Introduction
Three species of fish-borne trematodes belonging to the Opisthorchiidae family (Trematoda, Platyhelminthes, Digenea) rank 8th on the global list of 24 clinically important foodborne parasites (FAO/WHO, 2014). Adult worms parasitize the biliary tract of mammals, including humans, and cause damage to the hepatobiliary system, with possible serious complications.
The liver fluke Opisthorchis felineus (Rivolta, 1884) is a member of the triad of epidemiologically significant Opisthorchiidae parasites. The geographic range of O. felineus covers a major part of Eurasia from Western Siberia to Southern Europe. Natural foci of opisthorchiasis are active and tend to expand. Prevalence of human infection is highest in Russia, where up to 40 000 cases of O. felineus infection are diagnosed annually according to the official data from the Federal Service for Supervision of Consumer Rights Protection and Welfare (RF Rospotrebnadzor: http://rospotrebnadzor.ru/) [Gosudarstvennyj Doklad (The State Report), 2014]. In countries of the European Union, outbreaks of opisthorchiasis have been recorded (Pozio et al., Reference Pozio, Armignacco, Ferri and Gomez Morales2013; Scaramozzino et al., Reference Scaramozzino, Condoleo, Martini, Bossu, Aquilani, Spallucci, Aquilini and Marozzi2018).
Other members of the triad of epidemiologically significant liver flukes are Opisthorchis viverrini (Poirier, 1886) and Clonorchis sinensis (Loos, 1907). These flukes are common in Southeast Asia and several countries of the Far East and in endemic areas are considered the main risk factors of bile duct cancer, cholangiocarcinoma (CCA) (IARC, 2012). Opisthorchis felineus also has carcinogenic potential (Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017); however, the epidemiology of CCA in the foci of opisthorchiasis felinea is not well characterized. Nevertheless, data from long-term observations indicate that among people living in the O. felineus endemic area, CCA cases are registered much less frequently than among residents of O. viverrini or C. sinensis endemic areas (Mairiang et al., Reference Mairiang, Chaiyakum, Chamadol, Laopaiboon, Srinakarin, Kunpitaya, Sriamporn, Suwanrungruang and Vatanasapt2006; Fedorova et al., Reference Fedorova, Kovshirina, Kovshirina, Fedotova, Deev, Petrovsky, Filimonov, Dmitrieva, Kudyakov, Saltykova, Mikhalev, Odermatt and Ogorodova2016; Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016; Petrova et al., Reference Petrova, Starinskiy, Gretsova, Shahzadova and Samsonov2019). What is behind this difference? Is carcinogenic potential of O. felineus weaker than that of O. viverrini and C. sinensis or do other risk factors exist responsible for the comparatively high CCA prevalence in Southeast Asia? Can we compare pathogenicity of the 3 species and is there any difference in pathogenesis between opisthorchiasis and clonorchiasis?
It is not yet possible to answer these questions. The main reason, in our opinion, is the lack of comparative studies conducted on 1 model within 1 experiment and carried out without any research biases. There are only a few publications showing that histological changes characteristic of O. felineus and O. viverrini infections have much in common but are more pronounced and develop earlier in O. felineus-infected mammals than in O. viverrini-infected ones (Lvova et al., Reference Lvova, Tangkawattana, Balthaisong, Katokhin, Mordvinov and Sripa2012). Haemozoin, a haem detoxification product potentially involved in the induction of proinflammatory and immunomodulatory responses, was found in the gut of adult O. felineus and C. sinensis but not in O. viverrini (Lvova et al., Reference Lvova, Zhukova, Kiseleva, Mayboroda, Hensbergen, Kizilova, Ogienko, Besprozvannykh, Sripa, Katokhin and Mordvinov2016). These data indicate possible differences in the scenarios of the disorders caused by O. felineus and O. viverrini.
In this review, we focus on identifying similarities and differences among the 3 liver fluke infections; in particular, we concentrate on climatic differences among the endemic areas, characteristics of the life cycle, the range of intermediate hosts, clinical symptoms and morbidity of liver fluke infections in humans, carcinogenic potential and liver fluke genomic and transcriptomic features. We believe that this discussion will facilitate comparative studies on clinically important opisthorchids. Results of such research are needed for understanding the molecular mechanisms of liver fluke pathogenicity and helminth-associated carcinogenesis.
Materials and methods
Data on the absolute number of registered infections caused by O. felineus and the incidence of this infection per 100 000 people per year were retrieved from state annual reports of regional offices of Rospotrebnadzor (Russia) [Gosudarstvennyj Doklad (The State Report), 2014]. Annual reports on O. felineus infection are available on webpages of regional offices of Rospotrebnadzor (Russia). Data on cancer incidence, which provide both absolute numbers and the number of cases per 100 000 population per year in the Russian Federation, were analysed on the basis of annual reports issued by the Russian Center for Information Technology and Epidemiological Research in Oncology for 2011–2013 and 2017 (Kaprin et al., Reference Kaprin, Starinskiy and Petrova2013; Petrova et al., Reference Petrova, Starinskiy, Gretsova, Shahzadova and Samsonov2019). In accordance with the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), code C22 was chosen (cancer of the liver and intrahepatic bile ducts).
A large body of data has accumulated in the past several years on biomedical issues associated with C. sinensis and O. viverrini infection as well as the biology of these liver flukes. The information on O. felineus and opisthorchiasis felinea is still much less abundant. This review addresses the issues on these 3 species. Accordingly, we applied this filter to restrict the published data about C. sinensis and O. viverrini by the availability of corresponding published research on O. felineus.
A literature search for the other materials was conducted via a combination of a thorough manual search and searches in bibliographic databases PubMed, Elibrary (http://elibrary.ru/defaultx.asp) and Google Scholar (http://scholar.google.com/). There were no restrictions on publication date. When selecting bibliographic sources, we primarily focused on finding differences among the 3 species of opisthorchids, in particular, differences in the climate, geographical locations, life cycle, range of intermediate hosts, morbidity of the diseases, carcinogenicity, genome and transcriptome. We tried to cover all publications containing data from comparative analysis of liver flukes O. felineus, O. viverrini and C. sinensis. The following keywords were used: liver flukes, O. felineus, O. viverrini, Clonorchis sinensis, comparative studies, reservoir animals, opisthorchiasis, clonorchiasis, prevalence, clinical symptoms, morbidity, liver cancer, bile duct cancer, CCA, animal models, genomics and transcriptomics.
Full-length articles published in peer-reviewed journals were selected for the review. All cited articles included information about a study location and description of materials and methods including statistics. Articles about animal models had to contain data from experiments with a concurrent comparison of uninfected and infected animals along with pathomorphological images.
Results
A historical account
The liver fluke O. felineus is also called the ‘European liver fluke’. Indeed, the first detailed description of O. felineus was made by Italian scientist S. Rivolta in 1884, who discovered these parasites in cats and dogs in Pisa (Italy) and named them ‘Distoma felineum’. The existing species name and systematic position of the parasite were presented by R. Blanchard in 1885.
In humans, O. felineus was discovered in Western Siberia in 1891 by K.N. Vinogradov. He described the parasite in detail, considering it a new species, and named it the Siberian fluke: Distomum sibiricum (Vinogradov, Reference Vinogradov1892). This explains another rather common name of O. felineus: the Siberian liver fluke (Beer, Reference Beer2005; Schuster, Reference Schuster2010; Sripa et al., Reference Sripa, Tesana, Yurlova and Nawa2017).
The life cycle
The life cycle of O. felineus includes 5 stages, which sequentially develop in 2 intermediate and 1 definitive host (Fig. 1). The only infectious stage for the definitive host, humans and other mammals, is metacercaria (Figs 1 and 2A). Infection occurs after ingestion of raw or undercooked freshwater fish (the second intermediate host) infested with the metacercaria. In the gastrointestinal tract, newly excysted metacercariae are released and through the Vater ampulla migrate into the hepatobiliary system. After approximately a month, juvenile worms develop into adult worms (Fedorov et al., Reference Fedorov, Babueva and Karpenko1989; Beer, Reference Beer2005).
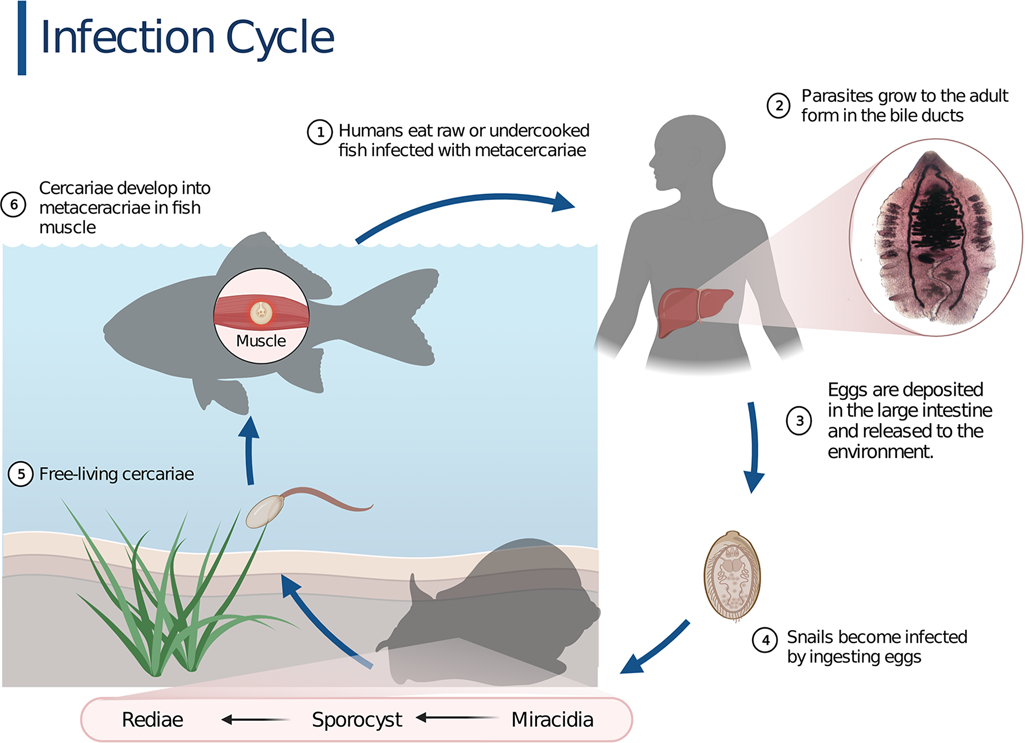
Fig. 1. The infection cycle of Opisthorchis felineus. The life cycle is typical for trematodes with 2 intermediate hosts (freshwater Bithynia spp. snails and Cyprinidae fish) and 1 definitive host (mammals including humans). Created with BioRender.com.
