 ppt, 8·1 and 1·0 mg/l, respectively, throughout the year.
Collected oncomiracidia were suspended in ice-cold PBS in 1·5 ml microcentrifuge tubes and were sonicated for 5 min on ice (7040 Ultrasonic Processor; Seiko Instruments, Inc., USA). The insoluble fraction was pelleted by centrifugation at 16000 g for 20 min at 4 °C. The pellet was resuspended and washed 5 times with ice-cold PBS and was stored at −80 °C until used.
The assays were performed essentially according to the method of Clark, Dickerson and Findly (1988). Filtered and UV-treated seawater (990 μl) containing approximately 100 oncomiracidia was added to each well of a 24-well tissue-culture plate. Rabbit or fish serum was serially diluted with filtered and UV-treated seawater (10 μl) and was added to each well. The plates were incubated for up to 1 h at 25 °C. Agglutination was monitored with a microscope (BX50; Olympus Optical Co., Japan) fitted with a CCD camera (FD-120M; Flovel Co. Ltd, Japan). After counting the number of agglutinated oncomiracidia, 50 μl of formaldehyde was added to each well and the total number of oncomiracidia was counted. The degree of agglutination of oncomiracidia was calculated as (the number of agglutinated oncomiracidia)/(the total number of oncomiracidia)×100 (%). Each fish serum was assayed in triplicate.
Sample protein concentrations were determined by the method of Bradford, using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Germany) (Bradford, 1976). Bovine serum albumin (BSA; Sigma-Aldrich, USA) was used as the protein standard for construction of the calibration curve.
SDS-PAGE using a discontinuous buffer system was performed on several types of gradient gels (Daiichi Pure Chemicals, Japan) (Laemmli, 1970). Gels were stained by either Coomassie brilliant blue (CBB) or a silver stain kit (Bio-Rad Laboratories, Germany). Molecular mass determinations were estimated by comparing the migration of the protein of interest with that of commercial molecular weight size standards (Bio-Rad Laboratories, Germany, and Invitrogen Corp., USA).
Antisera against an insoluble fraction of the oncomiracidia or Japanese flounder IgM were produced in female Japanese White rabbits. Five inoculations of 400 μg (first time only) or 200 μg each of an insoluble fraction of the oncomiracidia Japanese flounder IgM were given subcutaneously with Freund's incomplete adjuvant in multiple dorsal-lumbar sites at 2-week intervals. Blood was drawn at 8 weeks after the initial injection and coagulated at 4 °C and was subsequently centrifuged at 300 g for 5 min at 4 °C. Sera were collected, inactivated by heating (56 °C for 30 min) and stored at −80 °C.
To establish an enzyme-linked immunosorbent assay (ELISA) to detect the antibodies against ciliary proteins of oncomiracidia in Japanese flounder, young healthy fish (weighing 54·4–107·1 g) were immunized with a 1[ratio ]1 (v/v) emulsion of Freund's complete adjuvant (FCA; BD Diagnostic Systems, USA) and antigen fractions: intact cilia (n=10) and BSA (n=12; negative control). The dose of injected antigen was 0·5 mg/kg fish body weight. Each fish was immunized by an intraperitoneal injection of the corresponding emulsion. Two weeks after the first immunization, an intraperitoneal booster injection was given at the same antigen dose. Sera were collected 2 weeks after the booster injection. To monitor reactivities over time, fish (weighing 62·0–109·9 g) were immunized with a 1[ratio ]1 (v/v) emulsion of FCA and an antigen fraction: intact cilia (n=8), a sonicated ciliary fraction (n=6) or BSA as a negative control (n=10). All fish in the immunization experiments were bled on days 14, 39, 64 and 107 after the booster injection to obtain small serum samples. Blood was drawn from the caudal vein, and sera were stored at −80 °C.
On day 95 after the booster immunization (see above), fish immunized with intact cilia, with sonicated cilia or with BSA (as a negative control) were challenged with N. girellae oncomiracidia (13000 N per 500L tank). On day 107, each fish was treated with freshwater by dipping for 10 min to dislodge the parasites from the host (Leong, 1997), and the number of dislodged parasites was counted for each individual fish under a stereomicroscope. The total length of the fish was measured to calculate the fish surface area in order to obtain the number of parasites per square centimeter (cm2) of fish surface area. The fish surface area (cm2) was calculated using the following equation: total fish surface area of upper and lower surfaces (cm2)=0·290×(total length)2·0×2 (Kobayashi, 1980).
Pooled sera from pre-immune and uninfected female Japanese flounder were fractionated by ammonium sulfate precipitation and dialysed against 15 mM Tris-HCl (pH 8·0). Crude immunoglobulin fractions were loaded onto a DEAE DE-52 (Whatman, USA) ion-exchange column, previously equilibrated in starting buffer (15 mM Tris-HCl, pH 8·0). The elution was performed with a linear gradient of 0–0·2 M NaCl in starting buffer at a flow rate of 2 ml/min and was monitored by absorbance at 280 nm. Fractions thought to contain IgM heavy chains, as judged from molecular mass (~75 kDa) of the protein band on SDS-PAGE, were collected and dialysed against 20 mM Tris-HCl (pH 8·0)/300 mM NaCl. These fractions were subsequently applied to a Sephacryl S-200 column (Amersham Biosciences, Sweden) that was previously equilibrated in the same buffer. The elution was performed at a flow rate of 0·8 ml/min and was monitored by absorbance at 280 nm.
After the last blood sample was taken from each fish (day 107, see above), a sample of cutaneous mucus was collected by keeping the fish in a plastic bag for 10 min. The mucus was transferred from the bag to a 1·5 ml microcentrifuge tube. The mucus solution was centrifuged at 16000 g for 10 min at 4 °C, and the supernatant was collected and stored at −80 °C.
