INTRODUCTION
Digenetic trematodes possess complex life cycles that utilize two or three different hosts to complete their development (Shoop, 1988). In general, these cycles are initiated by the hatching of free-swimming miracidia from eggs produced by adult worms, followed by these larvae finding and entering (by direct penetration or ingestion) a mollusc, the most common first intermediate host. Within the molluscan host, the miracidial stage ‘transforms’ to a mother sporocyst, sometimes referred to as a germinal sac (Schutte, 1974), within which many germinal cells are produced, each capable of developing into embryos destined to become a second generation of larval stages called daughter sporocysts or rediae. Rediae are distinguished from sporocysts by their possessing a mouth (pharynx) and gut, and by their motile behaviour within the molluscan host. These larval stages undergo tissue-specific migration within their host where they either produce successive generations of these same stages, or produce a final intramolluscan stage, the cercaria. Cercariae then exit the molluscan host as free-swimming larvae that, depending on the species, will either encyst as a ‘resting’ metacercarial stage upon infecting a second intermediate host or contacting vegetation in the environment, or may skip the metacercarial stage altogether and penetrate the final or definitive host where they develop directly to adult worms. In the case of metacercaria-producing species, encysted stages must be ingested by the definitive host to complete their life cycle to the adult stage.
As illustrated by the above-generalized developmental cycle of trematode parasites, it is not difficult to imagine the myriad of sensory inputs being received by each of the different stages and immense complexity of how such inputs are coordinated and precisely targeted to the cells, tissues or organs responsible for mediating appropriate responses. Since muscular activity is presumed to play an important role in behavioural responses to external molecular or physical stimuli, this chapter seeks to explore the receptors and signal transduction pathways that may be involved in regulating motility and associated behavioural responses in trematode larvae. Because other chapters in this supplement will cover in detail the neurophysiology and neurochemistry of helminth muscle systems, it is the goal of the present chapter to emphasize the receptor-ligand systems involved in larval behavioural responses, and the putative cell signalling pathways associated with receptor activation. Thus the following topics will be explored: (1) molecular evidence for signal receptors and transduction systems in larval trematodes, (2) signalling pathways and parasite motility, and (3) what these receptors and their pathways tell us about the biology of trematodes.
The Digenea, with its approximately 8000 different species (Schell, 1985), represents a diverse group of organisms, and, unfortunately, the vast majority of research into their receptor repertoire and associated signalling molecules has focused only on a minute subset of trematode species; mainly the schistosomes and fasciolids. Therefore, it is worth bearing in mind that although the information and hypotheses presented here may be more generally applicable to members of this major helminth taxon, the data represented are based on a very narrow sampling of extant species. However, despite this limitation, with the recent publication of the annotated expressed sequence tag (EST) database representing approximately 90% of the complete transcriptomes of Schistosoma mansoni (Verjovski-Almeida et al. 2003) and S. japonicum (Hu et al. 2003), and ever-expanding EST databases for other species (e.g. ftp://ftp.sanger.ac.uk/pub/databases/Trematode/Fhep/ and ftp://ftp.sanger.ac.uk/pub/databases/Trematode/S.haematobium/), information regarding signal reception and associated transduction pathways will continue to increase, and potentially will serve as a foundation for extrapolating information to other trematode species. For the human schistosomes, a clear understanding of the molecular basis and role of cell signalling in the establishment of human infections by cercariae provide potential for immunological or chemical therapeutic intervention and control for the mammalian host. However, it might be argued that similar information on critical receptor/signal transduction systems involved in motility and early larval behaviour within the molluscan host also may provide ‘windows’ of opportunity for parasite-disruptive interventions through chemical or genetic alterations of the snail host (Lardans & Dissous, 1998).
PUTATIVE SIGNAL TRANSDUCTION PATHWAYS IN LARVAL TREMATODES
As alluded to in the introduction, larval digeneans possess complex life cycles requiring one or more intermediate hosts and a definitive host for completing successful development. Hence they must transit through and navigate within environments that can dramatically differ in their physical, chemical and physiological characteristics. That being the case, it is presumed that successful completion of their life cycles necessitates well-developed systems of reception capable of detecting a myriad of signals in their immediate environment, as well as systems capable of communicating these signals within and between cells to effect appropriate physiological or behavioural responses. However, despite a growing body of evidence that supports the existence of complex communication networks in a number of well-studied model invertebrates such as the fruit fly (Simon, Strathmann & Gautan, 1991; Agaisse & Perrimon, 2004; Huang & Klein, 2004), mosquito (Morton, 2004) and nematode (Sakaguchi, Matsumoto & Hisamoto, 2004), there is still very little information regarding such systems in trematode parasites, particularly when it comes to the larval stages of this helminth group.
Identification of gene homologues, representing potential orthologues, of signal transduction molecules and their receptors represents an effective methodological approach to determining whether or not trematodes possess the basic molecular machinery required for various specific functional signalling systems. As mentioned previously, the recently published EST sequencing and annotation projects for S. mansoni (Verjovski-Almeida et al. 2003) and S. japonicum (Hu et al. 2003), representing a significant sampling of the complete transcriptomes of these two major schistosome species, as well as other schistosome gene expression studies (Santos et al. 1999; Franco et al. 2000; Prosdocimi et al. 2002; Merrick et al. 2003), provide excellent resources for identifying genes related to potential signalling networks. For S. japonicum, the majority of the annotated ESTs was derived from adult worms, and therefore contained little direct information on larval stage expression. In contrast, the S. mansoni EST dataset incorporated, in addition to adult worm cDNAs, sequences derived from eggs, miracidia, germballs (daughter sporocysts, cercarial embryos) and cercariae. Perhaps not surprisingly, a search of this database for larval-derived ESTs revealed a number of genes with significant homology to a wide variety of transmembrane receptors and cytoplasmic proteins related to signal reception and transduction in other organisms (Table 1).

