INTRODUCTION
The parasite communities of fishes at infra, component and compound community levels (as defined by Bush et al. 1997) are affected by host and parasite phylogeny, historical ecological associations, and current ecological factors such as diet, habitat or niche overlap (Esch et al. 1990; Poulin, 1995). Thus, for example, host size or host age, schooling behaviour, and ecological overlap with other host species are factors affecting the parasite infracommunity structure of the hosts (Nelson and Dick, 2002). Likewise, the environmental characteristics such as latitude, temperature and depth can affect the parasite communities of fishes (Rohde et al. 1995; Oliva et al. 2004).
In the last decade, knowledge of the patterns and processes underlying the structure of parasite communities has progressed significantly (Sousa, 1994; Poulin, 1996, 2001). However, much of the evidence available to date on parasite community comes from unconfirmed studies on either temporal or spatial scales (Poulin and Valtonen, 2002). Therefore, the potential temporal variations in community structure remain an unresolved issue. Recently, some studies have analysed the structure of parasite communities in the same host, considering the variability in time and/or space (Carney and Dick, 2000; Poulin and Valtonen, 2002; Timi and Poulin, 2003; Vidal-Martinez and Poulin, 2003, Zelmer et al. 2004; González and Poulin, 2005). Those studies have yielded contradictory results; therefore, additional investigations are required to determine whether the observed patterns are consistent on a temporal scale.
Nested subset analyses are valuable descriptive tools for revealing ecologically meaningful non-random patterns, and are useful exploratory tools for suggesting mechanisms that may structure a particular community (Worthen, 1996). A nested pattern has been defined as a departure from a random association of species in which species that compose a depauperate island community constitute a proper subset of those species inhabiting richer islands (Patterson and Atmar, 1986). Nestedness has been well documented for parasites and has been used extensively to test for non-random patterns among species assemblages (Simková et al. 2001; Timi and Poulin, 2003; Zelmer et al. 2004; González and Poulin, 2005). Nestedness analyses can be used to assess the predictability of the parasite community structure in both space and time (Poulin and Valtonen, 2002).
In this study we examined the ectoparasite fauna of 2 related fish hosts (Sebastes capensis and Helicolenus lengerichi) from the southern Chilean coast to compare, through multivariate analyses, the degree of similarity of the ectoparasite fauna of these fish hosts at both component and infracommunity levels. Then, we determined, based on nested subset analyses, whether their ectoparasite infracommunities are structured. Lastly, we examined the temporal repeatability of ectoparasite community structure in these fish hosts. The scorpaenids, S. capensis and H. lengerichi, are the only species belonging to these genera found along the southeastern Pacific coast (Kong, 1985; Eschmeyer, 2000). These species are morphologically similar, but their bathymetric ranges differ. S. capensis inhabits shallow waters, from depths of 20 m to 150 m, and H. lengerichi inhabits deeper waters, between 50 m and 300 m in depth (Pequeño, 2000). Taking into account the characteristic of these hosts, it is hypothesized that similarity and structure, if any, of their ectoparasite communities can be expected as a consequence of their close phylogenetic relationship and habitat (bathymetric) overlap.
MATERIALS AND METHODS
From October to December 2001 and November to December 2002, and in April 2003 and September 2004, 189 specimens of S. capensis and 101 specimens of H. lengerichi, were examined. Fishes were captured as by-catch in the ray (Dipturus chilensis) fishery during 2001, 2002 and 2004; and by hand line during 2003 from Valdivia (40 °S). The fishes were captured from 40 m to 250 m depth.
Fishes were transported to the laboratory, identified according to Eschmeyer (2000), and by their otolith morphology. The fish were measured (total length (TL), precision 0·5 cm), and sexed macroscopically. The skin, fins, eyes, mouth, and gills were examined for parasites. The collection of parasites followed the traditional parasitological techniques outlined by Pritchard and Kruse (1982). The parasites were fixed in alcohol (70%), and identified using specialized literature (see González and Acuña, 1998).
For each parasite species, abundance and prevalence were estimated according to the protocol of Bush et al. (1997). Parasite richness (number of parasite species per host) and the similarity index of Bray Curtis (following log (n+1) transformation of mean abundance data) were calculated at infra- and component levels, respectively.