The adult liver fluke O. felineus (Figs 1 and 2C) is a hermaphrodite. In the definitive host, O. felineus individuals self-fertilize and produce a large number of eggs: miracidia enclosed in a tight shell (Figs 1 and 2B) (Fedorov, Reference Fedorov1979; Beer, Reference Beer2005). The eggs are excreted into the environment with feces.
To continue the life cycle, the eggs of the parasite (Fig. 2B) need to be carried to a freshwater reservoir and be eaten by the first intermediate host, Bithynia spp. snails (Bithyniidae family) (Fedorov, Reference Fedorov1979; Beer, Reference Beer2005).
In the digestive tract of a snail, miracidia penetrate the body of the mollusc through the intestinal wall and turn into sporocysts (Fedorov, Reference Fedorov1979). As a result of parthenogenesis, sporocysts can form their copies and motile larvae, rediae. The latter give rise to the next life stage, cercariae, the only free-living life stage. Cercariae emerge from molluscs into water and attach to the second intermediate host, freshwater Cyprinidae fish, penetrate into muscle and connective tissue, encyst and transform into metacercariae (Fedorov, Reference Fedorov1979; Beer, Reference Beer2005). It is important to note that fish species susceptible to O. felineus parasitism are either commercial species or recreational fishing targets. The highest infection rate is observed in the ide Leuciscus idus, common dace L. leuciscus and sunbleak Leucaspius delineatus (Sidorov, Reference Sidorov1983; Beer, Reference Beer2005; Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016).
Traditionally sun-dried fish, just as cold-smoked ones contain live metacercariae, and such fish delicacies can be a source of infection (Sidorov, Reference Sidorov1983). Major risk factors of human opisthorchiasis are the consumption of stockfish [odds ratio (OR) from multivariate analysis (mOR) 3.2, P < 0.001], smoked fish (mOR 1.5, P < 0.001), frozen fish (mOR 1.6, P < 0.001) and raw fish (mOR 1.4, P = 0.02) (Beer, Reference Beer2005; Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020). A habit of eating raw freshwater fish was found to be a major contributing factor for the persistence of clonorchiasis in Korea (mOR 3.2) (Park et al., Reference Park, Na, Cho, June, Cho and Lee2014) and of opisthorchiasis viverrini in Thailand (ORadj = 5.17) (Chudthaisong et al., Reference Chudthaisong, Promthet and Bradshaw2015). Moreover, a similar habit of family members (ORadj = 3.25) and unsafe disposal of food waste (ORadj = 2.1) are significant contributing factors in Thailand (Chudthaisong et al., Reference Chudthaisong, Promthet and Bradshaw2015).
The life cycles of C. sinensis and O. viverrini are similar to that of O. felineus and also include 2 intermediate developmental stages (freshwater gastropod molluscs and fish) and a definitive (usually piscivorous mammalian) host (Choi, Reference Choi1984; Harinasuta and Harinasuta, Reference Harinasuta and Harinasuta1984; Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005). Nevertheless, both intermediate developmental stages of the opisthorchids take place under different environmental conditions, and this state of affairs undoubtedly affects the basic metabolic characteristics of liver flukes. The following sections briefly describe natural foci of opisthorchiasis and features of the O. felineus life cycle in Northern Eurasia.
Opisthorchiasis foci
Foci of opisthorchiasis felinea have been found in many European countries and on the territory of the former USSR (Beer, Reference Beer2005; Mordvinov and Furman, Reference Mordvinov and Furman2010; Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016). The largest endemic area has been identified in Western Siberia in the basin of the Ob and Irtysh rivers, an area of ~3 million km2, abounding with swamps and lakes. All the factors necessary to maintain the life cycle of O. felineus are present in abundance here (Fedorov, Reference Fedorov1979; Fedorov et al., Reference Fedorov, Babueva and Karpenko1989; Beer, Reference Beer2005). The prevalence of opisthorchiasis in the population of this area remains extremely high: 50–80% of rural residents were infected with O. felineus according to the data from the 1950 to 1980s (Sidorov, Reference Sidorov1983; Beer, Reference Beer2005; Serbina, Reference Serbina2012). These data were recently confirmed in a community-based cross-sectional study in 2016–2017 (Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020), in particular, 60.2% of the 600 examined individuals were found to be infected. The vast majority of the infected individuals had mild infection (70.4%), but 3.1% of them had severe infection (Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020).
The second largest endemic area is located in the Dnieper River basin in the north-east of Ukraine (Zavoikin et al., Reference Zavoikin, Beer, Pliushcheva, Sholokhova and Nikiforova1989; Chemich et al., Reference Chemich, Ilina, Ilina and Sholohova2014). The prevalence of human opisthorchiasis is 21.6 cases per 100 000 people (Chemich et al., Reference Chemich, Ilina, Ilina and Sholohova2014). Another focus of opisthorchiasis is active and located in the basin of the Volga and Kama rivers. According to official data, the prevalence is 2.51–14.00 cases per 100 000 people.
Closer to the Ural Mountains, the prevalence of infection increases and reaches 22–52 cases per 100 000 people. In the 1960s, the problem of opisthorchiasis in this area was absent (Sidorov, Reference Sidorov1983). Nonetheless, already in the 1970s, frequent cases of infection in cats and humans were recorded (Kanunnikova and Solovyh, Reference Kanunnikova and Solovyh2007). Thus, it can be concluded that new territories endemic for opisthorchiasis have emerged, which may be due to the expansion of the major foci.
Over the past 20 years, quite a few original studies and literature reviews have been published confirming the existence of natural foci of opisthorchiasis in Europe (Schuster, Reference Schuster2010; Petney et al., Reference Petney, Andrews, Saijuntha, Wenz-Mücke and Sithithaworn2013; reviewed in Pozio et al., Reference Pozio, Armignacco, Ferri and Gomez Morales2013). In particular, in Portugal, Spain, Italy, Switzerland, Germany, the Netherlands, France and Belarus, infected red foxes, cats, muskrats and dogs have been found (Shimalov, Reference Shimalov2001; reviewed by Pozio et al., Reference Pozio, Armignacco, Ferri and Gomez Morales2013; Skripova, Reference Skripova2013; Schuster et al., Reference Schuster, Specht and Rieger2021). In addition, information on the detection of O. felineus in 3 species of mustelids, the Eurasian otter, American mink and European polecat in Lithuania, was recently published (Nugaraitė et al., Reference Nugaraitė, Mažeika and Paulauskas2019). The only European territory free from O. felineus is probably the Scandinavian Peninsula and the British Isles; we could not find any references to relevant studies.
In Italy, cases of human opisthorchiasis are recorded every now and then and are explained by amateur fishing and dishes from undercooked fish (Scaramozzino et al., Reference Scaramozzino, Condoleo, Martini, Bossu, Aquilani, Spallucci, Aquilini and Marozzi2018). From time to time, patients who get ill with opisthorchiasis felinea outside the location of permanent residence are admitted to hospitals in EU countries (Tselepatiotis et al., Reference Tselepatiotis, Mantadakis, Papoulis, Vassalou, Kotsakis and Samonis2003). Nevertheless, in general, the circulation of O. felineus in Europe occurs most likely without the participation of humans.
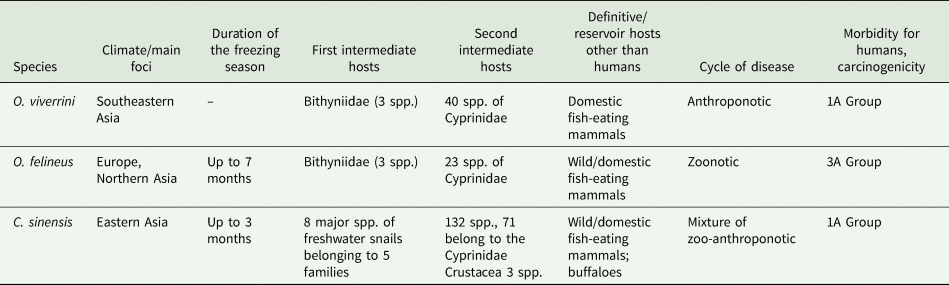
The endemic area of O. felineus differs significantly from those of O. viverrini and C. sinensis. It covers several climatic zones, radically differing in the duration and temperature of the winter freezing period (Table 1). In contrast, the endemic area of O. viverrini located in Southeast Asia does not go beyond the boundaries of 1 climatic zone. The endemic area of C. sinensis is rather wide from north to south, but its northern border is located at much lower latitudes than that of O. felineus, and in terms of the climatic range, it occupies an intermediate position between the areas of the 2 other liver flukes (Table 1). In addition, the reservoir hosts, which maintain the circulation of O. felineus throughout the whole endemic area, are wild mammals living near rivers, and we should keep in mind that the human population density is low here. In contrast, the foci of O. viverrini are considered anthropogenic, and C. sinensis foci have a mixed origin: zoonotic and anthropogenic (Petney et al., Reference Petney, Andrews, Saijuntha, Wenz-Mücke and Sithithaworn2013).