The collection of schistosome ESTs accumulated to date implies that many signalling proteins have been conserved through evolution, and indeed many of these ESTs share a high degree of sequence similarity across diverse taxa of eukaryotes and prokaryotes. This is especially true for elements of the signalling cascade that lie downstream of membrane receptors. For example, of the cytoplasmic signalling proteins represented in the larval stages, there is a preponderance of GTP-binding proteins such as trimeric G-proteins and the Ras-related family. Perhaps this apparent abundance stems from the degree of homology S. mansoni signalling proteins share with vertebrates, in that homologues of highly conserved proteins will be more easily recognized. The Ras superfamily and trimeric G-proteins display high homology across several phyla, such that the S. mansoni Ras (Genbank accession number U53177) shows 75·7% identity at the amino acid level with the human homologue. In addition, these families are very large, for instance there are 17 distinct types of Gα subunits in mammals and at least 150 proteins included in the Ras superfamily possibly increasing chances of finding homologous genes (http://www.ebi.ac.uk/interpro). With regards to intimate host-parasite relationships, similarities in gene sequences may be advantageous to either party. For example, parasites may obtain host molecules for their own use through their possession of specific host-like receptors, whereas the host may be able to modulate parasite behaviour or development by secreting parasite-related ligands capable of binding to parasite receptors, thereby triggering ill-timed or inappropriate responses (Salzet, Capron & Stefano, 2000).
In contrast to the families of downstream signalling molecules, relatively few genes encoding signalling-related receptors have been identified amongst the ESTs derived from trematode larval stages. As listed in Table 1, these include ESTs or full cDNA sequences representing three receptor families; receptor tyrosine kinases (RTKs), receptor serine threonine kinases (RSTKs) and the G protein-coupled receptors (GPCRs). Not surprising many of these receptor gene sequences were only recently discovered as a result of the recently published S. mansoni and S. japonicum transcriptome papers (Verjovski-Almeida et al. 2003; Hu et al. 2003). The relatively low number of identified receptors (5 RTKs, 2 RSTKs, 7 GPCRs; see Table 1) may be due to the fact that specific receptor classification relies on their displaying a high degree of similarity with receptors of other organisms, mostly vertebrates. Therefore, many schistosome ESTs that do indeed code for transmembrane receptors may not have been identified as such due to their low homology with known receptors. It also is a possibility that trematodes, perhaps by virtue of their parasitic lifestyle, may not have acquired the diversity of receptors of other free-living organisms. One only has to compare the number of GPCRs predicted in the proteomes of Drosophila (200 receptors; Brody & Cravchik, 2000), mosquitoes (276 receptors; Hill et al. 2002), and Caenorhabditis nematodes (>1000 receptors; Bargmann, 1998) to appreciate differences in range and number of potential signalling receptors present in free-living versus parasitic invertebrate organisms.
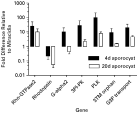
The number of ESTs sequenced from each of the different S. mansoni larval stages (eggs, miracidia, germ balls and cercariae) and annotated in the Schistosoma db (Verjovski-Almeida et al. 2003) was approximately equal, thus allowing a comparison to be made across stages with respect to gene expression. For example 14 ESTs similar to proteins involved in phospholipid signalling such as phospholipases were identified, and all 14 of these transcripts derived from either the egg or miracidial stages. Similarly, differential stage expression has been reported in other studies, for example elastase 2a, troponin I and tropomyosin transcripts were increased in daughter sporocyst germ balls (Verjovski-Almeida et al. 2003). In contrast, GTP-binding proteins appear to be ubiquitous across the larval stages examined, perhaps representing stage-independent constitutive expression (Table 1). However, because categories such as ‘Ras-related’ and ‘trimeric G proteins’ represent many members making up each family, differential expression levels may actually exist if individual proteins were examined on a gene-by-gene level. To illustrate this point, Vermeire, Boyle & Yoshino (2004) evaluated expression of 25 S. mansoni genes, many related to signalling molecules, using real-time quantitative polymerase chain reaction (qPCR) to assess gene expression in miracidia, and 4-day old and 20-day old in vitro cultured mother sporocysts. The genes chosen for analysis were from the S. mansoni EST databases or selected published sequences and included homologues of protein kinases, small G proteins, G protein-coupled receptors, and membrane transporters. Although steady-state transcript levels for the majority of genes included in this analysis initially showed a general trend of increasing transcript levels as a result of miracidial transformation to the mother sporocyst stage, statistical analyses revealed that transcript levels for only seven genes differed significantly between stages. As shown in Fig. 1, significant increases from 10- to 100-fold in 6 genes were observed in early sporocysts relative to miracidial transcript levels. Genes included a Rho GTPase (Rho-GTPase2), G-protein alpha subunit (G-alpha2), phosphoinositide-dependent protein kinase (3PI-PK), polo-like protein kinase (PLK), tri-spanning orphan receptor (3TM orphan) and glycerol-3-phosphate permease (G3P transporter). Interestingly, the opposite was true for the G-protein coupled rhodopsin receptor, which experienced a 40% decrease in transcript level at 4 days in culture, and >85% reduction in 20-day sporocysts (Fig. 1). In 20-day old cultured sporocysts, although there is a trend of decreasing transcript levels for the 6 genes upregulated at 4-days in culture, only 2 (G-alpha2, 3PI-PK) exhibited significant decreases between 4- and 20-day cultured sporocysts. These results serve to illustrate that steady-state transcript levels likely are responsive to changes in larval growth/development, and that larval stage-associated changes in gene expression under in vitro conditions occurs in a selective manner.