Frozen cilia from the oncomiracidia were thawed and suspended in PBS, and the protein concentration was adjusted to 50 μg/ml. The cilia solution (100 μl) was added to each well of the 96-well ELISA plates (Greiner Labortechnik, France), and plates were incubated at room temperature for 2 h. After blocking non-specific protein-binding sites with Block Ace (Dainippon Pharmaceutical Co., Ltd, Japan) at room temperature for 2 h, fish serum (1[ratio ]1000) or mucus (1[ratio ]10) diluted in Block Ace containing 0·1% (v/v) Tween 20 (Sigma-Aldrich, USA) was added, and plates were incubated overnight at 4 °C. Plates were washed 3 times with 0·1% (v/v) Tween 20 in PBS (PBS-T). Rabbit immunoglobulin G (IgG) anti-Japanese flounder IgM diluted to a final concentration of 1 μg/ml in PBS-T was added and incubated at room temperature for 2 h. After washing plates as above, goat anti-rabbit IgG conjugated to alkaline phosphatase (AP; Kirkegaard & Perry Laboratories, Inc., USA) diluted 1[ratio ]25000 (40 ng/ml) in PBS-T was added, and plates were incubated 2 h at room temperature. After washing as above, plates were analysed for AP using Alkaline-Phosphatase Substrate (Bio-Rad Laboratories, Germany). Absorbance was measured at 405 nm using a TECAN Rainbow Thermo (Wako Pure Chemical Industries, Ltd, Japan) according to the manufacturer's instructions. The background value at 405 nm (OD405) was defined as the average signal from 3 wells incubated with blocking solution in the absence of primary antibody. Each fish serum was assayed in triplicate.
The oncomiracidia of N. girellae were fixed by incubation for 30 min in 1% (v/v) formaldehyde in seawater and were collected by filtration with a Stericup-HV filter (Millipore). Formalin-fixed larvae were washed several times with distilled water before use. Approximately 100 formalin-fixed oncomiracidia were spread onto 2·5×7·5 cm glass slides and air dried at room temperature. Fixed and dried oncomiracidia were delineated on the slide by a square drawn with waterproof ink. After non-specific protein-binding sites were blocked with Block Ace, rabbit antiserum or pre-immune serum (negative control) diluted 1[ratio ]200 in PBS-T was added to each square, and slides were incubated in a humidified box for 2 h at room temperature. Slides were washed 3 times with PBS-T, and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1[ratio ]400; Sigma-Aldrich, USA) was added to each square. After a 2 h incubation at room temperature, slides were washed and mounted under cover-slips using the Prolong Antifade Kit (Molecular Probes). Oncomiracidia were examined with a confocal laser scanning microscope system (FLUOVIEW FV300; Olympus Optical Co., Japan).
Frozen cilia were thawed in distilled water, spread onto glass slides and air dried without prior fixation. Following incubation with rabbit antiserum as described above, cilia were incubated with AP-conjugated goat anti-rabbit IgG (Kirkegaard & Perry Laboratories, Inc.) and were subjected to AP colour development using the Black Alkaline Phosphatase Substrate Kit (Vector Laboratories, UK). Observations were made under a microscope (BX50; Olympus Optical Co., Japan) equipped with a CCD camera (FD-120M; Flovel Co. Ltd, Japan).
Ciliary integral membrane proteins of N. girellae were extracted by phase separation in Triton X-114 (Sigma-Aldrich, USA) solution, essentially as described previously (Border, 1981; Dickerson et al. 1989). Frozen cilia (~200 μg) were thawed and resuspended in 100 μl of ice-cold 10 mM Tris-HCl (pH 7·5) in a 1·5 ml microcentrifuge tube. An equal volume of ice-cold extraction buffer (10 mM Tris-HCl, pH 7·5, 300 mM NaCl, 2% (v/v) Triton X-114) was added, and cilia were incubated on ice for 1 h. The cytoskeletal components were removed by centrifugation at 16000 g for 20 min at 4 °C. The clear supernatant was overlaid on a 6% (w/v) sucrose cushion containing 10 mM Tris-HCl (pH 7·5), 150 mM NaCl and 0·06% (v/v) Triton X-114, and the tube was incubated at 32 °C for 5 min. The detergent phase was then separated by centrifugation at 300 g for 3 min at room temperature. The upper aqueous phase was removed from the tube and Triton X-114 was added to 0·5% (v/v). After incubation on ice, the mixture was again overlaid on the sucrose cushion used previously, and the tube was incubated at 32 °C for 5 min for condensation and centrifuged again to separate the detergent phase. Ciliary integral membrane proteins, which were contained in the detergent phase, were precipitated by adding 9 vols of ice-cold acetone. After incubation for 1 h on ice, the precipitate was pelleted by centrifugation at 16000 g for 20 min at 4 °C. After acetone removal, the pellet was vacuum dried, resuspended in 100 μl of 10 mM Tris-HCl (pH 7·5) and stored at −80 °C.
Protein bands from SDS-PAGE were electro-blotted onto Pall Fluoro Trans W Membranes (Nippon Genetics Co. Ltd, Japan) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad Laboratories, Germany) at 2 mA/cm2 for 1 h. Filters were blocked by incubation for 1 h at room temperature with Block Ace containing 0·1% (v/v) Tween 20. Filters were then incubated for 1 h with appropriate dilutions of the respective rabbit or fish sera. Bound fish antibodies were detected with a 1[ratio ]1000 dilution of rabbit IgG anti-Japanese flounder IgM for 1 h, followed by a 1[ratio ]25000 dilution of AP-conjugated goat anti-rabbit IgG for 1 h. Bound rabbit antibodies were detected with a 1[ratio ]25000 dilution of AP-conjugated goat anti-rabbit IgG for 1 h. Filters were then developed in nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Roche Diagnostics, Germany). All incubations were performed at room temperature.
Antisera from rabbits immunized with an insoluble fraction of the oncomiracidia promoted the agglutination of live oncomiracidia (Fig. 1). Serial dilutions of either pre-immune or immune rabbit sera were made in 24-well microtitre plates, and approximately 100 oncomiracidia were added to each well. Specific effects of the immunized rabbit sera were immediately evident when the oncomiracidia ceased to swim and began to form large aggregates and settle to the bottom of the wells. Agglutination of live oncomiracidia occurred in immunized rabbit serum diluted 1[ratio ]10000. In contrast, no agglutination occurred in the presence of pre-immune rabbit serum, even at a dilution of 1[ratio ]100 (data not shown).