Due to the non-normal distribution of the data, non-parametric tests were used (Zar, 1999). For each host fish, the sizes of the examined specimens and species richness per host among different sampling years were compared using the Kruskal-Wallis test. Spearman correlations (rs) were used to evaluate the association between fish size and species richness, and between fish size and abundances of each parasite species.
At the infracommunity level, patterns of similarity in ectoparasite fauna composition, both among years and between hosts, were investigated with multivariate discriminant analyses, using log (n+1) transformed abundance data for each parasite species. At the component community level, correspondence analyses were used to evaluate the association between all of the parasite species and the year of sampling (Digby and Kempton, 1987). The analyses were performed using the software Statistica 6.0.
Nested subset analyses were carried out for ectoparasite infracommunities for each fish host in the different years. The matrix “temperature” (T) proposed by Atmar and Patterson (1993) was calculated, using the Nestedness Temperature Calculator Program (NTCP, Atmar and Patterson, 1995). For each community of parasites, the observed matrix temperature was compared with the T values of 1000 randomly generated presence-absence matrices produced with Monte-Carlo simulations. The statistical probability of the observed pattern was given by the proportion of simulated T values that were lower than or equal to the observed T value and was used as a measure of the departure from the structure expected under random assembly (Guégan and Hugueny, 1994). A P value <0·05 indicates communities that are significantly nested, whereas P values >0·95 characterize significantly anti-nested patterns (Vidal-Martinez and Poulin, 2003).
RESULTS
Parasite community of Sebastes capensis
A total of 10 ectoparasite species was recovered from this host. During the years 2001, 2002 and 2003, 95%, 98% and 100% of the fish, respectively, were parasitized by at least 1 parasite species. The species richness per host varied between 1 and 7 species (X=3·0; S.D.=1·34). The most prevalent parasite species were Microcotyle sp. and Lepeophtheirus chilensis through all these years (Table 1). None of the abundances of ectoparasite species were significantly correlated with fish size (P>0·05 for all species).
Table 1. Mean abundances and prevalence (%) of the ectoparasites recorded on Sebastes capensis from Valdivia (40 °S) during the years 2001, 2002 and 2003 (S=skin, G=gill, M=mouth.)

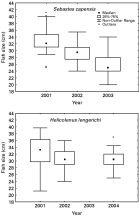
The size of S. capensis varied between 20 cm and 41 cm TL during the years of sampling (Fig. 1). Significant differences in fish sizes among years were recorded (H(2,189)=100·69; P<0·001). Also, there were significant differences in the parasite species richness per host among years (H(2,189)=24·90; P<0·001). The ectoparasite species richness per host was significantly correlated with host size during 2003 (rs=0·32; P=0·007; n=67), but there was no correlation between species richness per host and fish size during 2001 (rs=−0·07; P=0·55; n=61) and 2002 (rs=−0·15; P=0·23; n=61).

Fig. 1. Distribution of fish size of Sebastes capensis and Helicolenus lengerichi during sampling years.
Parasite community of Helicolenus lengerichi
A total of 9 ectoparasite species was recorded in H. lengerichi. During 2001, 2002 and 2004, 100%, 81%, and 100% respectively, of the specimens of this host were parasitized by at least 1 parasite species. The species richness per host varied between 1 and 5 species (X=2·0; S.D.=1·24). The most prevalent parasite species were Microcotyle sp. (Microcotyle specimens from both hosts belong to the same undescribed species), Juanetia continentalis, and Interniloculus chilensis (Table 2). In this host, the abundances of Gnathia sp. (rs=0·207; P=0·03; n=101), Microcotyle sp. (rs=0·214; P=0·03; n=101) and I. chilensis (rs=0·218; P=0·02; n=101) were significantly correlated with fish size.
Table 2. Mean abundances and prevalence (%) of the ectoparasites recorded on Helicolenus lengerichi from the Valdivia coast (40 °S) during the years 2001, 2002 and 2004 (S=skin, G=gill, M=mouth.)

The sizes of H. lengerichi varied between 21·2 cm and 39·7 cm during the studied years (Fig. 1). There was no significant difference in fish sizes between years of sampling (H(2,101)=4·835; P=0·089). There were significant differences in the parasite species richness per host between years (H(2,101)=27·18; P<0·001). The ectoparasite species richness per host was significantly correlated with host size during 2002 (r=0·34; P=0·009; n=56), but there was no correlation between species richness per host and fish size during 2001 (r=0·40; P=0·08; n=20) or during 2004 (rs=0·33; P=0·10; n=25).