Fig. 1. Differential expression of signalling-related transcripts in 4-day and 20-day Schistosoma mansoni mother sporocysts cultured in Biomphalaria glabrata embryonic (Bge) cell-conditioned sporocyst medium (SM) relative to miracidial transcript levels. Raw cycle threshold (Ct) values were normalized using an 18s rRNA Ct values as an internal standard and loading control. Fold differences for 4-day and 20-day sporocyst transcript levels are expressed relative to miracidia levels, which have been normalized to a baseline value of one. P<0·05 as determined by ANOVA and Least Squared Means post-tests on the delta Ct values. N=3/treatment miracidia, 4-day and 20-day sporocyst samples. Reprinted from Vermeire, J. J. et al., Differential gene expression and the effects of Biomphalaria glabrata embryonic (Bge) cell factors during larval Schistosoma mansoni development, Molecular and Biochemical Parasitology135, 153–157., Copyright (2004), with permission from Elsevier.
As a follow-up to the previous study, we have recently employed a DNA microarray approach to evaluate an even wider subset of cell signal-related genes in the free-living miracidium of S. mansoni as it ‘transforms’ in vitro to the parasitic mother sporocyst stage. These microarrays, developed and validated by Dr. Karl Hoffmann and colleagues (Department of Pathology, Cambridge University) (Fitzpatrick et al. 2005), were spotted with a total of 7335 unique S. mansoni sequences (oligonucleotides), of which approximately 5% represented signal transduction-related molecules based on gene ontology assignment. Results of three biological replicate experiments indicate that a large majority of signalling-related genes are expressed at similar levels in both the miracidium and mother sporocyst, and as reflected in the EST surveys mentioned above, Ras superfamily members (17) or Ras-associated proteins (activators/inhibitors; 6) and trimeric G proteins (5) were again highly represented in both miracidia and sporocysts. Other similarly expressed signalling-related transcripts included various receptors (EGF, TGF-β), adaptor proteins (14-3-3), nuclear receptors (RXR, RXRβ) and various cytoplasmic signalling kinases, lipases and phosphatases. However, based on a two-fold or greater increase in specific larval stage expression as the criterion for stage-associated gene expression, transformation and early development of the parasitic sporocyst stage appears to be accompanied by an upregulation in a number of signal-related genes (Fig. 2), thereby implying a functional role for such genes in the mother sporocyst. These preliminary data illustrate the enormous potential value of using a gene microarray approach as a general means of evaluating and comparing differential expression of large suites of genes (e.g. gene families or groups of pathway-related genes) during larval trematode development.

Fig. 2. Identification of differentially expressed stage-specific gene products in Schistosoma mansoni miracidial and sporocyst mRNA populations using DNA oligonucleotide microarray. Bar graph showing differential expression of signal-related receptors and molecules in developing S. mansoni larvae. BLASTx homologies describe each differentially transcribed gene. After initial normalization procedures, the log transformed fluorescence intensity ratios were plotted away from the line of identity (equal expression between stages). Results are based on averaged ratios from 3 biological replications of the experiment using separate parasite populations.
However, this sampling of the S. mansoni EST database is most probably not representative of the entire signalling repertoire of the Schistosoma spp. or of the many other digenean species yet to be studied. Thus it is expected that, with the addition of each new EST or genomic sequence database, many more signalling proteins and their pathways will continue to be identified well into the future. Given the previous summary of molecular evidence supporting the presence of signal reception- and transduction-related proteins in larval trematodes, the next questions to be explored are (1) based on what is known in other well-studied organisms, do the molecules identified to date comprise putative systems of signalling networks, and if so, (2) what might be their biological relevance in the life of these parasites? In order to address these questions, we have organized our review by first identifying putative receptors and elements of their known signal transduction systems, followed by a discussion of their potential roles in regulating larval motility and/or behaviour.
SIGNALLING RECEPTOR NETWORKS IN TREMATODE LARVAE
Trematodes are unique in comparison to other metazoan phyla in that all developmental stages, except the free-swimming miracidium, are covered by a single cellular syncitium (tegument), which represents the parasite's sole contact with the outside environment, be it either within or outside of its host. Therefore, it is presumed that the tegumental receptors play a vital role in receiving and transducing external signals to specific tissues within the parasite, thereby eliciting appropriate responses. However, many other cells/tissues comprising each parasite also possess receptors and associated signalling cascades, providing an extremely rich, but experimentally challenging, group of organisms for studying signal transduction networks.