Fig. 1. Agglutination of Neobenedenia girellae oncomiracidia in rabbit antisera. N. girellae oncomiracidia were incubated for 15 min at room temperature in a 1[ratio ]1000 dilution of immune sera from rabbits that were immunized with an insoluble fraction of the oncomiracidia.
Antisera from rabbits immunized with the insoluble oncomiracidium fraction that promoted agglutination seemed to react with ciliary proteins of oncomiracidia. To determine whether cilia contain an immobilization antigen that leads to this immune response, indirect immune staining was performed using rabbit antisera. The surface fluorescence of the oncomiracidia that were fixed with formalin indicated that immobilization antigens were located primarily on the surface of cilia (Fig. 2B, C). Ciliary surface proteins were strongly recognized by specific antibodies from rabbit antisera (Fig. 3B). Treatment with pre-immune rabbit sera followed by goat anti-rabbit IgG showed little fluorescence or staining (Figs 2A and 3A).

Fig. 2. Indirect immunofluorescence staining of Neobenedenia girellae oncomiracidia. Fixed oncomiracidia were incubated in rabbit pre-immune serum (A) or serum from rabbits immunized with an insoluble fraction of the oncomiracidia (B and C). Bound antibodies were detected with FITC-conjugated goat anti-rabbit IgG. (C) Higher-magnification image of the oncomiracidium from (B). Primary and secondary sera were used at dilutions of 1[ratio ]200 and 1[ratio ]400, respectively. The oncomiracidia were visualized by phase-contrast (left column) or fluorescence microscopy (right column).