Table 1. Biological similarities and differences among the liver flukes Opisthorchis felineus, O. viverrini and Clonorchis sinensis
Spp., species.
Features of the life cycle of O. felineus
Despite the general similarity of the life cycles of the 3 liver fluke species, it is worth noting the differences in the temperature of development of larval stages of the parasites as well as in the list of intermediate and definitive hosts.
Opisthorchis felineus eggs (Fig. 2B) can overwinter in lake water at 0–5°C, and 42% of eggs can survive for up to 160 days (Fedorov, Reference Fedorov1979). The eggs may maintain viability for 10 h at temperatures ranging from −2 to 3°C (Drozdov, Reference Drozdov1972).
Bithynia spp., the first intermediate host, become active at water temperatures above 15°C, which are typical for June (Fedorov, Reference Fedorov1979). After 30–48 days, sporocysts mature and rediae are formed. Nevertheless, the formation of cercariae does not occur by autumn. Development is inhibited due to the period of winter diapause of snails for the freezing season (Table 1). The diapause of molluscs lasts from September to April (Fedorov, Reference Fedorov1979). For the winter, molluscs burrow into silt at the bottom of a water reservoir.
The first cercariae appear in May next year. Mature cercariae are motile, emerge from molluscs in bright sunny weather and retain viability for 48–70 h (Beer, Reference Beer2005; Pelgunov et al., Reference Pelgunov, Kazakov, Fedorov, Bocharova, Sous, Pavlov and Mochek2006; Serbina, Reference Serbina2012). The morphogenesis of O. felineus metacercariae in fish continues from 3 weeks to 2 months (Fedorov, Reference Fedorov1979; Serbina, Reference Serbina2012) (Fig. 2A).
The optimum temperature for the development of O. viverrini and C. sinensis in snails differs from that of O. felineus. The highest level of infection of snails with O. viverrini eggs under experimental conditions is observed at temperatures above 30°C. When the water is cooled to 16°C, the level of infection in snails decreases 4–5-fold (Prasopdee et al., Reference Prasopdee, Kulsantiwong, Piratae, Khampoosa, Thammasiri, Suwannatrai, Laha, Grams, Loukas and Tesana2015). It has been experimentally established that at a temperature of 24–37°C, C. sinensis goes through all stages of development in molluscs in ~3 months. At temperatures of 20°C and below, the emergence of cercariae has not been detected (Liang et al., Reference Liang, Hu, Lv, Wu, Yu, Xu and Zheng2009).
The 3 liver flukes differ among themselves and in the range of intermediate hosts (Table 1). According to the mainstream opinion, only snails of the family Bithyniidae are susceptible to O. felineus and O. viverrini infestation (Serbina, Reference Serbina2012; Petney et al., Reference Petney, Andrews, Saijuntha, Wenz-Mücke and Sithithaworn2013). Nonetheless, species (Melanoides tuberculatus and Maningila sp.) belonging to other families were described also as the first intermediate host of O. viverrini (Doanh and Nawa, Reference Doanh and Nawa2016). The range of the first intermediate hosts of C. sinensis is much wider and includes at least 8 species of freshwater snails, which are representatives of 5 families (Assimineidae, Bithyniidae, Hydrobiidae, Melaniidae and Thiaridae) (Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005). It is noteworthy that except for M. tuberculatus, O. viverrini and C. sinensis infect distinct snail species (Doanh and Nawa, Reference Doanh and Nawa2016).
The second intermediate host for O. felineus is only Cyprinidae fish (Sidorov, Reference Sidorov1983; Beer, Reference Beer2005; Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016). Although O. viverrini also preferentially infects cyprinids (Petney et al., Reference Petney, Andrews, Saijuntha, Tesana, Prasopdee, Kiatsopit and Sithithaworn2018), O. viverrini metacercariae have also been found in the snakehead fish Channa striata from the Channidae family (Thu et al., Reference Thu, Dalsgaard, Loan and Murrell2007) as well as in the climbing perch Anabas testudineus from the Anabantidae family and in the moonbeam gourami Trichopodus microlepis from the Osphronemidae family (Eom et al., Reference Eom, Park, Lee, Sohn, Yong, Chai, Min, Rim, Insisiengmay and Phommasack2015).
The list of fish species susceptible to C. sinensis infestation is much longer. It includes more than 130 species, of which only approximately half of the species belong to the Cyprinidae family. It is known that 3 crustacean species can also serve as the second intermediate host of C. sinensis (Chen et al., Reference Chen, Wen, Lai, Wen, Wu, Yang, Yu, Hide and Lun2013).
Overall, 15 species of wild carnivorous mammals have been identified as definitive hosts of O. felineus (Fig. 2C), namely the wolf (Canis lupus), ermine (Mustela erminea), corsac fox (Vulpes corsac), fox (Vulpes vulpes), arctic fox (Alopex lagopus), muskrat (Ondatra zibethicus), water vole (Arvicola terrestris), otter (Lutra lutra), bear (Ursus arctos), sable (Martes zibellina), Siberian weasel (Mustela sibirica), marten (Martes sp.), steppe polecat (Mustela eversmanii), wild boar (Sus scrofa) and mink (Neovison vison) (Shuteev, Reference Shuteev1977; Safiullin and Shibitov, Reference Safiullin and Shibitov2012; Siben et al., Reference Siben, Domatsky, Fedorova and Klyatsky2019; Schuster et al., Reference Schuster, Specht and Rieger2021). Definitive hosts also include domestic cats and dogs fed raw fish. Nevertheless, the muskrat O. zibethicus is considered the main reservoir of O. felineus infection in Western Siberia (Shuteev, Reference Shuteev1977; Fedorov, Reference Fedorov1979). The muskrat is a medium-sized semiaquatic rodent settling along the banks of rivers, lakes and swamps. Plant materials constitute ~95% of its diet, but muskrats also eat small animals, such as freshwater mussels, frogs, crayfish and fish. The muskrat can get infected after consumption of weakened and dead fish accumulated near the banks of aquatic bodies after overwintering (Fedorov, Reference Fedorov1979; Fedorov et al., Reference Fedorov, Babueva and Karpenko1989).
The relatively low number of reservoir hosts of O. viverrini and C. sinensis is probably associated with the high population density in the endemic foci and scarcity of wild mammals. Of note, cattle (Bos taurus and indicus) are among the definitive hosts of C. sinensis (Petney et al., Reference Petney, Andrews, Saijuntha, Wenz-Mücke and Sithithaworn2013); no documented cases of infection of these mammals with other opisthorchids were found.
Although any fish-eating mammals may be potential definitive hosts of O. viverrini, only a few, especially domestic cats and dogs, are actually known reservoir hosts for this parasite (Tangkawattana and Tangkawattana, Reference Tangkawattana and Tangkawattana2018). The prevalence of O. viverrini infection is higher among cats (30.92%) than dogs (0.20%); accordingly, cats may play an important role in the transmission and maintenance of this disease in Thailand (Aunpromma et al., Reference Aunpromma, Kanjampa, Papirom, Tangkawattana, Tangkawattana, Tesana, Boonmars, Suwannatrai, Uopsai, Sukon and Sripa2016). Screening of wild animals in northeastern Thailand, in particular, macaques, rodents, small residential mammals and fish-eating birds, revealed the absence of O. viverrini infection (Tangkawattana et al., Reference Tangkawattana, Sereerak, Upontain, Tangkawattana and Sripa2021). This finding indicates that wild mammals and birds probably do not serve as alternate reservoir hosts of O. viverrini (Tangkawattana et al., Reference Tangkawattana, Sereerak, Upontain, Tangkawattana and Sripa2021).
Clinical symptoms and morbidity
The clinical picture of opisthorchiasis felinea varies from the absence of symptoms to pronounced manifestation. The incubation period is 2–3 weeks (Yablokov, Reference Yablokov1984; Bronshtein et al., Reference Bronshtein, Zolotukhin, Gitsu, Sabgaida and Parfenov1991). The disease can begin acutely with the appearance of pain in the right hypochondrium, a fever lasting up to 3 weeks, symptoms of toxicity, arthralgia, myalgia, skin involvement, hepatobiliary syndrome, eosinophilia 20–40% against the background of leucocytosis up to 20 000–60 000, bilirubinemia and increased levels of transaminases and alkaline phosphatase (Yablokov, Reference Yablokov1984; Bronshtein et al., Reference Bronshtein, Zolotukhin, Gitsu, Sabgaida and Parfenov1991).
The acute onset of opisthorchiasis is described thoroughly for O. felineus infection (Yablokov, Reference Yablokov1984; Bronshtein et al., Reference Bronshtein, Zolotukhin, Gitsu, Sabgaida and Parfenov1991; Mordvinov and Furman, Reference Mordvinov and Furman2010). Although acute onset of the disease has also been mentioned regarding O. viverrini and C. sinensis infection (Ip et al., Reference Ip, Leung and Cheng1995; Nishiura et al., Reference Nishiura, Tsunoda and Akao2003), there are no data on such a variety of symptoms as that in opisthorchiasis felinea. It seems that the acute onset of the disease is more common during O. felineus infection than during O. viverrini and C. sinensis infections.
The symptoms and signs of chronic opisthorchiasis and clonorchiasis are gastrointestinal (Choi, Reference Choi1984; Harinasuta et al., Reference Harinasuta, Riganti and Bunnag1984; Yablokov, Reference Yablokov1984; Bronshtein et al., Reference Bronshtein, Zolotukhin, Gitsu, Sabgaida and Parfenov1991). The presence of gallbladder stones (mOR = 2.8, P = 0.007) is associated with O. felineus infection (Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020), C. sinensis infection (OR = 7.8) (Choi et al., Reference Choi, Lim, Lee, Lee, Choi, Heo, Choi, Jang, Lee, Kim and Hong2008) and O. viverrini infection (Harinasuta et al., Reference Harinasuta, Riganti and Bunnag1984). Besides, periductal fibrosis and cholangitis are common consequences of all human liver fluke infections (Harinasuta et al., Reference Harinasuta, Riganti and Bunnag1984; Park et al., Reference Park, Na, Cho, June, Cho and Lee2014; Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020).