Receptor tyrosine kinases
Signalling through receptor tyrosine kinases (RTKs) regulates a wide range of biological processes, including cell proliferation and differentiation (for review see Wong & Guillard, 2004). As the prototypical RTK, epidermal growth factor receptor (EGFR) activation was first shown to be intimately involved in acceleration of developmental processes and many of the mechanisms associated with activation and recruitment of signalling pathways following growth factor stimulation were derived from studying the EGF receptor system. Activation of flatworm RTKs by exogenous EGF-like ligands emanating from the host could potentially serve to trigger physiological events crucial to parasite development. The classical EGFR pathway in mammalian cells is initiated upon ligand binding of EGFR resulting in dimerization and activation of its own tyrosine kinase domain by auto-phosphorylation of specific tyrosine residues in the cytoplasmic tail. These residues allow for the binding of adaptor proteins like Grb2 and Shc, which serve as intermediates between RTKs and downstream signal cascades by linking activated EGFR to the Ras signalling pathway. At the plasma membrane, Ras, activated via GDP to GTP exchange, induces a kinase cascade including Raf (Mek kinase), MAP kinase kinase (MAPKK or Mek) and MAP kinase (MAPK). Activated MAPK typically translocates to the nucleus, where it can phosphorylate and activate transcription factors such as Elk-1 and c-Myc (Wong & Guillard, 2004). Phospholipase Cγ (PLCγ) activation also may occur when it is phosphorylated on specific tyrosine residues upon binding to an activated EGFR. The generation of the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP-3) are produced by PLCγ hydrolysis of the membrane phospholipid phosphatidylinositol-4,5-bisphosphate. At the membrane DAG, activates protein kinase C (PKC), a serine/threonine protein kinase involved in a diverse array of cellular processes (Newton, 1995). IP-3 induces Ca2+ release from intracellular stores, affecting a variety of Ca2+-dependent chemical reactions in the cell.
A number of RTK genes have been identified in the blood flukes, S. mansoni (SER; Shoemaker et al. 1992) and S. japonicum (Hu et al. 2003). RTKs with homology to members of the epidermal growth factor (EGF) receptor (SER; Shoemaker et al. 1992), the insulin receptor (RTK-2; AF314754 unpublished) and a novel type of RTK containing a Venus flytrap (VFT) module (RTK-1; Vicogne et al. 2003) of which two are known to be expressed by sporocysts of S. mansoni (SER and RTK-1). This family of receptor kinases is thought to signal through multiple pathways, involving activation of PKC and following the Ras/MAPK pathway, and a number of pathway members have been described in several Schistosoma spp. (Kampkotter et al. 1999; Schussler, Grevelding & Kunz, 1997). It is presumed that flatworm RTKs binding exogenous EGF or EGF-like ligands from the host also serve as potential triggers of physiological events crucial to parasite development.
Immunohistochemical studies have localized S. mansoni SER primarily to the muscle layer and possibly the tegument of adult male and female worms (Ramachandran, Skelly & Shoemaker et al. 1996), drawing speculation about the receptor's role in host-parasite relationships. Drug therapies targeting receptor kinases with compounds that inhibit receptor kinase signalling hinge on the selective toxicity of certain compounds to block specific receptor kinase ligand binding. In a recent publication, Vicogne & Dissous (2003) have suggested such a strategy to specifically target parasite growth factor receptors. The authors point out the fact that praziquantel remains the only drug widely utilized to treat schistosome infections and that resistance in endemic areas has been reported. Further characterization of trematode RTKs may yield novel therapeutic strategies to treat infected individuals.
Recently, expression of the S. mansoni SER has been confirmed in in vitro-transformed S. mansoni mother sporocysts (Vermeire et al. 2004; Vicogne et al. 2004) suggesting a potential functional pathway in larval schistosome stages. Stimulation of sporocysts with human EGF resulted in phosphorylation of tyrosine residues in a protein of similar molecular weight as S. mansoni SER (Vicogne et al. 2004). These results suggest that human EGF serves as cognate ligand for SER expressed in sporocysts and ligand binding causes autophosphorylation within the cytoplasmic tail of the receptor. EGF stimulation also resulted in increased metabolic activity (i.e. protein and DNA synthesis), denoted by increased incorporation of [35S] methionine and [3H] thymidine. Although it is unknown if an EGF-like ligand is expressed by the intermediate host of S. mansoni, B. glabrata, a novel EGF-like homologue (L-EFG) with neurotrophic activity has been described in the pond snail, Lymnaea stagnalis (Wildering, Hermann & Bulloch, 2001). L-EGF is unique in that its structure is that of a secreted peptide, the first of its type to be described in an invertebrate system. A role in snail reproduction has been proposed for this molecule given that it is expressed in the albumen gland, an organ involved in the secretion of nutritive perivitelline fluid onto fertilized oocytes before they are packaged into eggs masses. Expression of a similar molecule in B. glabrata is plausible although, as is the case with SER expression in adult worms, the functional significance of snail EGF-like ligand binding to larval schistosomes is unknown. With the potential link between snail L-EGF expression and reproduction, it might be speculated that larval trematodes may be competing for snail EGF, thus leading to host ligand depletion and disruption of snail reproduction (De Jong-Brink, 1995). Therefore, expressing a parasite EGF receptor could afford a survival advantage to developing schistosome larvae.