Fig. 3. Indirect immune staining of ciliated epidermal cells from Neobenedenia girellae oncomiracidia. Isolated ciliated epidermal cells were incubated in rabbit pre-immune serum (A) or antiserum raised against an insoluble fraction of the oncomiracidia (B), followed by AP-conjugated goat anti-rabbit IgG. Primary and secondary sera were used at dilutions of 1[ratio ]200 and 1[ratio ]400, respectively.
Immunization of Japanese flounder with the oncomiracidium ciliary protein induced antibodies that promoted the agglutination of live oncomiracidia (Fig. 4). Agglutination activities varied between individuals, and agglutination of the oncomiracidia in fish immune sera occurred at a dilution of 1[ratio ]800. Pre-immune fish sera provoked little agglutination of live oncomiracidia (data not shown).
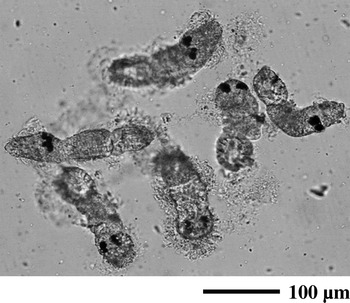
Fig. 4. Agglutination of Neobenedenia girellae oncomiracidia in Japanese flounder antisera. The oncomiracidia were incubated for 15 min at room temperature in a 1[ratio ]400 dilution of immune sera from Japanese flounder that were immunized with cilia.
In an ELISA, sera from fish immunized with cilia successfully showed reactivities to ciliary antigens. Optimal ELISA reactions contained 5 μg intact cilia as antigen, a 1[ratio ]10 dilution of fish cutaneous mucus, 1 μg/ml rabbit anti-Japanese flounder IgM and 40 ng/ml of AP-conjugated goat anti-rabbit IgG. The OD405 value and the degree of agglutination of the sera varied among the fish, but there was a clear correlation between these characteristics within a given individual (Fig. 5).

Fig. 5. Correlation between ELISA reactivity and agglutination activity of antisera from Japanese flounder. The graph examines the relationship between the degree of agglutination in a 1[ratio ]100 dilution and ELISA reactivity of sera from fish injected with intact cilia (closed circles) or BSA (open circles; negative control). The regression curve is shown. Generated linear regression equation (Y=280·99X, R2=0·914) is shown as dashed line.
Figure 6 shows the time-course of immunoreactivity in fish immunized using different protocols. Two weeks after booster immunization, detectable levels of antibodies against ciliary antigens were found in groups immunized with either intact or sonicated cilia. There was a significant difference between each of the groups and the control group of fish (ANOVA, Tukey's test; P<0·05). Antibody titres, as determined by ELISA, of sera from immunized fish reached a plateau at 39 days after booster immunization in both groups.