Cholangiocarcinoma
One of the possible consequences of liver fluke infection is the development of CCA, an aggressive human cancer. Opisthorchis viverrini and C. sinensis are recognized by the International Agency for Research on Cancer (IARC, 2012) as Group 1 biological carcinogens and are classified as major risk factors of CCA in endemic areas (IARC, 2012). Opisthorchis felineus is assigned to Group 3, which combines possible cancer-causing factors for which, according to the IARC data, there is no convincing evidence of oncogenic effects on humans and animals. Indeed, systematic studies on the carcinogenic potential of O. felineus and the association of opisthorchiasis felinea with CCA began relatively recently. Nevertheless, there are some data on the carcinogenic potential of O. felineus.
A comparison of the official data on the incidence of parasitic and oncological diseases in Russia as well as the results of retrospective analysis of autopsy records and medical records confirm the association between the O. felineus infection and liver cancer in residents of Western Siberia (Zubov et al., Reference Zubov, Belikov, Zaĭtseva, Kotrikov and Mezentsev1989; Brazhnikova and Tolkaeva, Reference Brazhnikova and Tolkaeva2002; Brazhnikova and Tskhai, Reference Brazhnikova and Tskhai2004; Fedorova et al., Reference Fedorova, Kovshirina, Kovshirina, Fedotova, Deev, Petrovsky, Filimonov, Dmitrieva, Kudyakov, Saltykova, Mikhalev, Odermatt and Ogorodova2016; Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016). On the other hand, early studies do not always decipher the diagnosis of liver cancer, and additional studies are required for definitive confirmation of the association between O. felineus infection and CCA. More recent studies provide evidence of CCA in patients with chronic opisthorchiasis (Kovshirina et al., Reference Kovshirina, Fedorova, Vtorushin, Kovshirina, Ivanov, Chizhikov, Onishchenko, Ogorodova and Odermatt2019).
To indirectly assess carcinogenic potential of liver flukes, data on the incidence and prevalence of CCA in endemic areas can be used. In particular, regarding O. viverrini, in a hospital-based prospective, case-controlled study of 227 hepatobiliary disease patients with O. viverrini infection, 8 patients (3.5%) developed CCA within 2 years of the follow-up period (Kurathong et al., Reference Kurathong, Lerdverasirikul, Wongpaitoon, Pramoolsinsap, Kanjanapitak, Varavithya, Phuapradit, Bunyaratvej, Upatham and Brockelman1985). Later, however, in a community study based on ultrasound screening of 4154 subjects residing in northeast Thailand, only 0.5% were suspected to have CCA (Mairiang et al., Reference Mairiang, Chaiyakum, Chamadol, Laopaiboon, Srinakarin, Kunpitaya, Sriamporn, Suwanrungruang and Vatanasapt2006).
The overall OR for CCA due to C. sinensis infection is 4.7 (P = 0.000) (Shin et al., Reference Shin, Oh, Lim, Shin, Kong, Jung, Won, Park, Park and Hong2010). Moreover, a case–control study out of Korea showed that C. sinensis infection is significantly associated with increased risk of CCA (OR = 7.3) (Choi et al., Reference Choi, Lim, Lee, Lee, Choi, Heo, Jang, Lee, Kim and Hong2006). In 2016, liver cancer in Korea ranked 5th among the main types of cancer, and in terms of mortality, it took the 2nd place (Jung et al., Reference Jung, Won, Kong and Lee2019).
If we compare these data with the details of cancer incidence and the main types of cancer in Russia, we can notice significant differences of that from Korea and Thailand. In 2017, 6429 cases of liver and intrahepatic bile duct cancer were registered in Russia, which is 1% of the total number of new cancer cases (Petrova et al., Reference Petrova, Starinskiy, Gretsova, Shahzadova and Samsonov2019). Among newly registered cases, this type of cancer ranked 18th (Petrova et al., Reference Petrova, Starinskiy, Gretsova, Shahzadova and Samsonov2019). According to official statistics, the prevalence of liver cancer in 2011–2013 was 4.8 ± 0.2 cases per 100 000 people. The highest rates were noted in the Republic of Sakha and the Tomsk region (14.5 and 9.3 per 100 000 people, respectively) (Kaprin et al., Reference Kaprin, Starinskiy and Petrova2013). The incidence of O. felineus infection was significantly associated with liver and intrahepatic bile duct cancers in Russia in 2011 (r = 0.25; P = 0.02) (Fedorova et al., Reference Fedorova, Kovshirina, Kovshirina, Fedotova, Deev, Petrovsky, Filimonov, Dmitrieva, Kudyakov, Saltykova, Mikhalev, Odermatt and Ogorodova2016). The relatively low carcinogenicity of O. felineus was also evidenced by a cross-sectional study in a rural endemic region, where 600 people were examined with 60.2% prevalence of infection (Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020). Those authors did not find patients with a liver mass or suspected CCA (Fedorova et al., Reference Fedorova, Fedotova, Zvonareva, Mazeina, Kovshirina, Sokolova, Golovach, Kovshirina, Konovalova, Kolomeets, Gutor, Petrov, Hattendorf, Ogorodova and Odermatt2020). Thus, the incidence rates of CCA differ significantly between Western Siberia and Southeast Asia, even though these regions are comparable in the prevalence of liver fluke infection of the population. This observation suggests that the carcinogenic potential of O. felineus is lower than that of O. viverrini and C. sinensis.
Aside from liver fluke infection, the most significant risk cofactors of CCA include cigarette smoking, alcohol consumption and foods containing carcinogenic nitrosamines (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a; Steele et al., Reference Steele, Richter, Echaubard, Saenna, Stout, Sithithaworn and Wilcox2018). Traditional dishes made from fermented fish with a high concentration of nitrosamines are extremely popular in Southeast Asia, and this practice correlates with the high prevalence of CCA in this area (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a). Inhabitants of areas endemic for O. felineus also have traditional dishes made from fermented vegetables; however, in our opinion, the proportion of such products in the diet of the population in Western Siberia is lower than that in Southeast Asia. It is possible that – among other reasons – this is an explanation for the low incidence of CCA in the foci of O. felineus infection.
Animal models
The generally accepted model for studying opisthorchiasis caused by O. felineus and O. viverrini is the Syrian hamster Mesocricetus auratus (Waterhouse, 1839) (Fig. 2D–G) (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a; Mordvinov et al., Reference Mordvinov, Shilov and Pakharukova2017b, Reference Mordvinov, Minkova, Kovner, Ponomarev, Lvova, Zaparina, Romanenko, Shilov and Pakharukova2021; Pakharukova et al., Reference Pakharukova, Pakharukov and Mordvinov2018, Reference Pakharukova, Samsonov, Serbina and Mordvinov2019a, Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b, Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c, Reference Pakharukova, Zaparina, Hong, Sripa and Mordvinov2021; Arunsan et al., Reference Arunsan, Ittiprasert, Smout, Cochran, Mann, Chaiyadet, Karinshak, Sripa, Young, Sotillo, Loukas, Brindley and Laha2019; Zaparina et al., Reference Zaparina, Rakhmetova, Kolosova, Cheng, Mordvinov and Pakharukova2021). These animals are susceptible to infection by liver flukes, which is not surprising because the main reservoir animal of O. felineus is the muskrat, of the Cricetidae family closely related to Syrian hamsters (Fedorov, Reference Fedorov1979; Fedorov et al., Reference Fedorov, Babueva and Karpenko1989). It must be noted that pathomorphological, histological and biochemical characteristics of O. felineus infection in hamsters (experimental opisthorchiasis) are similar to the manifestations of the disease in humans (Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019).
Hamsters infected with O. felineus and treated with low doses of a carcinogenic nitrosamine (dimethylnitrosamine) develop CCA tumours (Fig. 2G) (Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017; Mordvinov et al., Reference Mordvinov, Minkova, Kovner, Ponomarev, Lvova, Zaparina, Romanenko, Shilov and Pakharukova2021): the conditions under which tumours also develop under the influence of O. viverrini or C. sinensis (Boonmars et al., Reference Boonmars, Wu, Boonjaruspinyo, Pinlaor, Nagano, Takahashi, Kaewsamut and Yongvanit2009; Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a). The morphology and histological markers of O. felineus-promoted tumours fully match the characteristics of the CCA associated with O. viverrini or C. sinensis (Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017; Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019; Na et al., Reference Na, Pak and Hong2020). Thus, according to the hamster model, the carcinogenic properties of O. felineus are quite comparable to those of O. viverrini and C. sinensis.
What happens to the liver tissues when they are exposed to adult liver flukes O. felineus without any other damaging factors? It has been shown that during experimental opisthorchiasis in hamsters, the portal region of the liver increases, and inflammation, periductal fibrosis, cholangiofibrosis and biliary intraepithelial neoplasia take place (Figs 2D–E and 3) (Boonmars et al., Reference Boonmars, Wu, Boonjaruspinyo, Pinlaor, Nagano, Takahashi, Kaewsamut and Yongvanit2009; Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Brindley, Mordvinov, Amaro, Santos, Correia da Costa and Vale2017; Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017; Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b, Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c). In general, this set of pathomorphological changes matches the precancerous lesions of liver tissue that precede the onset of CCA (Zen et al., Reference Zen, Adsay, Bardadin, Colombari, Ferrell, Haga, Hong, Hytiroglou, Klöppel, Lauwers, van Leeuwen, Notohara, Oshima, Quaglia, Sasaki, Sessa, Suriawinata, Tsui, Atomi and Nakanuma2007).