Receptor serine threonine kinase (RSTK) family
The receptor serine threonine kinases (RSTKs) bind cytokines of the transforming growth factor-beta (TGF-β) superfamily including TGF-β, activins, and bone morphogenetic proteins (BMP) and are involved in the regulation of various physiological processes, including embryonic development, homeostasis, chemotaxis, and cell cycle control (for review see Leask & Abraham, 2004). Activation of RSTKs triggers phosphorylation of the Smad proteins, the intracellular effectors of TGF-β signalling. In mammalian cells the TGFR pathway is initiated by ligand binding at the cell surface to the TGF-β type II receptor, which in turn triggers the recruitment of the TGF-β type I RSTK. The type I receptor kinase is subsequently activated via phosphorylation within its GS domain by the ligand-type II receptor complex. This activation of the type I receptor kinase allows for the transduction of the signal into the cell via recruitment and phosphorylation of receptor-regulated Smad (R-Smads) proteins. The R-Smad proteins interact with type I receptor and co-Smad proteins in the signal cascade by virtue of conserved MH1 and MH2 domains at their N- and C-termini. These domains possess DNA binding and transcriptional activation functions, respectively. When inactive, these domains interact in an auto-inhibitory manner. Phosphorylation of the C-terminal region of R-Smads by the type I receptor alleviates this inhibitory association and the R-Smad is then able to interact with its appropriate co-Smad. The phosphorylated R-Smad/co-Smad complex is capable of translocation to the cell nucleus where, in coordination with nuclear factors, it is able to exert its transcriptional activation function.
The TGF-β signalling pathway in S. mansoni has been studied in detail by incorporating a combination of molecular and functional assays, and to date it represents the most complete characterization of a platyhelminth signalling pathway. Davies, Shoemaker & Pearce (1998) were the first to identify and characterize the TGF-β type I receptor (SmRK1) in adult S. mansoni and subsequently found that this receptor was capable of interacting with mammalian TGF-β type II receptor in transfected COS7 cells when bound by ligand (TGF-β) (Beall & Pearce, 2001). In this heterologous expression system, TGF-β ligand-binding to dimerized type I and II receptors also was capable of complexing with schistosome Smad2, a downstream signalling protein, clearly demonstrating not only a structural conservation of schistosome type I TGF-β receptor with its mammalian counterpart, but a functional conservation, with its ability to generate an active receptor and bind components of the downstream signalling cascade. In follow-up studies they identified a type II TGF-β receptor homologue (SmRK2; Forrester, Warfel & Pearce, 2004), thus providing further support for a fully functional TGF-β receptor complex in the schistosome tegument.
In addition, a number of SmRK1-interacting proteins associated with activated TGF-β receptor signalling recently have been identified in S. mansoni. These include Smad (Smad1, Smad2; Beall, McGoniak & Pearce, 2000; Osman, Niles & Loverde, 2001) and co-Smad (Smad4; Osman et al. 2004) homologues. Furthermore, using yeast two-hybrid assays employing human type I TGF-β receptor as bait, the adaptor protein 14-3-3ε (McGonigle et al. 2001a), SmRK1 interacting protein (SIP) (McGonigle et al. 2001b) and eukaryotic initiation factor subunit 2 α (eIF2α) (McGonigle et al. 2002) also have been found. In functional assays, 14-3-3ε and eIF2α were shown to interact both with each other and also with SmRK1 (McGonigle, Beall & Pearce, 2002). Perhaps one of the most interesting results to emerge from all of the above studies is that schistosome receptors and signalling molecules associated with the TGF-β receptor family can functionally interact with those of mammalian systems, again demonstrating a high level of functional conservation for this cellular signal transducing system.
Constituent members of the TGF-β pathway, including RK-1 and Smad1 and 2 are also expressed in the miracidial and sporocyst stages of S. mansoni (Vermeire et al. 2004). Given the large body of accumulated findings, there is strong evidence for a TGF-β signalling network in larval schistosomes. However, a major question still exists as to its specific function in the biology of these parasites. Receptor localization at the tegumental surface of adult worms would imply a potentially important role for TGF-β signalling in establishing and/or maintaining a compatible host-parasite relationship. Alternatively, as Osman et al. (2004) have speculated, TGF-β-like molecules and their receptors may also be involved in male worm-induced maturation of females during pairing. The role of the TGF-β pathway in the miracidium and sporocyst stages remains unknown. Clearly, additional work that incorporates rapidly developing transgenic technologies (Davis et al. 1999; Boyle & Yoshino, 2003; Correnti & Pearce, 2004) is needed to begin addressing questions of receptor functionality in this and other parasitic flatworm species.
Guanine protein-coupled receptor family (GPCRs)
The GPCR family of receptors are integral membrane proteins with seven transmembrane domains, and which can be divided into three major subfamilies, including the rhodopsin (Class A), secretin-related (Class B) and metabotropic (Class C) receptors (Brody & Cravchic, 2000). GPCRs are known to bind a wide range of signal-mediating ligands including photons, neurotransmitters and hormones in order to transduce external stimuli across membranes and on to downstream effectors (Gether & Kobilka, 1998). In eukaryotes, sensory perception involving chemical, olfactory and photoreception are attributed to this family of receptors which are involved in many biological processes, from neurotransmission to stress responses (Brody & Cravchic, 2000).