Fig. 6. Time-course of the humoral response of Japanese flounder immunized with intact cilia (closed triangles), sonicated ciliary fraction (closed diamonds) or BSA (open circles; negative control) plus FCA. Each point is the mean±S.E. of absorbances at 405 nm obtained in the assays of each individual serum sample. Asterisks indicate a significant difference (*P<0·05, **P<0·01, according to ANOVA, Tukey's test) with respect to the control fish.
Protein concentrations in fish cutaneous mucus were approximately 3 mg/ml. Fish cutaneous mucus was mixed with Laemmli sample buffer, resolved by SDS-PAGE and electroblotted onto PVDF membranes. IgM heavy chain (~75 kDa) was detected by immunoblotting with rabbit anti-Japanese flounder IgM (data not shown). Judged from the density of the IgM heavy chain band as resolved by SDS-PAGE, we estimate that ~50 μg of IgM accumulated in each 1 ml of fish cutaneous mucus. Cutaneous mucus from fish immunized with intact or sonicated cilia successfully showed reactivities to ciliary antigens. Optimal ELISA reactions contained 5 μg intact cilia as antigen, a 1[ratio ]10 dilution of fish cutaneous mucus, 1 μg/ml rabbit anti-Japanese flounder IgM and 40 ng/ml of AP-conjugated goat anti-rabbit IgG. The OD405 values from serum and cutaneous mucus isolated from the same individuals show a slight correlation (Fig. 7). The OD405 values of sera from fish injected with cilia were significantly different from those injected with BSA (as a negative control; P<0·05).

Fig. 7. Correlation between serum and mucus ELISA reactivity against Neobenedenia girellae oncomiracidia in Japanese flounder. Each point indicates immune reactivity of Japanese flounder immunized with intact cilia (closed triangles), sonicated ciliary fraction (closed diamonds) or BSA (open circles; negative control) plus FCA.
Parasites were mainly found on dorsal, anal and caudal fins, and the surface of mouth and eye regions. The number of parasites on the surface of fish immunized with intact cilia, with sonicated cilia or with BSA were 301·6±98·4 (N), 240·3±75·8 (N) and 316·0±215·1 (N), respectively. And the number of parasites per surface area (cm2) of fish in 3 groups were 1·10±0·34 (N/cm2: immunized with intact cilia), 0·90±0·29 (N/cm2: immunized with sonicated cilia) and 1·20±0·73 (N/cm2: immunized with BSA). There were no significant differences in parasite load among the 3 groups in both cases.
Cilia are the predominant feature of the oncomiracidia surface; they contain both external (membrane) and internal (axonemal) compartments. Because membrane compartments of cilia are thought to be associated with immobilization activities, they were extracted by differential centrifugation and phase separation in the nonionic detergent, Triton X-114. Figure 8A shows the protein composition of whole cilia (lane 1) and of integral membrane compartments of cilia (lane 2) as resolved by SDS-PAGE. Whole cilia were highly enriched in ~50 kDa protein thought to be tubulin, as expected, whereas the integral-membrane fraction was reduced in this component but contained a predominant glycoprotein of ~8 kDa. To characterize agglutination/immobilization antigens, immunoblot analyses were carried out with antigenic proteins of cilia that are recognized by rabbit and fish immune sera. Cilia were solubilized in Laemmli sample buffer, resolved by SDS-PAGE and electro-blotted onto PVDF membranes. The glycoprotein of ~8 kDa was strongly detected by immune sera from rabbit injected with an insoluble fraction of the oncomiracidia (Fig. 8B, lane 1) and by serum from fish injected with sonicated cilia (Fig. 8B, lane 3). There was no difference in band patterns when cilia were analysed under reducing or non-reducing conditions (data not shown).