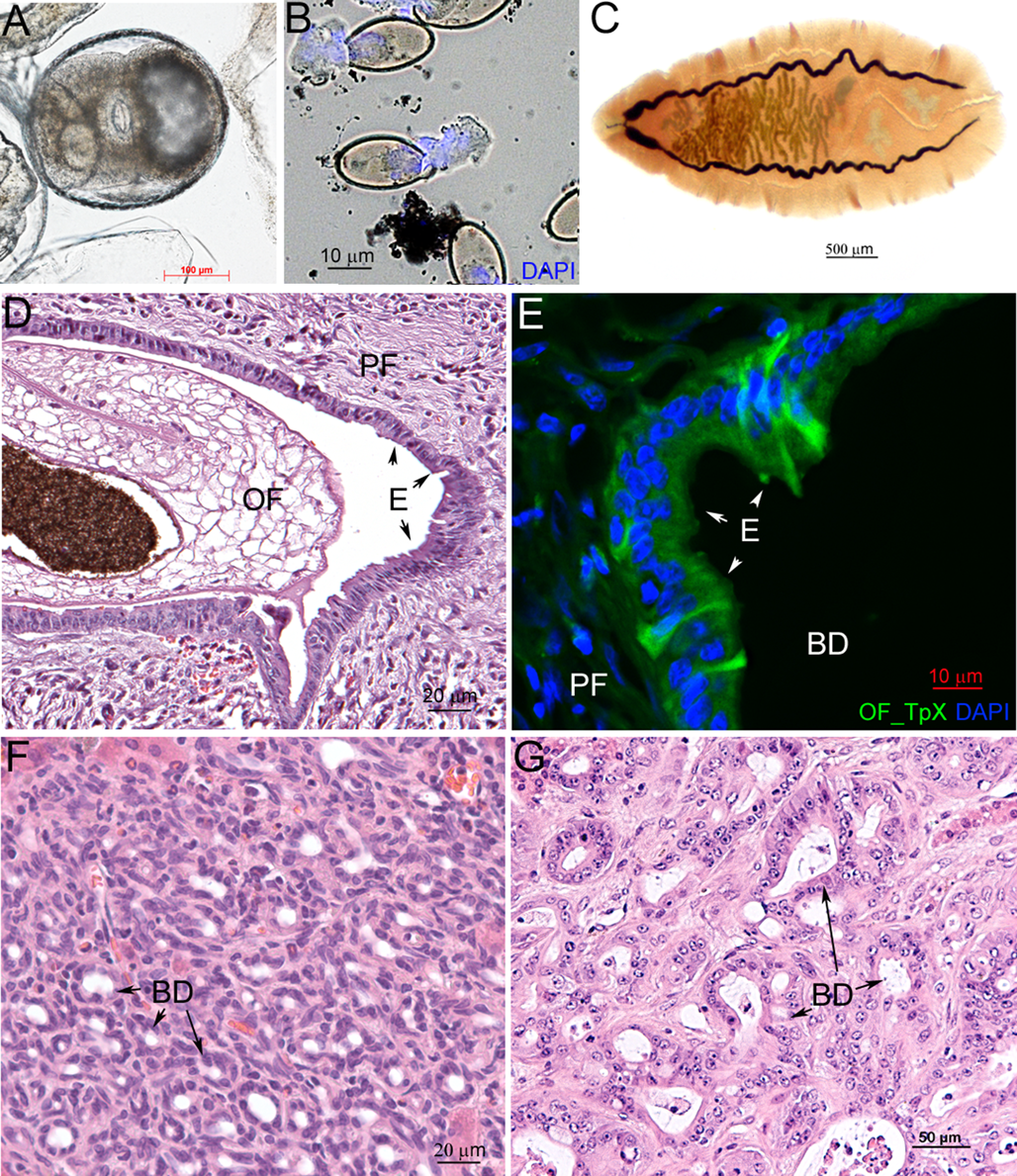
Fig. 2. Life stages of the liver fluke Opisthorchis felineus and histological consequences for mammalian infection. (A) Metacercariae; (B) hatching eggs with miracidia stained with 4′,6-diamidino-2-phenylindole (DAPI); (C) an adult worm; (D) a liver fluke inside a hamster bile duct (E, epithelium; OF, O. felineus; PF, periductal fibrosis); (E) an infected hamster liver stained with an anti-OF thioredoxin peroxidase (OF_TpX) antibody (BD, bile duct); (F) advanced cholangiofibrosis in an O. felineus-infected hamster at 6 months after infection initiation is recognized to be precancerous; (G) cholangiocarcinoma in an O. felineus-infected hamster after dimethylnitrosamine treatment at 6 months p.i.
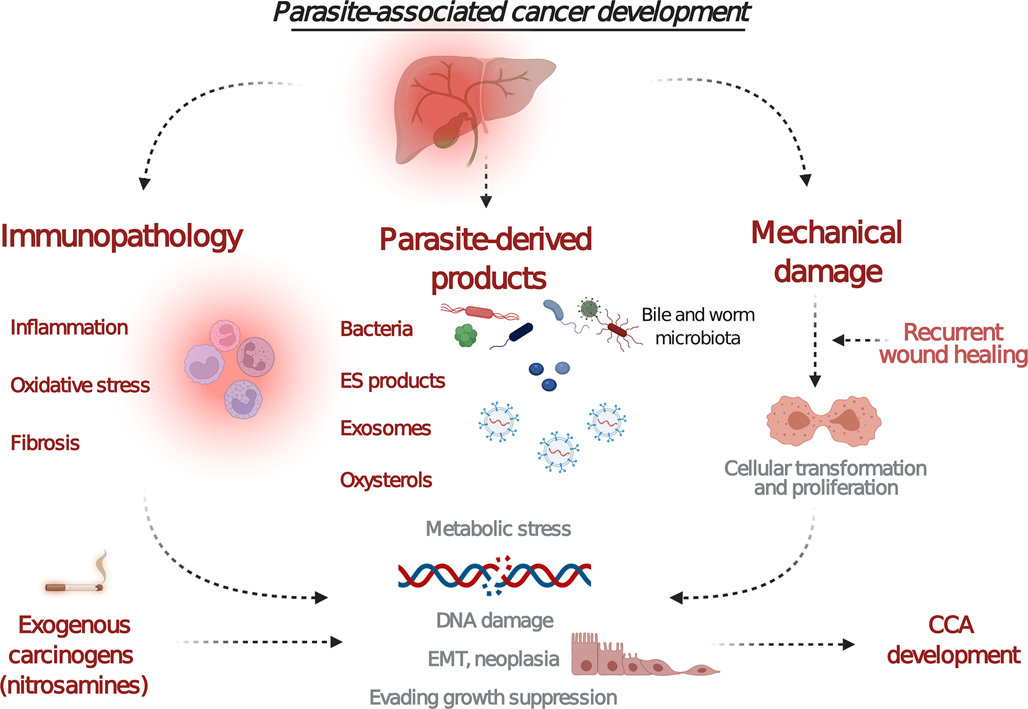
Fig. 3. Proposed mechanisms of carcinogenicity induced by the liver fluke infection. Chronic inflammation leads to the activation of signalling pathways that can induce oncogenes or stimulate epithelial–mesenchymal transition. Fluke-derived substances (extracellular vesicles and proteins) and metabolites secreted into the bile duct may induce oxidative lesions that facilitate DNA damage. In addition, physical damage to host tissues together with the active wound-healing process facilitates recurrent wound healing and cell proliferation. Cofactors such as dietary nitrosamines or changes in the microbiota may promote this pathology. Combined parasite–host interaction events (chronic inflammation, parasite-derived substances and physical damage) alter cell growth, proliferation and survival thereby possibly causing carcinogenesis. Created with BioRender.com.
Sometimes mice are used to study liver fluke infection, but liver fluke-associated CCA has not been obtained in this model. In particular, C3H/He mice develop extensive fibrosis rather than CCA when they are challenged by C. sinensis, N-nitrosodimethylamine and dicyclanil together (Uddin et al., Reference Uddin, Jang and Hong2016). Likewise, it has not been possible to obtain liver fluke-induced CCA in rats as a model animal, although these rodents are a common model for studying C. sinensis infection (Rim, Reference Rim2005).
Proposed mechanisms of CCA
Long-lasting interplay between liver flukes and host responses to the parasitic infection initiates carcinogenesis. Although indirect evidence suggests that carcinogenicity is different among the 3 species of liver flukes, published results of studies on molecular events and mechanisms of liver fluke-induced carcinogenesis are insufficient for identifying species-specific features of the infections. Nevertheless, we believe that the discussion of the proposed mechanisms of CCA development, which are probably largely common for 3 opisthorchiid species, is an important part of this review and does not contradict its logic.
The potential mechanisms of carcinogenesis include damage to the biliary epithelium by flukes, prolonged immune-system-mediated pathogenesis and effects of parasite-derived biomolecules on cholangiocytes, followed by modification of the cholangiocyte proliferation (Fig. 3).
Of the parasitic biomolecules that are potentially involved in CCA development, the secreted O. viverrini granulin, Ov-GRN-1, has attracted much research attention. This protein is a paralog of human progranulin, which is a glycoprotein involved in the regulation of cell division, motility and migration. Mammalian progranulin is known to participate in embryonic development, wound healing and carcinogenesis (Bateman et al., Reference Bateman, Cheung and Bennett2018). Ov-GRN-1 stimulates cell proliferation, angiogenesis and wound healing (Smout et al., Reference Smout, Laha, Mulvenna, Sripa, Suttiprapa, Jones, Brindley and Loukas2009, Reference Smout, Sotillo, Laha, Papatpremsiri, Rinaldi, Pimenta, Chan, Johnson, Turnbull, Whitchurch, Giacomin, Moran, Golledge, Daly, Sripa, Mulvenna, Brindley and Loukas2015; Bansal et al., Reference Bansal, Smout, Wilson, Cobos Caceres, Dastpeyman, Sotillo, Seifert, Brindley, Loukas and Daly2017; Dastpeyman et al., Reference Dastpeyman, Bansal, Wilson, Sotillo, Brindley, Loukas, Smout and Daly2018; Haugen et al., Reference Haugen, Karinshak, Mann, Popratiloff, Loukas, Brindley and Smout2018). Moreover, recombinant Ov-GRN-1 was shown to promote exosome-mediated crosstalk and a cellular microenvironment conducive to CCA (Arunsan et al., Reference Arunsan, Chaidee, Cochran, Mann, Tanno, Kumkhaek, Smout, Karinshak, Rodpai, Sotillo, Loukas, Laha, Brindley and Ittiprasert2020).