GPCRs accomplish signal propagation via their seven α-helical transmembrane domains (see Hamm, 1998 for review). Classical GPCR receptors are characterized by their close association with heterotrimeric G-protein complexes composed of Gα, Gβ, and Gγ subunits. Each subunit can be encoded by multiple gene products and the four major families of heterotrimeric Gα-proteins include Gαs, Gαi/Gαo, Gαq and Gα12–Gα13. Receptor activation is initiated by binding of an extracellular ligand inducing conformational changes in the transmembrane helices, which in turn expose cryptic G-protein binding sites on intracellular loops. The activated receptor facilitates an exchange of GDP for GTP, yielding an activated G-protein. The active conformation brought about by GTP binding leads to the dissociation of the Gα and Gβγ subunits. Both Gα and Gβγ subunits are able to act in highly specific manners to modulate a multitude of target enzymes. Receptor desensitization can occur in a number of ways including receptor endocytosis or through phosphorylation by second messenger protein kinases like protein kinase A (PKA) and protein kinase C (PKC) which prevent receptor coupling to G proteins and therefore inhibit further signalling.
Several guanine protein-coupled receptors (GPCRs) and a number of associated heterotrimeric G protein subunits have been identified and characterized in trematodes. Early studies provided evidence for GPCRs in the adult stages of the liver fluke, Fasciola hepatica, and in S. mansoni that were capable of binding serotonin (5-HT), resulting in the activation of adenylate cyclase and production of the second messenger cyclic 3′,5′ AMP (cAMP) (Mansour, 1984). Further investigations of this class of trematode membrane receptors, termed serotonin receptors by Mansour and colleagues, have yielded the identification of Gαs, Gαo and Gαi homologues in S. mansoni and F. hepatica by immunoblotting (Mansour and Mansour, 1989) and the cloning and characterization of Gαs (Iltzsch et al. 1992). Gαi (Genbank accession number AF540395) and Gαo (Genbank accession number AF540394) subunits have also been identified in S. mansoni.
What about the role of 5-HT receptor signalling in larval trematodes? Once again, using the S. mansoni system, recent work suggests that reception for 5-HT binding may not only involve interactions with larval muscle, but other target tissues as well. For example, the sporocyst tegument appears to be the site of both serotonin transport and serotonin receptor activation (Boyle, Zaide & Yoshino, 2000; Boyle, Hillyer & Yoshino, 2003), suggesting that 5-HT may be serving a multifunctional role at the larval surface. It is known that sporocysts are capable of transporting exogenous 5-HT via a high affinity mechanism that is both Na+- and Cl−-dependent (Boyle et al. 2003) and is blocked by serotonin transporter (SERT)-inhibiting antidepressant compounds (Table 2). Therefore, it was hypothesized that 5-HT-mediated augmentation of muscle contractile activity may be linked directly to importation of exogenous 5-HT via tegumental SERT-like proteins for delivery to the subtending muscle layers. However, 5-HT transport blockers were unable to inhibit 5-HT-mediated muscle contractility (Boyle & Yoshino, 2005), suggesting, to the contrary, that these tegumental transporters are not involved, at least directly, in the modulation of muscle activity. This notion is further supported by results of labelled 5-HT tracer experiments showing that [3H]5-HT is transported through the tegument, but localizes mainly in the apical gland of in vitro transformed sporocysts, and not to the muscle layer (Boyle et al. 2003). An alternative explanation, based on earlier findings that exposure of in vitro-transformed sporocysts to 5-HT receptor antagonists reduces both the frequency and duration of sporocyst muscular contractions (Boyle et al. 2000), may be that exogenous 5-HT binds to tegumental 5-HT receptors that are directly coupled to subtending muscle cells through some yet unknown signalling mechanism (Boyle & Yoshino, 2005). Typically metabotropic 5-HT receptors involve G protein-mediated activation of adenylate cyclase and production of cAMP (Noda et al. 2004). Receptor activation could potentially lead to the production of second messengers that would diffuse directly to the subtending muscle layer to increase muscle contractility. Alternatively, these same second messengers could alter the ionic permeability of the tegumental membrane by modulating existing ion channels through activation of specific protein kinases (Pax et al. 1983). Clearly further follow-up studies are warranted to resolve these intriguing questions.

The ability of larval trematodes to respond to changes in light intensity is likely to be facilitated by GPCR photoreceptors. A GPCR rhodopsin gene encoding a putative photoreceptor has been identified and cloned by Hoffmann and colleagues (Hoffmann et al. 2001) from S. mansoni. Expression of this receptor was found to be developmentally regulated in that rhodopsin transcript levels were higher in both free-swimming cercarial and miracidial stages compared to the parasitic adult worms or the mother sporocyst stages (Hoffmann et al. 2001; Vermeire et al. 2004). The biological implications of the differential expression patterns of the rhodopsin gene in schistosomes will be discussed in the next section.
Another GPCR capable of specifically binding histamine was identified and described in adult worms of S. mansoni by Hamdan et al. (2002). Follow-up experiments further showed that expression and activation of this receptor in a heterologous mammalian cell line result in elevation of intracellular Ca2+ levels, demonstrating its ability to manifest cellular signals in a manner typical of other GPCRs. Results of preliminary studies conducted in our laboratory have demonstrated stage-specific expression of this receptor in in vitro-derived mother sporocysts, but not the miracidium, suggesting a functional role of the histamine GPCR and its signalling system in the establishment of intramolluscan larval infections (unpublished data in collaboration with P. Ribeiro, McGill University).