Fig. 8. SDS-PAGE and immunoblot analyses of intact cilia and ciliary integral membrane proteins from Neobenedenia girellae oncomiracidia using rabbit and fish sera. (A) Intact cilia (lane 1) and integral membrane proteins from cilia (lane 2) were solubilized in Laemmli sample buffer, resolved by reducing SDS-PAGE on 4-20% polyacrylamide gradient gels (15 μg/lane) and silver stained. (B) For immunoblot analysis, 5 μg of integral membrane proteins of cilia were resolved by reducing SDS-PAGE and were electro-blotted onto PVDF membranes. Each lane was incubated with the following sera: lane 1, rabbit antiserum raised against an insoluble fraction of oncomiracidia; lane 2, fish antiserum injected with BSA as a negative control; lane 3, fish antiserum raised against sonicated cilia. The signal was developed with NBT-BCIP. Relative molecular masses of standard markers are indicated on the left. The position of the 8 kDa antigen is indicated with arrows on the right side of each panel.
In the present study, antisera from fish immunized with cilia and antiserum from rabbits immunized with an insoluble fraction of N. girellae oncomiracidia led to agglutination of this parasite within a few minutes in vitro. This agglutination activity demonstrates that N. girellae oncomiracidia express agglutination/immobilization antigens on their surface, as do T. thermophila, P. aurelia, I. multifiliis and P. dicentrarchi. Microscopy revealed that these antigens are located on the surface of the cilia of N. girellae oncomiracidia. The pre-adsorption of rabbit antiserum with high concentrations of these cilia diminished the agglutination/immobilization activity of the rabbit antiserum (data not shown). Ciliary protein from N. girellae oncomiracidia induces the production of antibodies that have agglutination/immobilization activities in fish, as does ciliary protein of I. multifiliis and P. dicentrarchi.
An inoculation of ciliary protein of the oncomiracidia did not show an effect on the number of N. girellae present on the surface of the fish. Purified immobilization-antigen of I. multifiliis prevents fish from infection following a lethal challenge with parasites. In the case of P. dicentrarchi, fish that were vaccinated with ciliary protein had an improved survival rate against a lethal challenge. Although OD405 values for cutaneous mucus taken on day 107 after booster immunization showed a significant difference (P<0·05) between cilia-immunized and control fish, the cilia-immunized mucus did not possess agglutination/immobilization activities, even at a 1[ratio ]10 dilution after several hours of incubation.
Although the systemic and mucosal antibody response of fish seemed to occur separately, the actual mechanisms and sites of cutaneous antibody induction, production and secretion have not been determined (Diconza and Halliday, 1971; Rombout et al. 1986; Xu and Dickerson, 2002). Intraperitoneal injection of agglutination/immobilization antigen into channel catfish did not stimulate as great a mucosal response as that generated by active infection with I. multifiliis (Maki and Dickerson, 2003). In the present study, sera and cutaneous mucus samples from individual fish showed a slight correlation with respect to their reactivity against N. girellae oncomiracidia ciliary protein. This phenomenon suggests that a different method of immunizing, such as bath or oral immunization, should be considered to elicit an effective immune response against N. girellae oncomiracidia in the cutaneous mucus of fish.
We found that antisera from fish infected with N. girellae did not have agglutination/immobilization activity; however, this was not the case for I. multifiliis and P. dicentrarchi (Hines and Spira, 1974; Iglesias et al. 2002). It may be that N. girellae expresses different antigenic proteins at different stages in its life-cycle, like the variant surface glycoprotein (VSG) of the African trypanosomes (McLaren, 1992). This phenomenon also occurs in the parasitic species Toxoplasma gondii, Entamoeba histolytica, Leishmania spp. and Plasmodium spp. (McLaren, 1992). In a challenge trial, cilia were collected from N. girellae oncomiracidia immediately after hatching and were used as antigen. Antisera from fish immunized with these cilia had agglutination/immobilization activity against N. girellae oncomiracidia at the swimming stage, but were thought to be inactive against N. girellae at different life-stages. These antisera may not have agglutination/immobilization activity against this parasite when it is on the surface of fish, because agglutination/immobilization on the surface of cilia may be exchanged.
The ~8 kDa protein (on SDS-PAGE), a major component of the integral membrane fraction of cilia, was detected by immunoblot analyses with rabbit and fish antisera, each of which had agglutination/immobilization activity. The sizes of agglutination/immobilization antigens of T. thermophila, P. aurelia and I. multifiliis vary from approximately 40 to 250 kDa (Hansma, 1975; Doerder, Berkowitz and Skalican-Crowe, 1985; Dickerson et al. 1989). This protein was detected by silver staining and periodic acid-Schiff staining according to the method of Zacharius et al. (1969) (data not shown), but not by Coomassie staining. These results suggest that this protein lacks aromatic amino acids and has a sugar chain(s). We are pursuing further investigation of both the physical and the functional properties of this interesting protein.
In conclusion, this study demonstrates that N. girellae expresses an immobilization/agglutination antigen on its ciliary surface. The immobilization/agglutination antigen is recognized by the Japanese flounder immune system and may be useful for vaccination, although it is not yet clear whether fish antibodies against this antigen prevent infection of N. girellae.