It is likely that Ov-GRN-1 is an important tool in the O. viverrini parasitism strategy and contributes to the pathogenesis. Indeed, hepatobiliary morbidities were less pronounced in hamsters experimentally infected with Ov-Grn-1 gene knockout O. viverrini than in those with wild-type flukes (Arunsan et al., Reference Arunsan, Ittiprasert, Smout, Cochran, Mann, Chaiyadet, Karinshak, Sripa, Young, Sotillo, Loukas, Brindley and Laha2019).
Clonorchis sinensis granulin is localized in the tegument and testes of adult worms and is a component of the excretory–secretory product (ESP) and has been found in surrounding host tissues too. Moreover, a recombinant C. sinensis granulin can promote metastasis of malignant liver tumours and malignant transformation of hepatocytes (Wang et al., Reference Wang, Lei, Tian, Shang, Wu, Li, Zhao, Shi, Tang, Chen, Lv, Huang, Tang, Yu and Li2017, Reference Wang, He, Yin, Wu and Li2021; Shi et al., Reference Shi, Yu, Liang, Huang, Ou, Wei, Wan, Yang, Zhang and Jiang2020). The properties of O. felineus granulin have not yet been studied.
The ESPs of all 3 species include antioxidant defence proteins thioredoxin and thioredoxin peroxidase (TPx) (Suttiprapa et al., Reference Suttiprapa, Loukas, Laha, Wongkham, Kaewkes, Gaze, Brindley and Sripa2008; Ju et al., Reference Ju, Joo, Lee, Cho, Cheun, Kim, Lee, Lee, Sohn, Kim, Kim, Park and Kim2009; L'vova et al., Reference L'vova, Duzhak, Tsentalovich, Katokhin and Mordvinov2014). Opisthorchis viverrini TPx and O. felineus TPx have been found in the bile duct epithelium of infected animals, and this protein is located not only in the host tissues surrounding the parasites but also in small first-order bile ducts, which are substantially smaller than adult worms. Furthermore, O. viverrini TPx was shown to decrease apoptosis, which – when cell proliferation is activated by other parasite-derived products – may contribute to malignancy (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a).
Together with the liver fluke infection, such cofactors as environmental or exotic microbes in the biliary system that are resistant to host inflammatory responses may also contribute to carcinogenesis (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a, Chng et al., Reference Chng, Chan, Ng, Li, Jusakul, Bertrand, Wilm, Choo, Tan, Lim, Soetinko, Ong, Duda, Dima, Popescu, Wongkham, Feng, Yeoh, Teh, Yongvanit, Wongkham, Bhudhisawasdi, Khuntikeo, Tan, Pairojkul, Ngeow and Nagarajan2016; Pakharukova et al., Reference Pakharukova, Zaparina, Hong, Sripa and Mordvinov2021). Cofactors such as dietary nitrosamines could significantly facilitate the pathology in question (Fig. 3) (Boonmars et al., Reference Boonmars, Wu, Boonjaruspinyo, Pinlaor, Nagano, Takahashi, Kaewsamut and Yongvanit2009; Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a; Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017; Mordvinov et al., Reference Mordvinov, Minkova, Kovner, Ponomarev, Lvova, Zaparina, Romanenko, Shilov and Pakharukova2021).
The following mechanism can explain the association between liver fluke infection and CCA: parasite-derived substances can lead to uncontrolled growth of host cells. The parasite-derived proteins like granulin 1 of O. viverrini can promote proliferation of biliary cells, whereas other proteins, e.g. thioredoxin and TPx, can prevent apoptosis (Sripa et al., Reference Sripa, Brindley, Mulvenna, Laha, Smout, Mairiang, Bethony and Loukas2012a). Opisthorchis viverrini- and O. felineus-derived substances are internalized by the bile duct epithelium (Chaiyadet et al., Reference Chaiyadet, Sotillo, Smout, Cantacessi, Jones, Johnson, Turnbull, Whitchurch, Potriquet, Laohaviroj, Mulvenna, Brindley, Bethony, Laha, Sripa and Loukas2015; Petrenko et al., Reference Petrenko, Pakharukova, Kovner, Lvova, Lyakhovich and Mordvinov2017; Pakharukova et al., Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c). Cholangiocytes in culture can capture exosomes of O. viverrini, which is followed by elevated cell proliferation, IL-6 expression and pronounced alterations of the cell phenotype (Chaiyadet et al., Reference Chaiyadet, Sotillo, Smout, Cantacessi, Jones, Johnson, Turnbull, Whitchurch, Potriquet, Laohaviroj, Mulvenna, Brindley, Bethony, Laha, Sripa and Loukas2015). Elevated IL-6 production was demonstrated in O. viverrini-infected patients with CCA as compared to those without (Sripa et al., Reference Sripa, Thinkhamrop, Mairiang, Laha, Kaewkes, Sithithaworn, Periago, Bhudhisawasdi, Yonglitthipagon, Mulvenna, Brindley, Loukas and Bethony2012b).
Additionally, an analysis of adult worm lysates has identified novel oxysterol derivatives in O. viverrini and O. felineus, which are potential promutagenic compounds (Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013; Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Brindley, Mordvinov, Amaro, Santos, Correia da Costa and Vale2017, Reference Gouveia, Pakharukova, Rinaldi, Mordvinov, Brindley, Gärtner and Vale2020).
On the other hand, the host response is also a crucial determinant of liver fluke infection outcomes and of cholangiocarcinogenesis initiation. In particular, it was demonstrated that O. viverrini infection downregulates RB1 (retinoblastoma 1) and p16INK4 (cyclin-dependent kinase inhibitor 2A) expression and upregulates cyclin D1 and CDK4 (cyclin-dependent kinase 4) during CCA progression (Boonmars et al., Reference Boonmars, Wu, Boonjaruspinyo, Pinlaor, Nagano, Takahashi, Kaewsamut and Yongvanit2009). There is also evidence of elevated activity of PI3K–AKT and Wnt–β-catenin signalling pathways during chronic opisthorchiasis caused by O. viverrini (Yothaisong et al., Reference Yothaisong, Thanee, Namwat, Yongvanit, Boonmars, Puapairoj and Loilome2014).
Concerning C. sinensis infection morbidity, strong stimulation of T helper 2-associated inflammation by C. sinensis infection was revealed (Kim et al., Reference Kim, Bae, Choi and Hong2012). During C. sinensis infection, peroxiredoxin 6 expression inversely correlates with NF-κB activation due to the response to C. sinensis-derived ESP (Pak et al., Reference Pak, Son, Seo, Hong, Sohn, Na and Kim2016).
The liver flukes have strong proinflammatory properties, which increase the risk of carcinogenesis (Maeng et al., Reference Maeng, Lee, Bashir, Kim, Hong, Lee, Sohn, Na, Kim and Pak2016; Pak et al., Reference Pak, Son, Seo, Hong, Sohn, Na and Kim2016; Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b, Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c). During chronic inflammation, inflammatory cells are recruited to injury sites, thereby enhancing the release and accumulation of free radicals, resulting in the formation of lipid peroxidation byproducts, which can induce the formation of oxidative DNA lesions, e.g. 8-hydroxy-2′-deoxyguanosine (Maeng et al., Reference Maeng, Lee, Bashir, Kim, Hong, Lee, Sohn, Na, Kim and Pak2016; Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b; Zaparina et al., Reference Zaparina, Rakhmetova, Kolosova, Cheng, Mordvinov and Pakharukova2021).
Major factors contributing to inflammation are macrophage-derived proinflammatory cytokines. Increased mRNA expression levels of IL-1b, TGF-β and TNF (formerly known as TNF-α) were revealed in the liver of O. viverrini-infected hamsters (Prakobwong et al., Reference Prakobwong, Pinlaor, Yongvanit, Sithithaworn, Pairojkul and Hiraku2009). Moreover, elevated levels of TNF are reported to correlate with morbidity in O. viverrini-infected hamsters (Dangtakot et al., Reference Dangtakot, Pinlaor, Itthitaetrakool, Chaidee, Chomvarin, Sangka, Wilailuckana and Pinlaor2017). The same correlation between TNF upregulation and liver problems was observed in a time course study on O. felineus-infected hamsters (Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b). This cytokine is also known as an activator of fibroblast proliferation and stimulates epithelial–mesenchymal transition factor SNAIL in human O. viverrini-associated-CCA cells (Techasen et al., Reference Techasen, Namwat, Loilome, Bungkanjana, Khuntikeo, Puapairoj, Jearanaikoon, Saya and Yongvanit2012).
Continuous production of proinflammatory cytokines and growth factors that facilitate the activation of myofibroblasts has been revealed during O. viverrini infection and O. felineus infection (Prakobwong et al., Reference Prakobwong, Pinlaor, Yongvanit, Sithithaworn, Pairojkul and Hiraku2009; Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019; Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b). Furthermore, the activation of epithelial–mesenchymal transition, extracellular-matrix deposition and fibrogenesis was demonstrated in patients and experimental animals with opisthorchiasis (Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019). Myofibroblasts produce α-smooth muscle actin and deposit extracellular-matrix components. In O. felineus-infected animals, the number of these cells is elevated during the infection as evidenced by excessive fibrogenesis (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Brindley, Mordvinov, Amaro, Santos, Correia da Costa and Vale2017; Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019; Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b).