Similarity of the ectoparasite community within and between fish hosts
The infracommunities of S. capensis harboured higher total numbers of parasite individuals (U=6,459; P<0·001), and showed higher species richness (U=5,777; P<0·001) than the infracommunities of H. lengerichi. The similarity between infracommunities within the same host among years, and between infracommunities of both hosts (considering all study periods) were evaluated using multivariate discriminant analyses. In addition to abundance (previously log(n+1) transformed), the year and host size were also considered as potential explanatory variables. The infracommunities of S. capensis showed significant differences in parasite abundances among years (F(20,354)=25·40; P<0·0001; Wilks' Lambda=0·168) (Fig. 2). Eighty-three percent of infracommunities of this host were correctly assigned in each sampling year. The discriminant variables (those with highest F- to remove) were abundances of Gnathia sp. and C. cheilodactylus, and fish size. Likewise, the infracommunities of H. lengerichi showed significant differences in parasite abundances among years (F(12,186)=9·94; P<0·0001; Wilks' Lambda=0·361) (Fig. 3). Seventy-four percent of infracommunities of this host were correctly assigned at each sampling year. The discriminant variables were the abundances of I. chilensis and Microcotyle sp. The multivariate discriminant analysis considering the variables: host species, host size and the abundances (log(n+1) transformed) of each of the parasite species showed significant differences between infracommunities of each host species (F(12,277)=28·438; P<0·0002; Wilks' Lambda=0·448). Overall, 89% of the infracommunities were correctly assigned to their respective hosts. The discriminant variables corresponded to those parasite species whose abundances and prevalences are significantly different between fish hosts: J. continentalis, I. chilensis, and L. chilensis (see Tables 1 and 2).

Fig. 2. Discriminant analysis for infracommunities of Sebsates capensis during years 2001, 2002 and 2003 using abundances of all parasites recovered during each year of sampling.

Fig. 3. Discriminant analysis for infracommunities of Helicolenus lengerichi during years 2001, 2002 and 2004 using abundances of all parasites recovered during each year of sampling.
At the component community (CC) level, S. capensis and H. lengerichi shared 60% of their parasites. The Bray-Curtis index of similarity of CC composition among years varied between 60% and 81% for S. capensis, and between 63% and 74% for H. lengerichi. Of the shared ectoparasite species 3 are specific to these scorpaeniformes: the copepod J. continentalis, and the monogeneans I. chilensis and Microcotyle sp. The correspondence analyses considering all parasite species (10 and 9 species, respectively) showed that in both hosts there were significant differences among years in their parasite prevalences (S. capensis: X2=178·88; D.F.=18, P<0·001; H. lengerichi: X2=117·26, D.F.=16, P<0·001). In S. capensis the differences were produced by the samples of 2003. In this year U. australis and C. cheilodactylus were most prevalent. In the case of H. lengerichi, the difference resulted mainly from the high prevalence of I. chilensis during 2004 (Fig. 4). Likewise, multiple correspondence analyses for component communities of each host considering all parasite prevalences and sampling years showed significant differences between hosts: S. capensis was strongly associated with the parasites L. chilensis, C. cheilodactylus, N. melleni and U. australis, while H. lengerichi was associated with their specific parasites: I. chilensis and J. continentalis (Fig. 4). Thus, although the taxonomic composition of parasites was similar during the studied years, the most prevalent species changed over time.

Fig. 4. Multiple correspondence analysis for the parasite component communities of Sebastes capensis and Helicolenus lengerichi using prevalence of all parasites recovered during each year of sampling.
Temporal nestedness analyses for each fish host
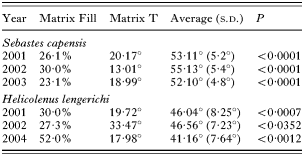
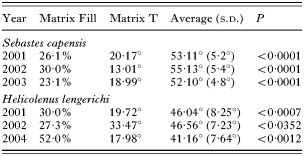
Significant nestedness was found in the ectoparasite infracommunities of S. capensis and H. lengerichi during all years of sampling (Table 3), indicating that common species are found in infracommunities of varying richness, but rare species mainly occur in more diverse infracommunities, and that the ectoparasite community structure departs from randomness through all years of sampling.