Fig. 1. Agglutination of Neobenedenia girellae oncomiracidia in rabbit antisera. N. girellae oncomiracidia were incubated for 15 min at room temperature in a 1[ratio ]1000 dilution of immune sera from rabbits that were immunized with an insoluble fraction of the oncomiracidia.

Fig. 2. Indirect immunofluorescence staining of Neobenedenia girellae oncomiracidia. Fixed oncomiracidia were incubated in rabbit pre-immune serum (A) or serum from rabbits immunized with an insoluble fraction of the oncomiracidia (B and C). Bound antibodies were detected with FITC-conjugated goat anti-rabbit IgG. (C) Higher-magnification image of the oncomiracidium from (B). Primary and secondary sera were used at dilutions of 1[ratio ]200 and 1[ratio ]400, respectively. The oncomiracidia were visualized by phase-contrast (left column) or fluorescence microscopy (right column).

Fig. 3. Indirect immune staining of ciliated epidermal cells from Neobenedenia girellae oncomiracidia. Isolated ciliated epidermal cells were incubated in rabbit pre-immune serum (A) or antiserum raised against an insoluble fraction of the oncomiracidia (B), followed by AP-conjugated goat anti-rabbit IgG. Primary and secondary sera were used at dilutions of 1[ratio ]200 and 1[ratio ]400, respectively.

Fig. 4. Agglutination of Neobenedenia girellae oncomiracidia in Japanese flounder antisera. The oncomiracidia were incubated for 15 min at room temperature in a 1[ratio ]400 dilution of immune sera from Japanese flounder that were immunized with cilia.

Fig. 5. Correlation between ELISA reactivity and agglutination activity of antisera from Japanese flounder. The graph examines the relationship between the degree of agglutination in a 1[ratio ]100 dilution and ELISA reactivity of sera from fish injected with intact cilia (closed circles) or BSA (open circles; negative control). The regression curve is shown. Generated linear regression equation (Y=280·99X, R2=0·914) is shown as dashed line.

Fig. 6. Time-course of the humoral response of Japanese flounder immunized with intact cilia (closed triangles), sonicated ciliary fraction (closed diamonds) or BSA (open circles; negative control) plus FCA. Each point is the mean±S.E. of absorbances at 405 nm obtained in the assays of each individual serum sample. Asterisks indicate a significant difference (*P<0·05, **P<0·01, according to ANOVA, Tukey's test) with respect to the control fish.

Fig. 7. Correlation between serum and mucus ELISA reactivity against Neobenedenia girellae oncomiracidia in Japanese flounder. Each point indicates immune reactivity of Japanese flounder immunized with intact cilia (closed triangles), sonicated ciliary fraction (closed diamonds) or BSA (open circles; negative control) plus FCA.

Fig. 8. SDS-PAGE and immunoblot analyses of intact cilia and ciliary integral membrane proteins from Neobenedenia girellae oncomiracidia using rabbit and fish sera. (A) Intact cilia (lane 1) and integral membrane proteins from cilia (lane 2) were solubilized in Laemmli sample buffer, resolved by reducing SDS-PAGE on 4-20% polyacrylamide gradient gels (15 μg/lane) and silver stained. (B) For immunoblot analysis, 5 μg of integral membrane proteins of cilia were resolved by reducing SDS-PAGE and were electro-blotted onto PVDF membranes. Each lane was incubated with the following sera: lane 1, rabbit antiserum raised against an insoluble fraction of oncomiracidia; lane 2, fish antiserum injected with BSA as a negative control; lane 3, fish antiserum raised against sonicated cilia. The signal was developed with NBT-BCIP. Relative molecular masses of standard markers are indicated on the left. The position of the 8 kDa antigen is indicated with arrows on the right side of each panel.