In O. felineus-infected animals, the amount of CD163 significantly increases with time (Pakharukova et al., Reference Pakharukova, Zaparina, Kapushchak, Baginskaya and Mordvinov2019b). CD163-positive cells can infiltrate tumour tissues, in particular in CCA associated with O. viverrini infection, and the CD163 protein is reported to be a marker of macrophages having an alternative activated phenotype (tumour-associated macrophages) (Thanee et al., Reference Thanee, Loilome, Techasen, Namwat, Boonmars, Pairojkul and Yongvanit2015). Alternatively, activated CD163+ macrophages contribute to the control of an inflammatory process through a release of anti-inflammatory cytokines: a process that promotes tissue regeneration (Braga et al., Reference Braga, Agudelo and Camara2015).
Genetics and genomics
One of the intriguing features of O. felineus is the absence of well-defined population structure over a significant part of its range. Results of large-scale genotyping using marker DNA fragments from mitochondrial (cox1 and cox3) and nuclear internal transcribed spacer sequences revealed that genetic diversity of this species is very low across Eastern Europe, Siberia and Northern Kazakhstan (Brusentsov et al., Reference Brusentsov, Katokhin, Brusentsova, Shekhovtsov, Borovikov, Goncharenko, Lider, Romashov, Rusinek, Shibitov, Suleymanov, Yevtushenko and Mordvinov2013). In contrast, population genetic differentiation is well-pronounced in O. viverrini (Laoprom et al., Reference Laoprom, Sithithaworn, Andrews, Ando, Laha, Klinbunga, Webster and Petney2012). An in-depth analysis of the population genetics of O. viverrini and of its first intermediate host, Bithynia siamensis, was published recently (Saijuntha et al., Reference Saijuntha, Andrews, Sithithaworn and Petney2022).
The genetic diversity of C. sinensis is not as pronounced as that of O. viverrini, but the population structure of C. sinensis does exist (Sun et al., Reference Sun, Huang, Huang, Liang, Wang, Mao, Men, Chen, Deng, Zhou, Lv, Zhou, Zhang, Li, Tian, Lei, Liang, Hu, Xu, Li and Yu X2013).
Microsatellites, a powerful tool for population-genetic studies, were utilized for O. viverrini research (Laoprom et al., Reference Laoprom, Sithithaworn, Ando, Sithithaworn, Wongkham, Laha, Klinbunga, Webster and Andrews2010). It was shown that cyprinids – the second intermediate host – could contribute to the genetic diversity of O. viverrini. In particular, there are differences in O. viverrini genetic parameters between different species of fish within and between some regions (Pitaksakulrat et al., Reference Pitaksakulrat, Webster, Webster, Laha, Saijuntha, Lamberton, Kiatsopit, Andrews, Petney and Sithithaworn2018). Advances in the investigation into the population genetics of O. viverrini indicate that microsatellite DNA markers should be utilized for in-depth studies on the genetic diversity of O. felineus.
There are differences in the chromosome number among the 3 opisthorchids. The karyotype of O. felineus (Siberian isolate) and C. sinensis (Russian Far East isolate) consists of 7 pairs of chromosomes (2n = 14) (Zadesenets et al., Reference Zadesenets, Katokhin, Mordvinov and Rubtsov2012), whereas the karyotype of C. sinensis (Korea and China isolate) is represented by 28 chromosome pairs (2n = 56) (Park et al., Reference Park, Im, Huh and Yong2000). This fact contradicts the data on the final C. sinensis genome assembly from a Korean isolate comprising 7 largest scaffolds corresponding to a karyotype of 2n = 14 (Young et al., Reference Young, Stroehlein, Kinkar, Wang, Sohn, Chang, Kaur, Weisz, Dudchenko, Aiden, Korhonen and Gasser2021). In contrast, the karyotype of O. viverrini is composed of 6 pairs of chromosomes (2n = 12) (Zadesenets et al., Reference Zadesenets, Katokhin, Mordvinov and Rubtsov2012).
The size of the O. felineus nuclear genome is 684 Mbp, and 30.3% of the genome is represented by repetitive elements, mainly retrotransposons. In the genome, 11 455 thousand protein-coding genes are annotated (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019), as are 55 genes coding for microRNAs (Ovchinnikov et al., Reference Ovchinnikov, Afonnikov, Vasiliev, Kashina, Sripa, Mordvinov and Katokhin2015). In terms of the size and abundance of repetitive elements, the O. felineus genome is close to the genomes of O. viverrini and C. sinensis, the sizes of which are 634.5 and 558 Mbp, respectively. Nonetheless, judging by the number of annotated genes, the genomes of O. viverrini and C. sinensis surpass the genome of O. felineus and contain 16 379 and 13 489 genes, respectively (Young et al., Reference Young, Nagarajan, Lin, Korhonen, Jex, Hall, Safavi-Hemami, Kaewkong, Bertrand, Gao, Seet, Wongkham, Teh, Wongkham, Intapan, Maleewong, Yang, Hu, Wang, Hofmann, Sternberg, Tan, Wang and Gasser2014, Reference Young, Stroehlein, Kinkar, Wang, Sohn, Chang, Kaur, Weisz, Dudchenko, Aiden, Korhonen and Gasser2021). This is probably due to the differences in genome assembly technologies. As a result of orthology analysis, it was found that the published versions of assembly of the genomes are not exhaustive, and the gene annotation for all 3 species needs to be improved (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019).
Unusually high heterozygosity was found in the O. felineus genome in samples obtained both from single and pooled worms (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019). In this regard, the O. felineus genome significantly differs from the O. viverrini genome, whose sequencing and assembly revealed low-sequence heterozygosity (Young et al., Reference Young, Nagarajan, Lin, Korhonen, Jex, Hall, Safavi-Hemami, Kaewkong, Bertrand, Gao, Seet, Wongkham, Teh, Wongkham, Intapan, Maleewong, Yang, Hu, Wang, Hofmann, Sternberg, Tan, Wang and Gasser2014). In an analysis of genome-wide synteny among the 3 opisthorchids, it was revealed that in the arrangement of homologous loci, the similarity of the genomes of O. felineus and C. sinensis is higher than that of O. viverrini with O. felineus or C. sinensis (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019).
The data on genome synteny are confirmed by the results of phylogenetic studies involving individual genetic markers and genome-wide data from the 3 opisthorchids (Shekhovtsov et al., Reference Shekhovtsov, Katokhin, Romanov, Besprozvannykh, Fedorov, Yurlova, Serbina, Sithithaworn, Kolchanov and Mordvinov2009; Cai et al., Reference Cai, Liu, Song, Wu, Zou, Yan, Yuan, Lin and Zhu2012; Pomaznoy et al., Reference Pomaznoy, Logacheva, Young, Penin, Ershov, Katokhin and Mordvinov2016). Thus, according to the above findings, O. felineus and C. sinensis are closer species than O. felineus and O. viverrini are. This is inconsistent with the separation of C. sinensis from the genus Opisthorchis and suggests that this species occupies an intermediate position between O. felineus and O. viverrini. Moreover, the geographic ranges of O. felineus and C. sinensis are also closer than those of O. felineus and O. viverrini.
As mentioned above, parasitic granulins are believed to be directly involved in the development of O. viverrini-associated CCA (Arunsan et al., Reference Arunsan, Ittiprasert, Smout, Cochran, Mann, Chaiyadet, Karinshak, Sripa, Young, Sotillo, Loukas, Brindley and Laha2019). Initially, 3 genes coding for single-domain granulins and 1 gene of a multidomain progranulin (PGRN) were found in the O. viverrini genome (Young et al., Reference Young, Nagarajan, Lin, Korhonen, Jex, Hall, Safavi-Hemami, Kaewkong, Bertrand, Gao, Seet, Wongkham, Teh, Wongkham, Intapan, Maleewong, Yang, Hu, Wang, Hofmann, Sternberg, Tan, Wang and Gasser2014). Nevertheless, later, 4 genes (grn-1 through grn-4) coding for single-domain granulins as well as 1 gene of PGRN have been identified in O. viverrini, O. felineus and C. sinensis genomes. Genes grn-1–grn-4 in all 3 genomes are situated in the same chromosomal locus and constitute a syntenic group of genes. Genes grn-4 and grn-1 share 95% nucleotide sequence identity (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019). Functional activity of the Ov-GRN-1 protein and its probable role in CCA development have been investigated (Smout et al., Reference Smout, Laha, Mulvenna, Sripa, Suttiprapa, Jones, Brindley and Loukas2009, Reference Smout, Sotillo, Laha, Papatpremsiri, Rinaldi, Pimenta, Chan, Johnson, Turnbull, Whitchurch, Giacomin, Moran, Golledge, Daly, Sripa, Mulvenna, Brindley and Loukas2015; Mulvenna et al., Reference Mulvenna, Sripa, Brindley, Gorman, Jones, Colgrave, Jones, Nawaratna, Laha, Suttiprapa, Smout and Loukas2010; Arunsan et al., Reference Arunsan, Ittiprasert, Smout, Cochran, Mann, Chaiyadet, Karinshak, Sripa, Young, Sotillo, Loukas, Brindley and Laha2019). The functional activity of other opisthorchiid granulins has not yet been studied.
Comparative transcriptomics
In a comparison of the transcriptomes of adult O. felineus, O. viverrini and C. sinensis worms, it was found that the expression of the overwhelming majority of genes does not have significant differences among the 3 opisthorchiid species (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019). This result indicates high similarity in the mechanisms that ensure normal physiological processes of helminths in the definitive host. Nevertheless, the expression of several dozen genes proved to be species-specific. It is worth mentioning that most of these genes encode proteins of the opisthorchids' ESP, an essential component of the parasite–host interaction mechanisms (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019).