Table 3. Summary of the results of nestedness analyses within each ectoparasite component community of Sebastes capensis and Helicolenus lengerichi from the Valdivia coast (40 °S) for different years (P<0·05 indicates nested communities. Average=mean of 1000 simulations matrix, and (S.D.)=standard deviation.)
DISCUSSION
To describe and explain patterns of community structure is one of the central themes in ecology and in fish parasite communities remain an unresolved issue. In this context, the temporal predictability of the parasite communities in fish has not been extensively evaluated because almost all studies are carried out in a short period of time (Poulin and Morand, 2000; Poulin and Valtonen, 2002). Some studies of parasite communities on a temporal scale have evaluated the similarity of infracommunities in terms of parasite abundances, prevalences and species richness, mainly focusing upon the endohelminth fauna of the hosts (e.g. Balboa and George-Nascimento, 1998; Garcías et al. 2001, Karvonen et al. 2005, but see Bagge et al. 2004). The endohelminth communities of fish can be determined by the feeding habits of the hosts (e.g. specialist versus generalist predators) and their ontogenetic changes, as well as by the availability of different prey species (intermediate hosts) in a given environment (Poulin, 1995), but the transmission of ectoparasites is not affected by such factors. The ectoparasite communities are affected by host habitat, host behaviour (e.g. migratory or sedentary habits), host density, and by environmental characteristics (e.g. depth and temperature) (Poulin, 1995; Oliva et al. 2004). Fish ectoparasite infracommunities represent colonization-dominated systems (Rohde et al. 1995). In this study, although the temporal scale might still be restricted, the ectoparasite composition of S. capensis and H. lengerichi showed between 60 and 80% of taxonomic similarity among years respectively, but significant differences were evident in the abundances and prevalences of their ectoparasite species. For S. capensis, the differences in these community descriptors can be explained either because in 2003, fish were captured in shallow water (25 m) and/or in early Autumn, whereas the rest of the samples were captured in Spring. The copepods L. chilensis and C. cheilodactylus and their hyper-parasite U. australis can be most abundant in shallow water as these ectoparasites are also commonly present in other littoral fishes (González and Poulin, 2005). Additionally, seasonal variations in the abundances and prevalences of copepods of these genera have been reported (Boxshall, 1974; Schram et al. 1998). In the case of H. lengerichi the high intensity of infection of I. chilensis during samples of 2004 is responsible for the differences observed among years. Therefore, parasite abundance is not a useful tool to predict fish infracommunities due to their variability.
In spite of the above-mentioned difficulties, during all the years of study a nestedness pattern was observed in infracommunities of both hosts; that is, rare species mainly occur in more diverse infracommunities, which suggests that the ectoparasite communities of these hosts are structured and might be predictable in terms of specific composition. Rohde et al. (1998) concluded that parasite assemblages in marine fish were commonly unstructured and unpredictable. However, other studies have shown that an increase in parasite species richness did not occur at random but followed a predictable pattern of parasite infracommunity structure (Guégan and Hugueny, 1994; González and Poulin, 2005). Rohde et al. (1998) indicated that nested patterns in fish parasite assemblages have been found only because fish samples include both juvenile and adult hosts, and considered that a nested pattern is merely a consequence of an increased parasite diversity associated with ontogeny of the host. Our data include only adult fish, avoiding the influence of host ontogeny suggested by Rohde et al. (1998). The analysis of parasite communities considering fish of different developmental stages is likely to generate apparent nestedness because of differential colonization probabilities among parasites (Poulin and Valtonen, 2001; Vidal-Martinez and Poulin, 2003). This assumption can be applied to ectoparasite infracommunities because larger fish present more space to be colonized by new ectoparasite species (Poulin, 1995). In this study, a significant correlation between fish size and species richness was observed only for S. capensis during 2003 and H. lengerichi during 2002. Species richness was not correlated with fish size during the other studied years; in these years the examined fish were larger and covered a wider host range size. However, these infracommunities were not the richest. Therefore, in these hosts, larger fish do not harbour infracommunities richer than those of smaller fish, and the observed nested patterns are not influenced by fish size.