CELL SIGNALLING: PUTATIVE ROLES IN LARVAL MOTILITY AND HOST-FINDING BEHAVIOUR
The free-living larval stages of the Trematoda exhibit behaviours that allow them to locate and invade their appropriate hosts. These behaviours include increases in the rate of change of direction when stimulated by chemical agents and a turn-back response upon sensing a decreasing concentration gradient. Representing a critical link in the life cycle of digeneans, this host-finding behaviour is dictated by a multitude of sensory perceptions including chemotactic orientation, temperature gradients and light stimuli (Sopott-Ehlers, Haas & Ehlers, 2003). A number of molecules have been identified in snail-conditioned water (SCW) and described as chemo-attractants for miracidia of S. mansoni, S. haematobium, Trichobilharzia ocellata, Fasciola hepatica and Echinostoma caproni, all of which respond to macromolecules present in SCW (Haberl et al. 1999; Kalbe, Haberl & Haas, 2000).
Species-specific host finding behaviour has been described for some larval trematodes including F. hepatica and T. ocellata. These two trematodes display specificity for SCW from their host snail species, Lymnaea truncatula or L. stagnalis, respectively, and not to SCW from other sympatric snail species (Kalbe et al. 2000). Similarly, the chemical host signals that stimulate invasion and transformation are not the same for trematode miracidial and cercarial stages. In fact, the cercariae and miracidia of E. caproni have been shown to utilize different signals when locating the same host, Biomphalaria alexandrina (Haberl et al. 1999). Miracidia of E. caproni are stimulated by high molecular weight glycoconjugates, while cercariae respond to amino acids, peptides, sugars and other small molecular weight molecules. It seems clear then that E. caproni miracidia and cercariae must utilize different receptor systems to respond to these separate stimuli. Although a large body of work has focused upon the host-finding behaviour of miracidia and cercariae of the Trematoda, less has been done to understand its molecular underpinnings. Receptors and their associated signal transduction pathways presumably link the parasites to ability to perceive the various stimuli and this behaviour, although the nature of these systems remains an open and unexplored area.
Because the GPCR rhodopsin has been ascribed a putative function as part of a light-sensing organ system localized to sub-tegumental structures found near the anterior end of cercariae (Hoffmann et al. 2001), it is speculated that its up-regulation specifically in the free-living larval stages is associated with photoreception and photoreactive behaviour associated with host-finding. Photoreceptors are broadly split into two main types, the ciliary and the rhabdomeric, both having been described in trematode larvae. Rhabdomeric photoreceptors are present in the cercarial stages of Trichobilharzia ocellata (Sopott-Ehlers et al. 2003) and are found in ocelli cup cell structures, consisting of pigment granule subunits and are positioned in a dorsal-lateral orientation close to the front of the cerebral ganglion. Analogous structures have also been described in cercariae of S. mansoni (Dorsey et al. 2002). Previous studies have also drawn attention to many forms of putative sensory non-ciliate, uniciliate, and multiciliate bulb structures located in the tegumental and subtegumental regions of S. mansoni and E. revolutum cercariae (Short & Gagne, 1975; Pan, 1980; Zdarska, 1992). Moreover, in a recent study more than 13 types of structural sensory receptors were identified in the tegument of Austrobilharzia sp. cercariae using scanning electron microscopy (Abdul-Salam & Sreelatha, 2004). The existence of distinctly different sensory structures on the surface of larval trematodes is thought to correlate with responses to variant stimuli, both light and shadow. Rhabdomeric receptors associated with sensory structures are believed to allow for the perception of increases in light stimuli whereas ciliary bulb structures may sense increases in shadow (Short & Gagne, 1975). However, the relationship between these structures and their coincidence with molecular receptor systems remains an open question. Research in this area will undoubtedly increase our understanding of how larval trematodes perceive their environment and seek out new hosts.
The presence of such sensory structures alone does not explain the ability of trematode larvae to respond external stimuli. They represent the external termini of the intricate sensory systems used by larval trematodes to orient themselves in the pursuit of a suitable intermediate host. Receptors must be linked to signal transduction pathways or neural networks that allow for propagation of a signal downstream in order to affect cellular processes or parasite movement. Ultrastructural investigations carried out in conjunction with studies of receptor position relative to neuronal networks draw a connection between sensory perception and the locomotive behaviour involved in host finding. One such study involving a species of Allopodocotyle (Digenea: Opecoelidae), showed a correlation between location of major transverse commissures and nerve cords with various types of sensory structures located in cercaria by cholinesterase and silver nitrate staining (Bogea & Caira, 2001). The topics of trematode nervous communication are covered in detail in review articles in this supplement. It seems clear that stimuli perceived by receptors located in these structures could have a direct impact on musculature controlled by associated nervous connections. These types of observations are necessary to begin to understand fully the relationship between how sensory structures detect external stimuli and how those signals are transduced and interpreted to effect parasite behaviour.