In particular, a group of differentially expressed genes among the 3 species included CAP superfamily genes. Expression levels of some CAP domain-containing proteins (cysteine-rich secretory proteins, antigen 5 and pathogenesis-related 1 proteins) are higher in O. felineus than in C. sinensis and O. viverrini (Fig. 4B) (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019). CAP domain-containing proteins represent a highly evolutionarily diversified superfamily whose members share specific sequence motifs (Gibbs et al., Reference Gibbs, Roelants and O'Bryan2008; Chalmers and Hoffmann, Reference Chalmers and Hoffmann2012). Ant and wasp allergens, snake venom toxins and plant antifungal proteins are included in this superfamily. Although nematodes' CAP proteins are known as Ancylostoma-secreted proteins, some parasitic CAP proteins are known as venom allergen-like (VAL) proteins (Fig. 4A).

Fig. 4. The phylogenetic tree and mRNA abundance of genes encoding CAP domain-containing proteins. (A) The neighbour-joining phylogenetic tree without distance correction. A comparison of O. felineus venom allergen-like proteins with selected members of the CAP superfamily. The sequences were aligned with clustalw2. Pry1 (NP_012456.1, sterol-binding protein of Saccharomyces cerevisiae), SmVAL-4 [XP_018652935.1, venom allergen-like (VAL) 4 protein of Schistosoma mansoni], CsVAL-28 (AWV55762.1, venom allergen-like protein 28 of Clonorchis sinensis), BmVAL-1 (AAK12274.1, venom allergen antigen-like protein 1 of Brugia malayi) and HpVAL-4 (AEP82919.1, venom allergen/ancylostoma secreted protein-like 4 of Heligmosomoides polygyrus bakeri) were used. (B) Interspecies differences in mRNA abundance of genes coding for VAL proteins among adult liver flukes. Normalized data were taken from the paper (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019) and were formatted as a heatmap using the heatmap.2 (v.2.38) R package. OF, O. felineus; OV, O. viverrini; CS, C. sinensis. GenBank accession numbers for sequences encoding CAP domain-containing proteins in the Opisthorchiidae liver flukes are presented in Supplementary Table ST1.
VALs are ubiquitously present in ESPs of helminths that parasitize animals and seem to be highly expressed at stages of the parasite life cycle where maximal contact occurs between the parasite and host, e.g. transmission, tissue migration or feeding (Wilbers et al., Reference Wilbers, Schneiter, Holterman, Drurey, Smant, Asojo, Maizels and Lozano-Torres2018). Roles of VALs in protease activity and modulation of the immune response during mammalian host invasion in Schistosoma mansoni have been proposed too (Chalmers and Hoffmann, Reference Chalmers and Hoffmann2012). In particular, S. mansoni VAL9 affects the expression of extracellular-matrix-modifying gene products (metalloproteinases and tissue inhibitors of metalloproteinases) in both Biomphalaria glabrata embryonic cells and Mus musculus bone marrow-derived macrophage populations (Yoshino et al., Reference Yoshino, Brown, Wu, Jackson, Ocadiz-Ruiz, Chalmers, Kolb, Hokke and Hoffmann2014). Two VAL proteins of C. sinensis (VAL13 and VAL28) are thought to hold promise for serodiagnostics; their functional motifs and structural details have been characterized in silico (Woo et al., Reference Woo, Kim, Sohn and Yong2015; Lee et al., Reference Lee, Yoo, Kim, Chung, Cho and Ju2018).
These data may form a basis for the research into functional significance of these secreted proteins in the pathogenesis of opisthorchiasis and clonorchiasis as well as for the studies on the species-specific features of the pathologies caused by these liver flukes.
In the genome of O. felineus, 4 genes encoding glutathione-S-transferases (GSTs) have been found, coding for xenobiotic metabolism enzymes capable of catalysing the conjugation of the reduced form of glutathione to substrates. GST sigma (molecular weight 28 kDa) has a conserved domain of prostaglandin synthase and retains its activity in the in vitro incubation medium of helminths (Pakharukova et al., Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c) and accumulates in the bile duct epithelium of mammals infected with O. felineus and patients with opisthorchiasis (Kovner et al., Reference Kovner, Pakharukova, Maksimova and Mordvinov2019; Pakharukova et al., Reference Pakharukova, Zaparina, Kovner and Mordvinov2019c). According to a comparative analysis of the transcriptomes of adult O. felineus, O. viverrini and C. sinensis worms, mRNA abundance of GST sigma in the transcriptome is many times higher in O. felineus than in the other opisthorchids (Ershov et al., Reference Ershov, Mordvinov, Prokhortchouk, Pakharukova, Gunbin, Ustyantsev, Genaev, Blinov, Mazur, Boulygina, Tsygankova, Khrameeva, Chekanov, Fan, Xiao, Zhang, Xu, Yang, Solovyev, Lee, Liu, Afonnikov and Skryabin2019). It has been hypothesized that this enzyme plays an important part in the parasite–host interaction system.
Conclusions/future directions
In this review, we provided a recent update of our knowledge on the liver fluke O. felineus and drew attention to its differences from the closely related liver flukes O. viverrini and C. sinensis.
The fish-borne trematode O. felineus occurs across a large territory of Eurasia and poses a threat to the health of the population in a number of countries. The prevalence of opisthorchiasis in Russia remains high. Active human intervention into nature, global climate change and ever-increasing migration of the population can change the current situation, provoke the expansion of geographic foci and the resumption of outbreaks of opisthorchiasis in other regions. Awareness of the population about the risk of opisthorchiasis from eating raw or undercooked freshwater fish and about the clinical manifestations of the human infection is essential for the control and prevention of this disease.
Liver fluke infections caused by O. felineus, C. sinensis and O. viverrini seem to have similar clinical symptoms, and today, the same anthelmintic agents are used to treat them. This situation can give the impression that there is no difference among the disorders. Nevertheless, these disorders, in addition to similar characteristics, have distinct clinical manifestations, probably due to the biological characteristics of the causative agents. The most important issue of the liver fluke pathogenicity is their carcinogenic potential.
The carcinogenicity of C. sinensis and O. viverrini is well documented, but for O. felineus, there is still no convincing epidemiological data confirming the direct link of opisthorchiasis felinea with cancer of the biliary tract. There are many publications pointing to relatively elevated prevalence of CCA in territories endemic for opisthorchiasis in the Russian Federation (Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016). In contrast to the O. viverrini endemic areas in Thailand, CCA in Western Siberia is not the leading type of cancer (Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2016; Petrova et al., Reference Petrova, Starinskiy, Gretsova, Shahzadova and Samsonov2019). Although, at present, there are no comparative studies on their carcinogenicity under the same conditions, it can already be said judging by indirect signs that O. felineus, O. viverrini and C. sinensis have pronounced differences in carcinogenicity for humans.
On the other hand, it is necessary to take into account national traditions and dietary habits of the population in endemic areas. In addition to the traditional consumption of raw or undercooked fish, which is a major risk factor of liver fluke infection, exogenous nitrosamines in the diet significantly facilitate the initiation of CCA. Fermented foods are more popular in countries where O. viverrini and C. sinensis are common than in O. felineus endemic areas. It is possible that these dietary patterns contribute to CCA incidence too.
The mechanisms of biological carcinogenesis need to be investigated further. Is chronic inflammation alone a necessary and sufficient condition for the formation of CCA? If so, then 3 epidemiologically significant species of liver flukes O. viverrini, C. sinensis and O. felineus would cause carcinogenesis in the same way. Experimental infection caused by O. felineus, O. viverrini or C. sinensis represents a promising model for comparative studies of helminth-associated carcinogenesis. The adaptation of liver flukes to life cycle conditions in different climatic zones with several intermediate and final hosts may be reflected in their basic molecular and biochemical processes. Such adaptive changes may in turn be associated with the pathogenicity of certain species. Species-specific composition of parasitic ESPs components, in particular, proteins and metabolites as well as small RNAs in exosome-like vesicles, can determine carcinogenic potential of the opisthorchids. The worm microbiota and its effects on the host microbiota may also influence carcinogenic potential. Taking into account the complex life cycle and climatic differences of endemic areas, relevant differences in their microbiomes can be found as well.
In this regard, the need for comparative bias-free studies on the 3 epidemiologically significant liver fluke species is obvious. Research developments on comparative analysis – in particular, high-throughput mRNA sequencing aimed at identifying differences in host–parasite interaction and differentially expressed genes during the host response caused by closely related species with moderate and high carcinogenicity – will improve the understanding of the mechanisms behind helminth-associated carcinogenesis and will help to answer the question ‘What are the general mechanisms of biological carcinogenesis?’
To investigate the mechanisms of helminth-associated carcinogenesis, in particular, to identify key pathogenesis-related proteins, active use of functional-genomics approaches is required. These approaches have already manifested their effectiveness in studies on the xenobiotic metabolism of O. felineus. For instance, using RNA interference, it was shown that the genes encoding CYP and ABC proteins are important for normal physiological processes of adults O. felineus and can be considered drug targets (Pakharukova et al., Reference Pakharukova, Vavilin, Sripa, Laha, Brindley and Mordvinov2015; Mordvinov et al., Reference Mordvinov, Ershov, Pirozhkova, Pakharukov and Pakharukova2017a). In a recent report on O. viverrini granulin 1, CRISPR/Cas9 technology was utilized, which is a new genetic tool that has revolutionized the investigation of gene functions. Those authors confirmed the important role of granulin 1 in the development of O. viverrini opisthorchiasis (Arunsan et al., Reference Arunsan, Ittiprasert, Smout, Cochran, Mann, Chaiyadet, Karinshak, Sripa, Young, Sotillo, Loukas, Brindley and Laha2019).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000397.
Acknowledgements
We are thankful to Nikolai Shevchuk (http://shevchuk-editing.com) for the English-language editing of this manuscript.
Author contributions
M. Y. P. and V. A. M. contributed equally to this manuscript.
Financial support
This work was supported by the Russian Science Foundation (grant number 18-15-00098 to V. A. M.). The funding agencies had no role in this study, e.g. in study design, data collection or decision to publish.
Conflict of interest
None.