The nestedness in ectoparasite communities of marine fish has been explained as a consequence of epidemiological processes that in turn depend of the demographic characteristics of each parasite species in an assemblage (Morand et al. 2002). Thus, a nested pattern can be the result of different factors that affect the probability of a given host being infested by a particular parasite. In this sense, host biology may be a key determinant of parasite infracommunity structure (Zelmer and Arai, 2004; González and Poulin, 2005). The rockfish S. capensis is an abundant and sedentary species, without migratory movements, inhabiting the rocky subtidal zone, where it waits motionless in order to capture prey (Moreno, 1981; Barrientos et al. 2006). Individual fish (between 20 cm and 35 cm TL) should occupy a similar and restricted habitat, ensuring the recruitment of the same parasite species in individual hosts of these sizes. Thus, the gregarious and sedentary habits of this fish species (added to their abundance) should favour a richer and predictable ectoparasite community structure, through direct contact between individual hosts. On the other hand, H. lengerichi is a fish species distributed across a broader depth range (from 50 m to 300 m depth), which could allow wide-ranging movements of individual fish. So, it is also likely that larger fish occupy a different habitat (deeper water) than smaller fish (Massutí et al. 2001). This differential use of habitat could affect the colonization rate of ectoparasites in this host and generate the observed nested patterns (Guégan and Hugueny, 1994).
Exchanges of parasite species may occur over evolutionary time between phylogenetically related host species, and therefore related hosts living in the same geographical area could harbour richer parasite communities, sharing similar parasites (Kennedy and Bush, 1994). At the component community level, the ectoparasite taxonomic composition of S. capensis along the southern Chilean coast is similar to those recorded along the northern (González and Acuña, 1998), and central Chilean coasts (González and Poulin, 2005). Fish from the southern coast, inhabiting deeper waters, harbour only 1 additional (although rare) parasite species, the copepod Juanetia continentalis, a species originally described from H. lengerichi (Villalba and Fernández, 1985). However, the species richness of the infracommunities of S. capensis on the southern Chilean coast is higher than those recorded on the northern and central Chilean coasts. Similarly, the parasite species richness of H. lengerichi on the southern coast is higher than those recorded from central Chile (George-Nascimento and Iriarte, 1989). On the southern coast (40 °S), at the component community level these rockfish species share between 60 and 80% of their ectoparasite species. Then, the higher species richness per host and similarity in the ectoparasite faunas of both host species in this geographical area might be explained because the habitats of S. capensis and H. lengerichi overlap between 50 m and 150 m in depth, which makes possible the interchange of specific ectoparasites (e.g. Microcotyle sp., I. chilensis, J. continentalis) as well as generalist species (e.g. L. chilensis, C. cheilodactylus, Gnathia sp.) between these rockfish hosts. Additionally, S. capensis inhabits the rocky-littoral zone with diverse fish species (e.g. Prolatilus jugularis, Eleginops maclovinus, Cheilodactylus variegatus, Chromis crusma) that also harbour the copepods C. cheilodactylus and L. chilensis (Fernández and Villalba, 1986), thus facilitating the exchange of these caligid parasites between these fish hosts. On the contrary, H. lengerichi inhabits the deeper water, near the bottom (demersal), where a higher diversity of fish species is present (e.g. Merluccius spp., Genypterus spp.) (Pequeño, 2000). It is well known that these fish host harbour specific ectoparasite species that are not found in Scorpaenids. Therefore, for H. lengerichi the exchange of ectoparasite species among different fish hosts does not play an important role in the composition or species richness of their ectoparasite community.
According to Kennedy and Bush (1994), generalist parasite species should reduce the predictability of the parasite community of a host species, whereas host-specific parasites should produce predictable parasite communities. Host specificity determines whether a parasite is able to colonize a host and therefore may be a factor influencing the nestedness structure in parasite communities (Matejusová et al. 2000). This argument is supported for parasite infracommunities of H. lengerichi whose specific parasites were predominant during all the years of study. However, it is equally possible that generalist parasites can be evenly distributed between ranges of host species producing predictable parasite communities both within and between these hosts. This seems be the case for S. capensis where both specific and generalist parasites were equally present during the whole period of study. Thus, independent of the degree of specificity of their ectoparasites, the consistent temporal nested patterns observed in their infracommunities support the hypothesis that the ectoparasite communities of these rockfishes are structured and, therefore, might be predictable over time, at least in the southern Chilean coast where they share the same habitat.
We thank Professor Robert Poulin of the University of Otago for his comments and criticism. Thanks also to Julio Lamilla and Roberto Licandeo of the Zoology Department of Universidad Austral de Chile for their help with the collection of fish samples from the ray Fishery. This study was funded by fellowships CONICYT AT-4040092 and D.I.D. of the Universidad Austral of Chile granted to the principal author.