As alluded to above, although physical and chemical attractants such as light, amino acids and fatty acids are thought to be involved in host finding (Sturrock, 2001), little is known about the specific receptors and signalling pathways used by parasites in directing their behaviour. One intriguing finding resulting from a search of the S. mansoni EST database is the presence of numerous sequences in larval S. mansoni that share significant homology with signalling proteins involved in a two-component bacterial chemotaxis pathway. In this pathway, ligand is bound by a methyl accepting receptor, and the signal passes through a histidine kinase then onto a response regulatory protein, which ultimately modulates the flagellar motor (Fig. 4) (Falke et al. 1997). The phosphorylated/activated regulatory response protein (che Y) binds to the motor and changes the direction of flagellar rotation to produce ‘tumbling’ or random movement until a new direction is taken (Falke & Hazelbauer, 2001). Such bacterial chemotaxis pathways respond to a variety of ligands including aspartate, ribose, galactose, serine, dipeptides, citrate and redox potential (Falke & Hazelbauer, 2001). Since miracidia demonstrate specific swimming patterns in their search for hosts, it is not inconceivable that they utilize such a chemotactic signalling pathway or something similar to it. Interestingly, although components of this pathway are found almost exclusively in bacteria, in eukaryotes they appear to be confined to plants and free-living microbial organisms, such as yeasts, fungi and protozoa (Wolanin, Thomason & Stock, 2002), although two EST sequences from Anopheles gambiae bear similarity to members of two component systems (Swiss-Prot/TrEMBL accession numbers Q7PE76 and Q7P455). No homologues of this chemotaxis pathway have been identified in the completed genomes of Caenorhabditis elegans, Drosophila melanogaster or Homo sapiens, and it is thought that these molecules are not represented in the animal kingdom (Wolanin et al. 2002). However, we may need to re-think the existence and possible functional role of this pathway in animals in view of the finding of gene homologues in mosquitoes and schistosomes.

Fig. 3. (a) Photomicrograph of a daughter sporocyst (DS) emerging from a mother sporocyst (MS) under control conditions. The daughter sporocyst was observed forcing itself through the body wall of the mother, and this photo was taken after full emergence. Scale bar: 25 μm. (b) Photomicrograph of a mother sporocyst ‘brood chamber’ containing developing daughter sporocysts (DS) in the presence of 5 μM fluoxetine. Scale bar: 50 μm. Reprinted from Boyle, J. P. & Yoshino, T. P., Serotonin-induced muscular activity in Schistosoma mansoni larval stages: importance of 5-HT transport and role in daughter sporocyst production, Journal of Parasitology91, Copyright (2005), with permission from Allen Press.

Fig. 4. Diagram depicting a generalized bacterial chemotaxis pathway. Ligand is bound by a methyl accepting receptor (MCP), and relays a signal through a histidine kinase (Che A) then a response regulatory protein (Che Y). Che Y can modulate flagellar movement by binding to the flagellar motor. MCP methylation is regulated by Che B and Che R. Asterisks denote pathway components that may be represented by homologues in the S. mansoni EST database.
In addition to host finding, another GPCR, the 5-HT receptor, and its signalling system has been implicated in the in vitro development/production of S. mansoni daughter sporocysts. Bayne & Grevelding (2003) recently observed that exposure of mother sporocysts to exogenous 5-HT stimulated the release of daughter sporocysts from the mother stages. Moreover, reduced levels of endogenous 5-HT appear to correlate with the inhibition of daughter sporocyst release in vitro (Boyle & Yoshino, 2005). In this study, daughter sporocyst production, endogenous 5-HT levels and parasite motility were significantly reduced in mother sporocysts treated with 5-HT transport inhibitors when compared to controls (Table 2). Under control in vitro culture conditions, daughter sporocysts were observed emerging from mother sporocyst brood chambers by forcing themselves directly through the body wall of the mother sporocyst (Fig. 3). In contrast, the majority of daughters developing in mother sporocysts treated with the 5-HT transport inhibitor, fluoxetine, did not emerge from brood chambers, even though they appeared morphologically comparable to their control stages. Overall, these findings support a role of endogenous 5-HT in the development/production of daughter sporocyst stages that involves 5-HT transporter activity. In this case, it is speculated that daughter sporocyst muscle contractility is dependent upon a supply of endogenous 5-HT provided to the brood chamber compartment via membrane 5-HT transporters.
CONCLUDING REMARKS
Throughout their complex life cycles, the Trematoda rely upon receptor systems to sense and integrate a complexity of physical and chemical cues found in their external and internal environments. Their ability to transition successfully from their free-living to parasitic forms and establish successful infections within new hosts constitutes an essential link in their development and continued survival. Ongoing research in this area has produced data that has only just begun to describe the putative signal transduction pathways utilized by these specialized organisms. From what we presently know, it is clear that larval trematodes possess many of the signalling cascades present in other metazoans, including mammals. These cascades include transmembrane receptors and their associated pathway members that are necessary to activate downstream effector molecules that ultimately modulate cellular processes governing larval motility and behaviour. As new and effective tools (e.g. in vitro culture of helminths, RNA interference, DNA/protein microarrays) are developed to explore the intricate details of these systems, our capability to address important hypotheses pertaining to such cellular signalling processes will be greatly expanded. Increasing amounts of gene sequence information and genome-wide gene expression analyses, coupled with burgeoning proteomics tools, will surely contribute to our understanding of the biology of larval trematodes.